

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lanosterol 14-alpha demethylase (Candida albicans (strain SC5314 / ATCC MYA-2876) (...) | BDBM213825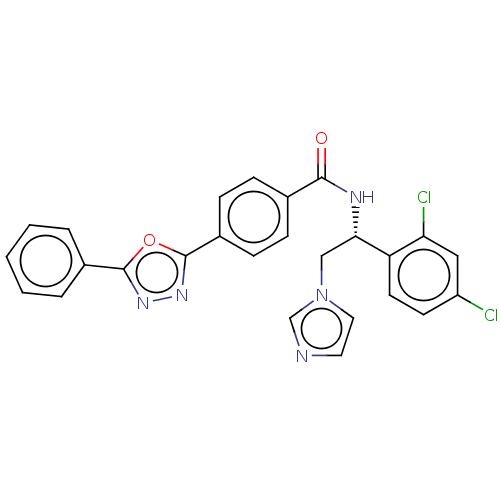 (VNI) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Candida albicans CYP51 assessed as reduction in [3-3H]lanosterol 14alpha-demethylation preincubated for 60 secs followed by NADPH addit... | J Med Chem 61: 5679-5691 (2018) Article DOI: 10.1021/acs.jmedchem.8b00641 BindingDB Entry DOI: 10.7270/Q26H4KX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505758 (CHEMBL4444489) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 min using [3H] lanosterol as substrate by HPLC analys... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50465948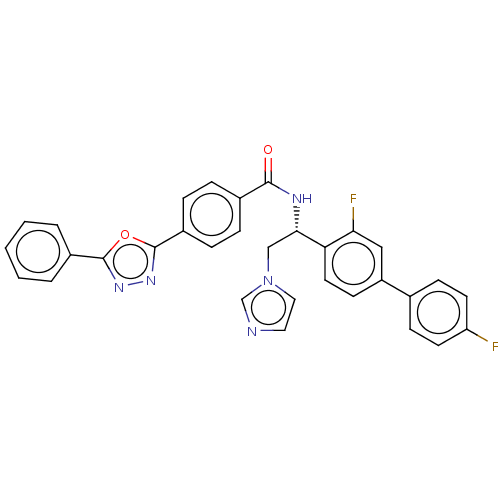 (CHEMBL3629567) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 min using [3H] lanosterol as substrate by HPLC analys... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505766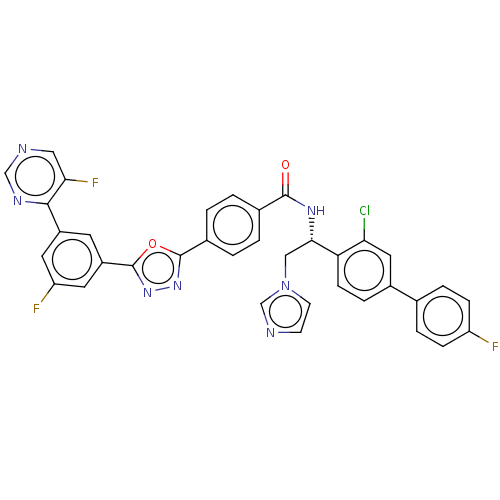 (CHEMBL4522116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 min using [3H] lanosterol as substrate by HPLC analys... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505763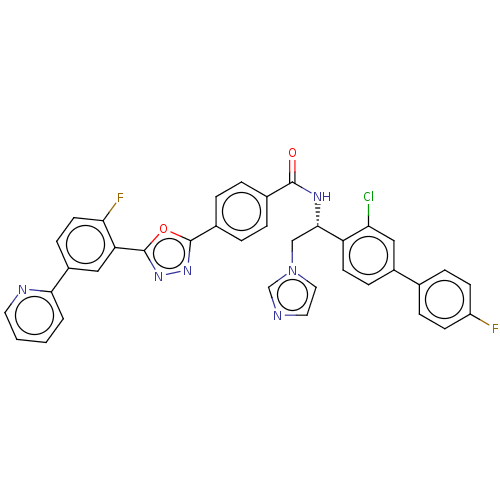 (CHEMBL4466288) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 min using [3H] lanosterol as substrate by HPLC analys... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505764 (CHEMBL4461447) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 min using [3H] lanosterol as substrate by HPLC analys... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505761 (CHEMBL4277680) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 min using [3H] lanosterol as substrate by HPLC analys... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505768 (CHEMBL4439225) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 min using [3H] lanosterol as substrate by HPLC analys... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505765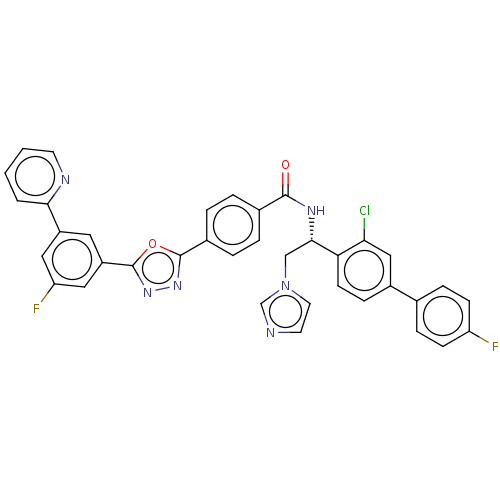 (CHEMBL4435160) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 min using [3H] lanosterol as substrate by HPLC analys... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505766 (CHEMBL4522116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 hr using [3H] lanosterol as substrate by HPLC analysi... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505765 (CHEMBL4435160) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 hr using [3H] lanosterol as substrate by HPLC analysi... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505760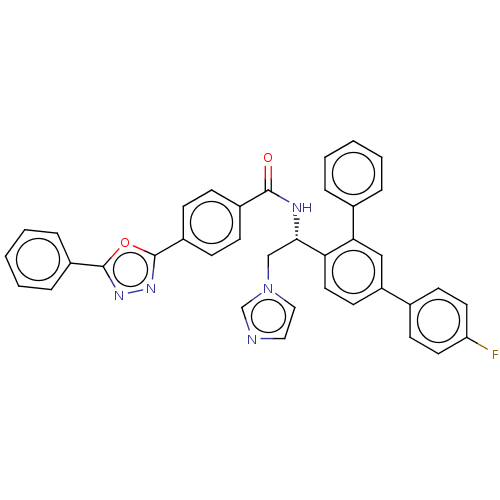 (CHEMBL4458275) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 min using [3H] lanosterol as substrate by HPLC analys... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505759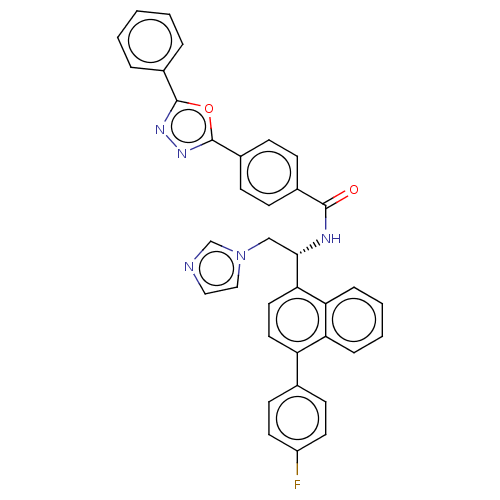 (CHEMBL4593688) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 min using [3H] lanosterol as substrate by HPLC analys... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505768 (CHEMBL4439225) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 hr using [3H] lanosterol as substrate by HPLC analysi... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505764 (CHEMBL4461447) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 hr using [3H] lanosterol as substrate by HPLC analysi... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505762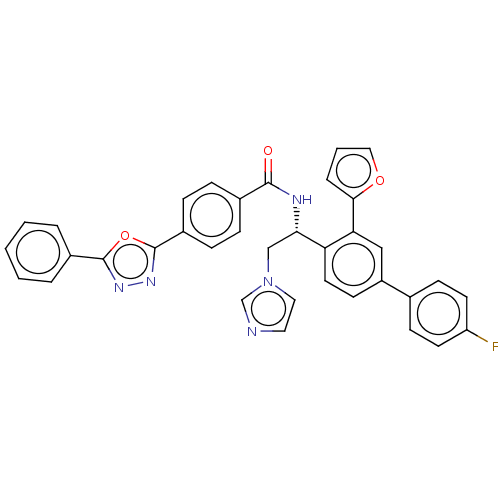 (CHEMBL4452294) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 min using [3H] lanosterol as substrate by HPLC analys... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505761 (CHEMBL4277680) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 hr using [3H] lanosterol as substrate by HPLC analysi... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505767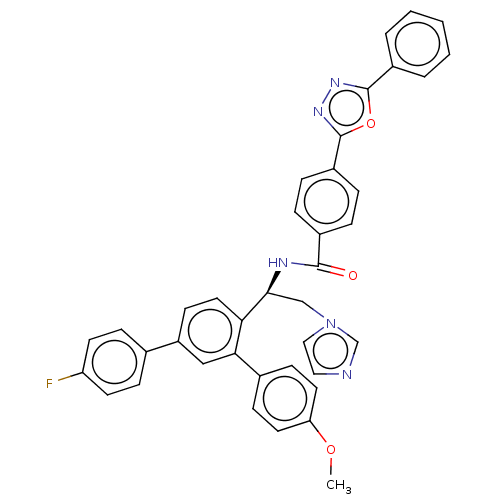 (CHEMBL4472919) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 min using [3H] lanosterol as substrate by HPLC analys... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505758 (CHEMBL4444489) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 hr using [3H] lanosterol as substrate by HPLC analysi... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505763 (CHEMBL4466288) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 hr using [3H] lanosterol as substrate by HPLC analysi... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50465948 (CHEMBL3629567) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 hr using [3H] lanosterol as substrate by HPLC analysi... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505767 (CHEMBL4472919) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 hr using [3H] lanosterol as substrate by HPLC analysi... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505762 (CHEMBL4452294) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 hr using [3H] lanosterol as substrate by HPLC analysi... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505759 (CHEMBL4593688) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 hr using [3H] lanosterol as substrate by HPLC analysi... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50505760 (CHEMBL4458275) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant full length human CYP51 expressed in Escherichia coli incubated for 1 hr using [3H] lanosterol as substrate by HPLC analysi... | J Med Chem 62: 10391-10401 (2019) Article DOI: 10.1021/acs.jmedchem.9b01485 BindingDB Entry DOI: 10.7270/Q2TM7FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM25810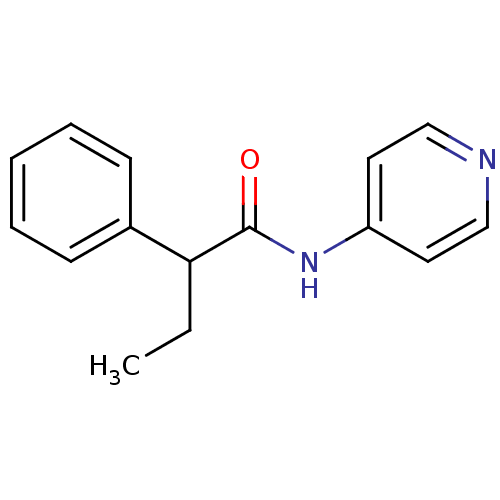 (2-phenyl-N-(pyridin-4-yl)butanamide | 2-phenyl-N-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 440 | n/a | n/a | n/a | 7.4 | 24 |
Vanderbilt University | Assay Description As a cysteine-coordinated hemoprotein, sterol 14-alpha-demethylase responds spectrally to any perturbations in the area surrounding the heme iron. Th... | J Med Chem 52: 2846-53 (2009) Article DOI: 10.1021/jm801643b BindingDB Entry DOI: 10.7270/Q2MP51MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM29341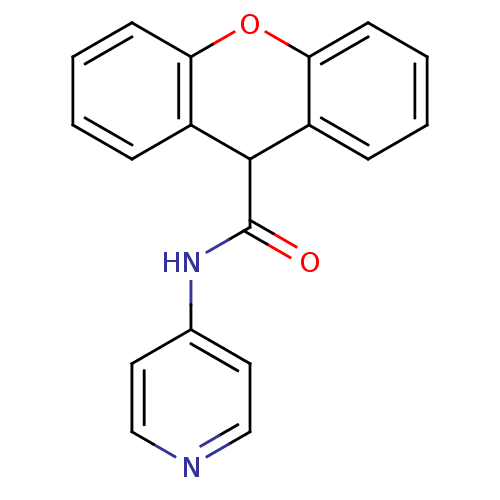 (ChemDiv 5556-0480, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 750 | n/a | n/a | n/a | 7.4 | 24 |
Vanderbilt University | Assay Description As a cysteine-coordinated hemoprotein, sterol 14-alpha-demethylase responds spectrally to any perturbations in the area surrounding the heme iron. Th... | J Med Chem 52: 2846-53 (2009) Article DOI: 10.1021/jm801643b BindingDB Entry DOI: 10.7270/Q2MP51MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM29342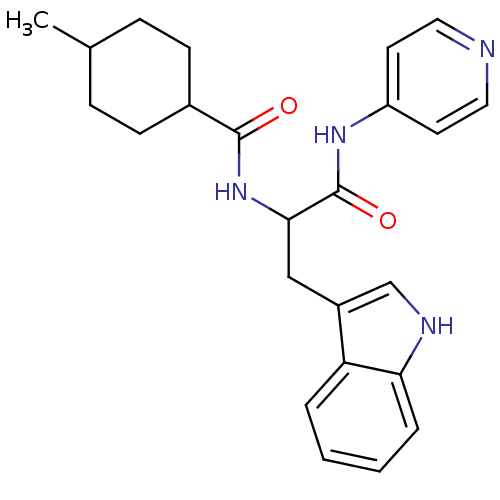 (ChemDiv C155-0123, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 240 | n/a | n/a | n/a | 7.4 | 24 |
Vanderbilt University | Assay Description As a cysteine-coordinated hemoprotein, sterol 14-alpha-demethylase responds spectrally to any perturbations in the area surrounding the heme iron. Th... | J Med Chem 52: 2846-53 (2009) Article DOI: 10.1021/jm801643b BindingDB Entry DOI: 10.7270/Q2MP51MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM22962 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 290 | n/a | n/a | n/a | 7.4 | 24 |
Vanderbilt University | Assay Description As a cysteine-coordinated hemoprotein, sterol 14-alpha-demethylase responds spectrally to any perturbations in the area surrounding the heme iron. Th... | J Med Chem 52: 2846-53 (2009) Article DOI: 10.1021/jm801643b BindingDB Entry DOI: 10.7270/Q2MP51MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM29343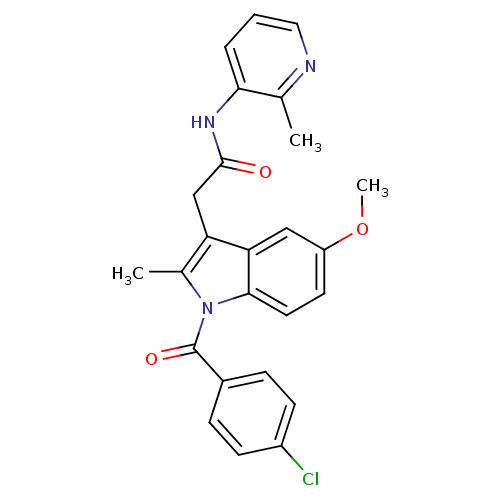 (Indomethacin-amide derivative, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | 7.4 | 24 |
Vanderbilt University | Assay Description As a cysteine-coordinated hemoprotein, sterol 14-alpha-demethylase responds spectrally to any perturbations in the area surrounding the heme iron. Th... | J Med Chem 52: 2846-53 (2009) Article DOI: 10.1021/jm801643b BindingDB Entry DOI: 10.7270/Q2MP51MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM29344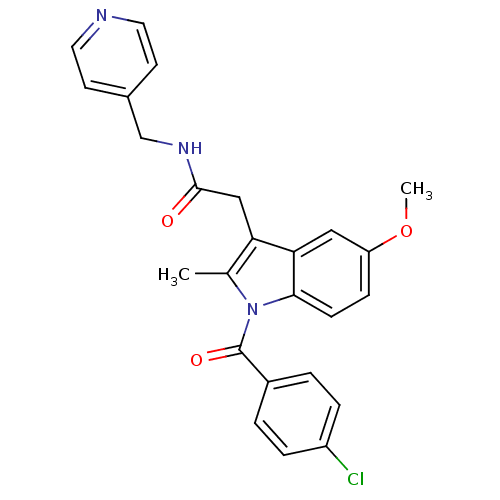 (Indomethacin-amide derivative, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 540 | n/a | n/a | n/a | 7.4 | 24 |
Vanderbilt University | Assay Description As a cysteine-coordinated hemoprotein, sterol 14-alpha-demethylase responds spectrally to any perturbations in the area surrounding the heme iron. Th... | J Med Chem 52: 2846-53 (2009) Article DOI: 10.1021/jm801643b BindingDB Entry DOI: 10.7270/Q2MP51MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM29345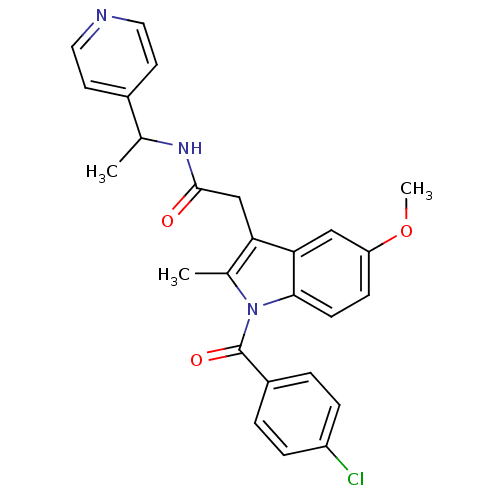 (Indomethacin-amide derivative, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 370 | n/a | n/a | n/a | 7.4 | 24 |
Vanderbilt University | Assay Description As a cysteine-coordinated hemoprotein, sterol 14-alpha-demethylase responds spectrally to any perturbations in the area surrounding the heme iron. Th... | J Med Chem 52: 2846-53 (2009) Article DOI: 10.1021/jm801643b BindingDB Entry DOI: 10.7270/Q2MP51MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM29346 (Indomethacin-amide derivative, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | 7.4 | 24 |
Vanderbilt University | Assay Description As a cysteine-coordinated hemoprotein, sterol 14-alpha-demethylase responds spectrally to any perturbations in the area surrounding the heme iron. Th... | J Med Chem 52: 2846-53 (2009) Article DOI: 10.1021/jm801643b BindingDB Entry DOI: 10.7270/Q2MP51MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM29347 (Indomethacin-amide derivative, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 560 | n/a | n/a | n/a | 7.4 | 24 |
Vanderbilt University | Assay Description As a cysteine-coordinated hemoprotein, sterol 14-alpha-demethylase responds spectrally to any perturbations in the area surrounding the heme iron. Th... | J Med Chem 52: 2846-53 (2009) Article DOI: 10.1021/jm801643b BindingDB Entry DOI: 10.7270/Q2MP51MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM29348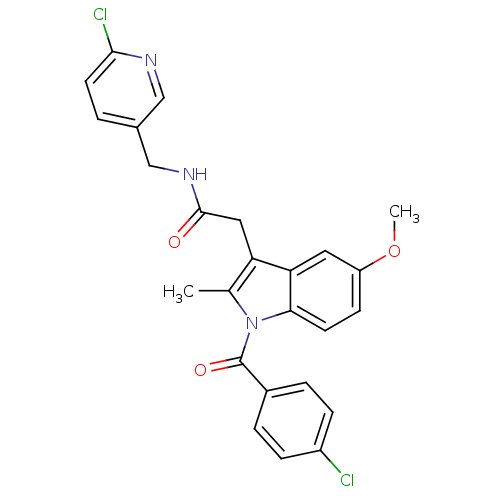 (Indomethacin-amide derivative, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 260 | n/a | n/a | n/a | 7.4 | 24 |
Vanderbilt University | Assay Description As a cysteine-coordinated hemoprotein, sterol 14-alpha-demethylase responds spectrally to any perturbations in the area surrounding the heme iron. Th... | J Med Chem 52: 2846-53 (2009) Article DOI: 10.1021/jm801643b BindingDB Entry DOI: 10.7270/Q2MP51MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM29349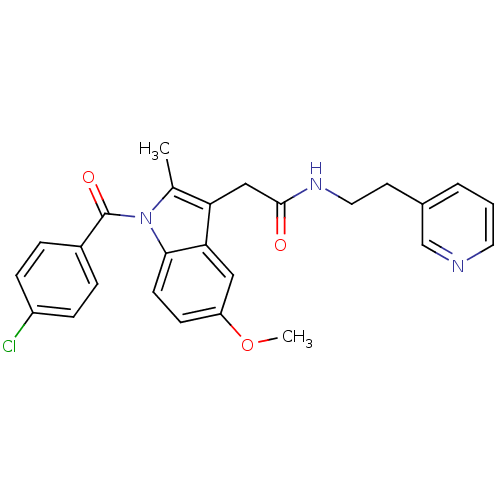 (Indomethacin-amide derivative, 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | 7.4 | 24 |
Vanderbilt University | Assay Description As a cysteine-coordinated hemoprotein, sterol 14-alpha-demethylase responds spectrally to any perturbations in the area surrounding the heme iron. Th... | J Med Chem 52: 2846-53 (2009) Article DOI: 10.1021/jm801643b BindingDB Entry DOI: 10.7270/Q2MP51MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM29350 (Indomethacin-amide derivative, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 360 | n/a | n/a | n/a | 7.4 | 24 |
Vanderbilt University | Assay Description As a cysteine-coordinated hemoprotein, sterol 14-alpha-demethylase responds spectrally to any perturbations in the area surrounding the heme iron. Th... | J Med Chem 52: 2846-53 (2009) Article DOI: 10.1021/jm801643b BindingDB Entry DOI: 10.7270/Q2MP51MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM29351 (Indomethacin-amide derivative, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 150 | n/a | n/a | n/a | 7.4 | 24 |
Vanderbilt University | Assay Description As a cysteine-coordinated hemoprotein, sterol 14-alpha-demethylase responds spectrally to any perturbations in the area surrounding the heme iron. Th... | J Med Chem 52: 2846-53 (2009) Article DOI: 10.1021/jm801643b BindingDB Entry DOI: 10.7270/Q2MP51MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM81297 (Azole, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 320 | n/a | n/a | n/a | 6.0 | n/a |
Vanderbilt University | Assay Description Azole derivatives used as an inhibitor of TB and TC CYP51. | Chem Biol 14: 1283-93 (2007) Article DOI: 10.1016/j.chembiol.2007.10.011 BindingDB Entry DOI: 10.7270/Q22J69B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha-demethylase (Trypanosoma brucei) | BDBM81297 (Azole, 1) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | 6.0 | n/a |
Vanderbilt University | Assay Description Azole derivatives used as an inhibitor of TB and TC CYP51. | Chem Biol 14: 1283-93 (2007) Article DOI: 10.1016/j.chembiol.2007.10.011 BindingDB Entry DOI: 10.7270/Q22J69B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 119 total ) | Next | Last >> |