

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098322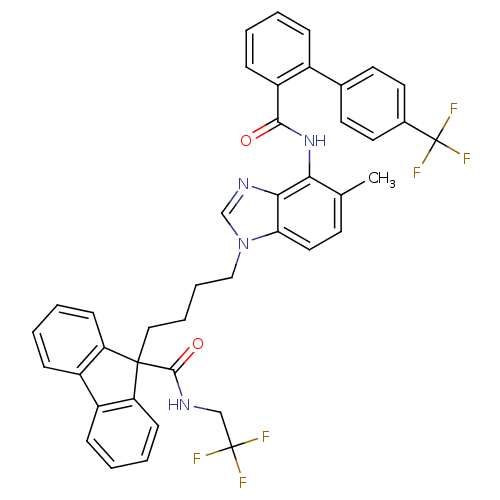 (9-(4-{5-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098321 (9-(4-{2,5-Dimethyl-4-[(4'-trifluoromethyl-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098326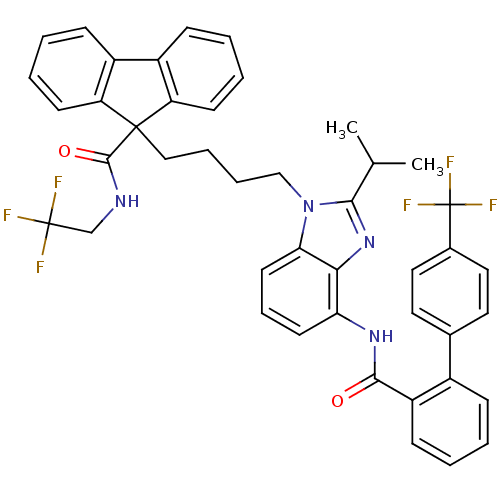 (9-(4-{2-Isopropyl-4-[(4'-trifluoromethyl-biphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098324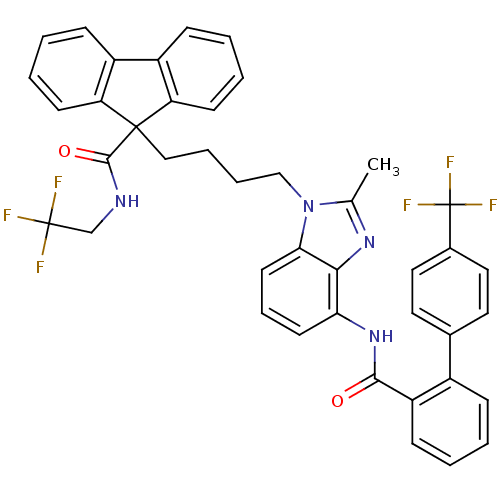 (9-(4-{2-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098325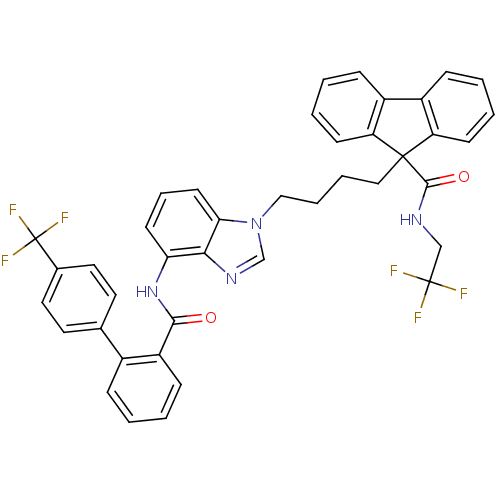 (9-(4-{4-[(4'-Trifluoromethyl-biphenyl-2-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031845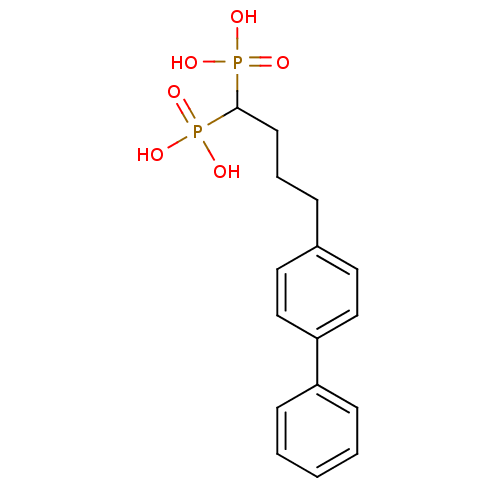 ((4-Biphenyl-4-yl-1-phosphono-butyl)-phosphonic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031845 ((4-Biphenyl-4-yl-1-phosphono-butyl)-phosphonic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098320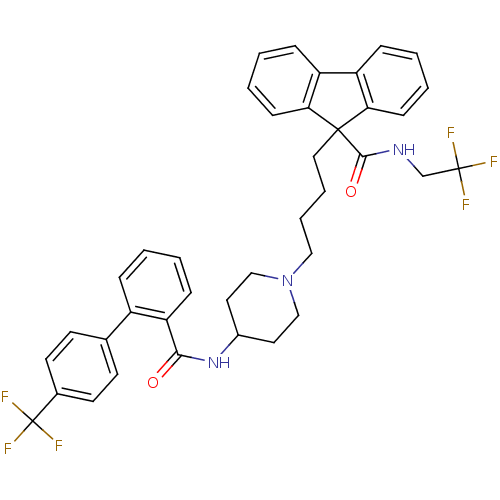 (9-(4-{4-[(4''-Trifluoromethyl-biphenyl-2-carbonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098322 (9-(4-{5-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098324 (9-(4-{2-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098321 (9-(4-{2,5-Dimethyl-4-[(4'-trifluoromethyl-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049232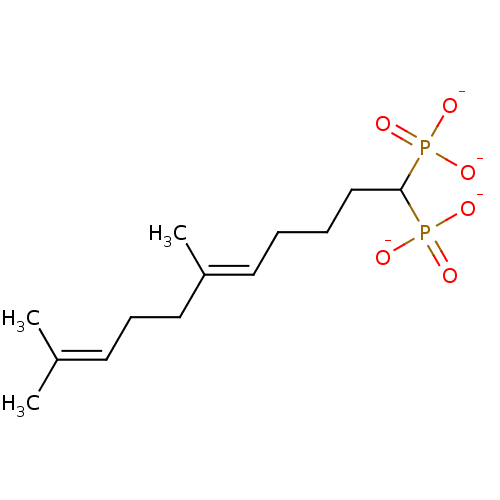 (CHEMBL348349 | Tetrasodium salt of 4-(4'-Methyl-bi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031839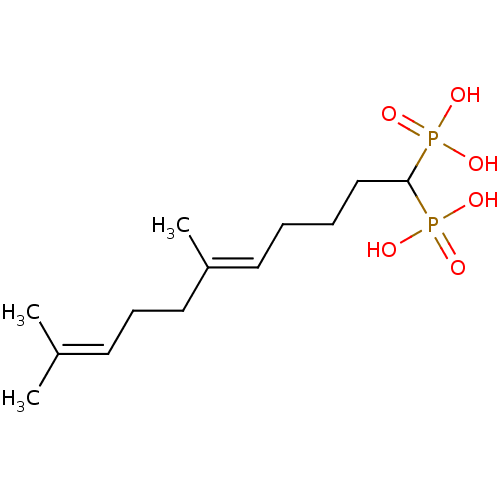 (((E)-6,10-Dimethyl-1-phosphono-undeca-5,9-dienyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098323 (9-(4-{2-Propyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031843 (((E)-8,12-Dimethyl-1-phosphono-trideca-7,11-dienyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031848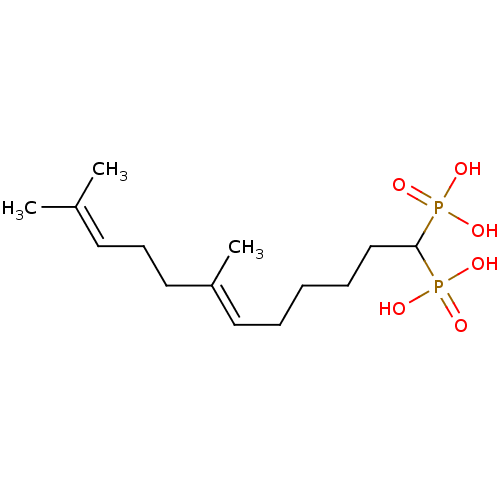 (((E)-7,11-Dimethyl-1-phosphono-dodeca-6,10-dienyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50153193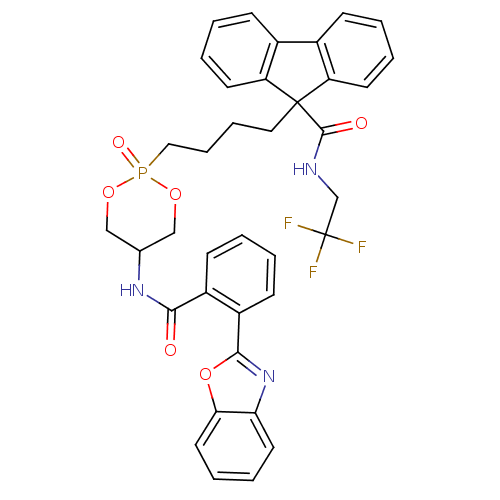 (CHEMBL186836 | Trans-9-{4-[5-(2-Benzooxazol-2-yl-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human Microsomal triglyceride transfer protein | Bioorg Med Chem Lett 14: 5067-70 (2004) Article DOI: 10.1016/j.bmcl.2004.07.069 BindingDB Entry DOI: 10.7270/Q2H70F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM205027 (US9249096, 40) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031844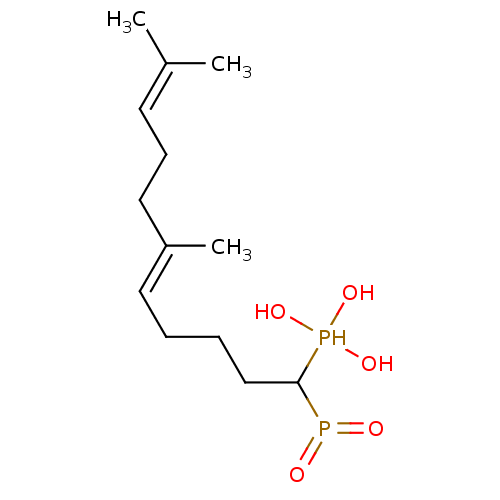 (((E)-1-Hydroxyphosphinoyl-6,10-dimethyl-undeca-5,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098326 (9-(4-{2-Isopropyl-4-[(4'-trifluoromethyl-biphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031851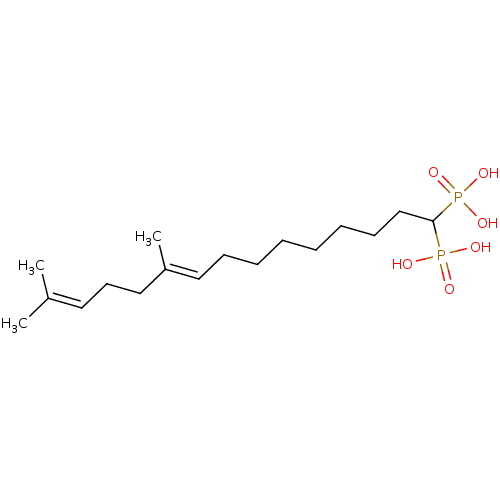 (((E)-10,14-Dimethyl-1-phosphono-pentadeca-9,13-die...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031842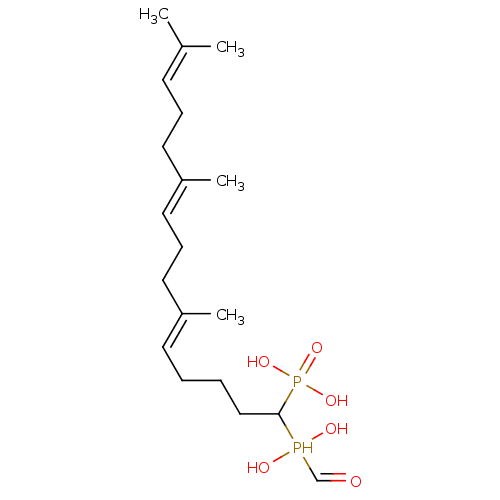 (CHEMBL86867 | [(5E,9E)-1-(Hydroxy-hydroxymethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50153191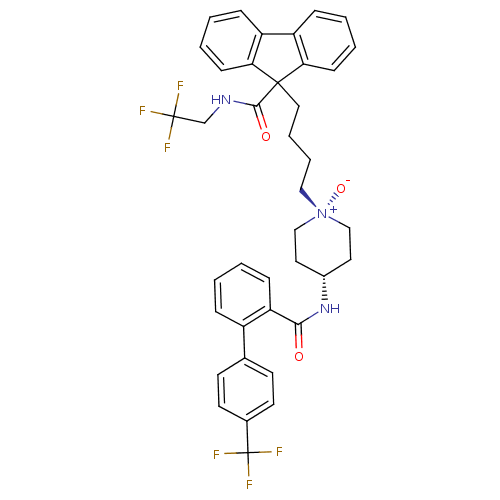 (9-(4-{1-Oxy-4-[(4''-trifluoromethyl-biphenyl-2-car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human Microsomal triglyceride transfer protein | Bioorg Med Chem Lett 14: 5067-70 (2004) Article DOI: 10.1016/j.bmcl.2004.07.069 BindingDB Entry DOI: 10.7270/Q2H70F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098325 (9-(4-{4-[(4'-Trifluoromethyl-biphenyl-2-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458638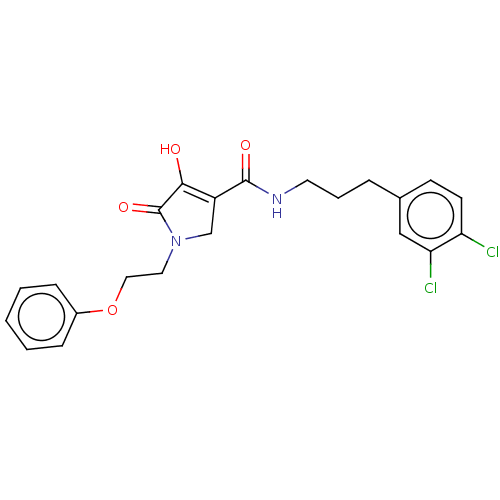 (CHEMBL4203395) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049227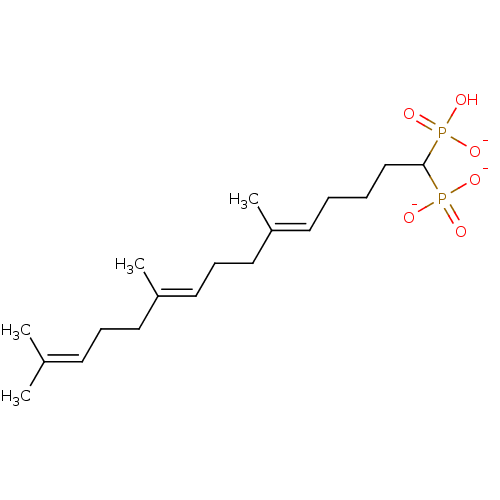 (CHEMBL158707 | Trisodium salt of [1-(Dimethoxy-pho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031847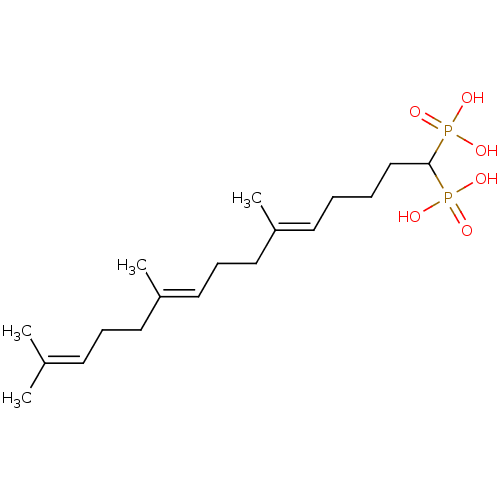 (((5E,9E)-6,10,14-Trimethyl-1-phosphono-pentadeca-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031832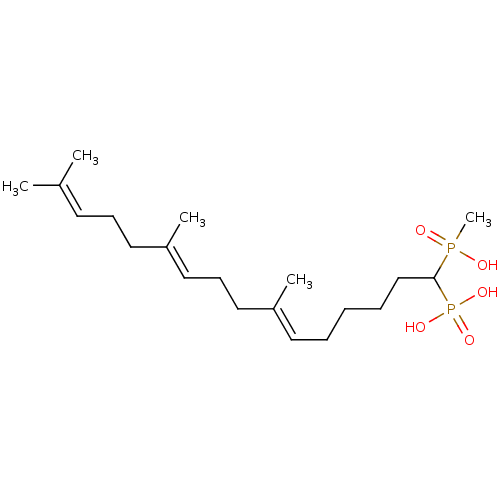 (CHEMBL87922 | [(6E,10E)-1-(Hydroxy-methyl-phosphin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031859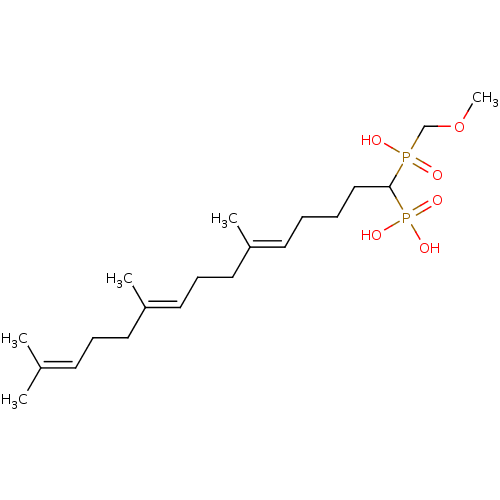 (CHEMBL314797 | [(5E,9E)-1-(Hydroxy-methoxymethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50153189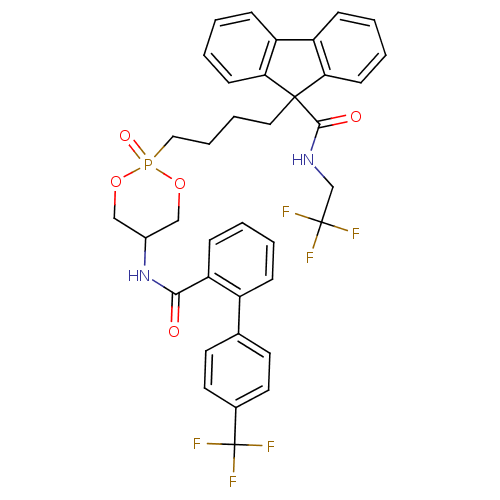 (CHEMBL365687 | Cis-9-(4-{2-Oxo-5-[(4''-trifluorome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human Microsomal triglyceride transfer protein | Bioorg Med Chem Lett 14: 5067-70 (2004) Article DOI: 10.1016/j.bmcl.2004.07.069 BindingDB Entry DOI: 10.7270/Q2H70F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50153189 (CHEMBL365687 | Cis-9-(4-{2-Oxo-5-[(4''-trifluorome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human Microsomal triglyceride transfer protein | Bioorg Med Chem Lett 14: 5067-70 (2004) Article DOI: 10.1016/j.bmcl.2004.07.069 BindingDB Entry DOI: 10.7270/Q2H70F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098320 (9-(4-{4-[(4''-Trifluoromethyl-biphenyl-2-carbonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human Microsomal triglyceride transfer protein | Bioorg Med Chem Lett 14: 5067-70 (2004) Article DOI: 10.1016/j.bmcl.2004.07.069 BindingDB Entry DOI: 10.7270/Q2H70F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098320 (9-(4-{4-[(4''-Trifluoromethyl-biphenyl-2-carbonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein using triglyceride transfer assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031862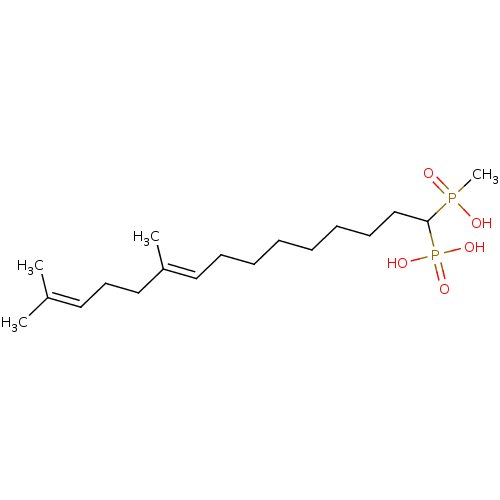 (CHEMBL84629 | [(E)-1-(Hydroxy-methyl-phosphinoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50153188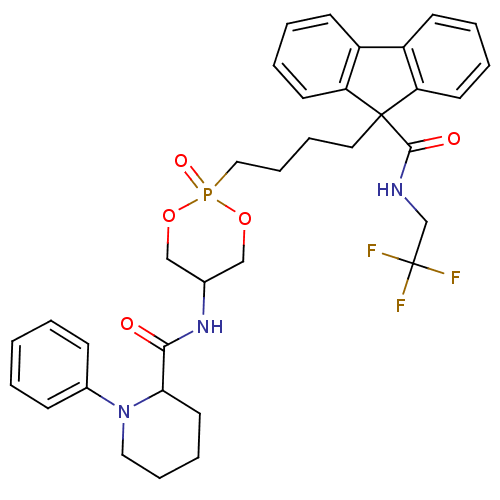 (CHEMBL360146 | Trans-1-Phenyl-piperidine-2-carboxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human Microsomal triglyceride transfer protein | Bioorg Med Chem Lett 14: 5067-70 (2004) Article DOI: 10.1016/j.bmcl.2004.07.069 BindingDB Entry DOI: 10.7270/Q2H70F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50153195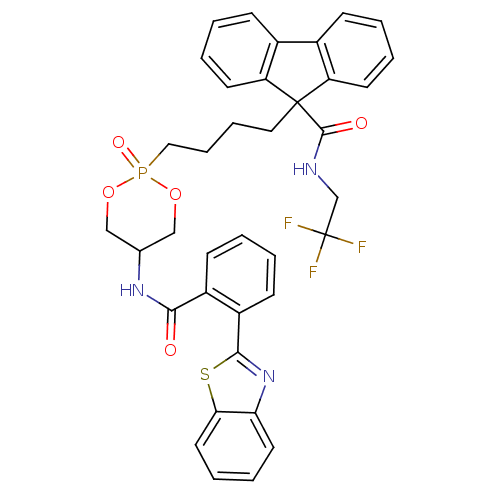 (CHEMBL186494 | Trans-9-{4-[5-(2-Benzothiazol-2-yl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human Microsomal triglyceride transfer protein | Bioorg Med Chem Lett 14: 5067-70 (2004) Article DOI: 10.1016/j.bmcl.2004.07.069 BindingDB Entry DOI: 10.7270/Q2H70F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098327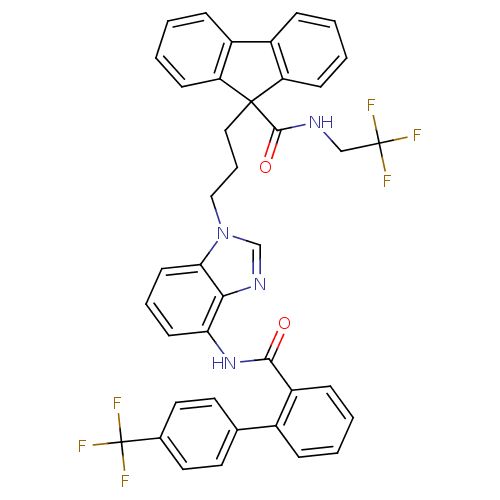 (9-(3-{4-[(4'-Trifluoromethyl-biphenyl-2-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458637 (CHEMBL4210998) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Bos taurus) | BDBM50126193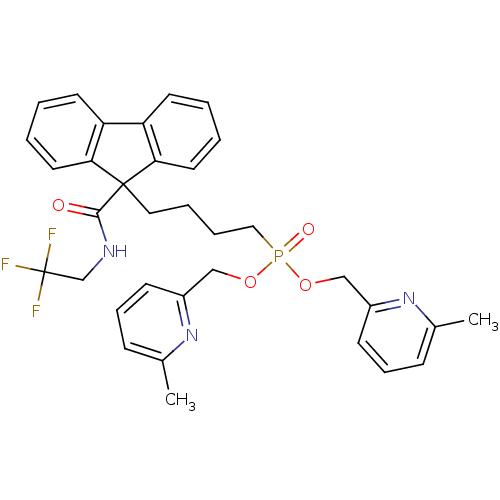 (CHEMBL27660 | {4-[9-(2,2,2-Trifluoro-ethylcarbamoy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against microsomal triglyceride transfer protein (MTP) | Bioorg Med Chem Lett 13: 1337-40 (2003) BindingDB Entry DOI: 10.7270/Q2NC60K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50153190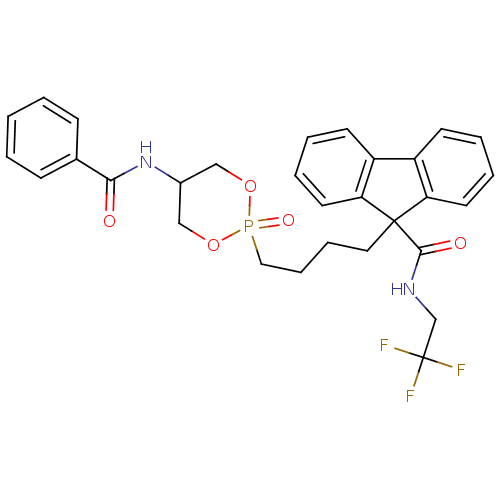 (CHEMBL185866 | Trans-9-[4-(5-Benzoylamino-2-oxo-2l...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human Microsomal triglyceride transfer protein | Bioorg Med Chem Lett 14: 5067-70 (2004) Article DOI: 10.1016/j.bmcl.2004.07.069 BindingDB Entry DOI: 10.7270/Q2H70F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031836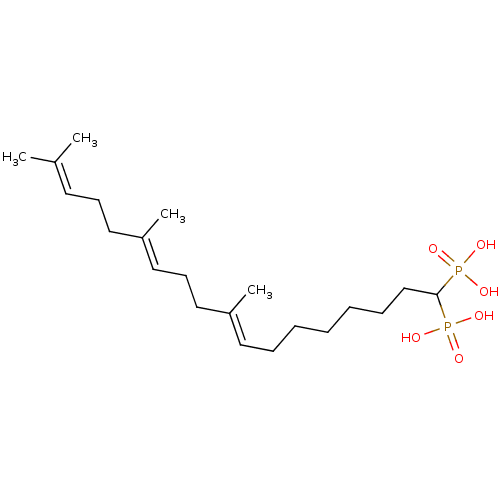 (((8E,12E)-9,13,17-Trimethyl-1-phosphono-octadeca-8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50153196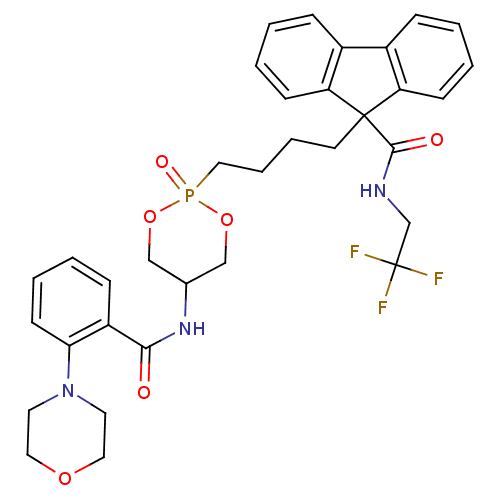 (CHEMBL187076 | Trans-9-{4-[5-(2-Morpholin-4-yl-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human Microsomal triglyceride transfer protein | Bioorg Med Chem Lett 14: 5067-70 (2004) Article DOI: 10.1016/j.bmcl.2004.07.069 BindingDB Entry DOI: 10.7270/Q2H70F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031849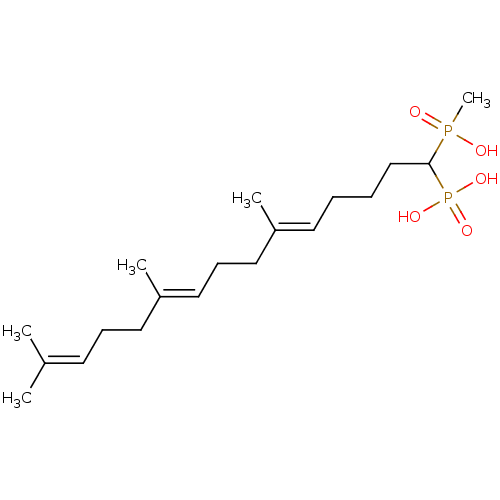 (CHEMBL86918 | [(5E,9E)-1-(Hydroxy-methyl-phosphino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049217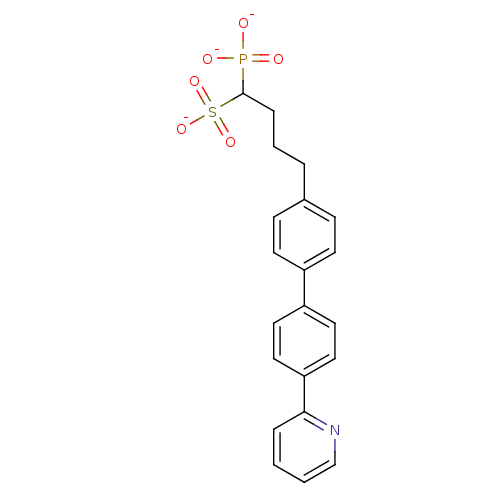 (CHEMBL158517 | Tripotassium salt of 1-Phosphono-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031837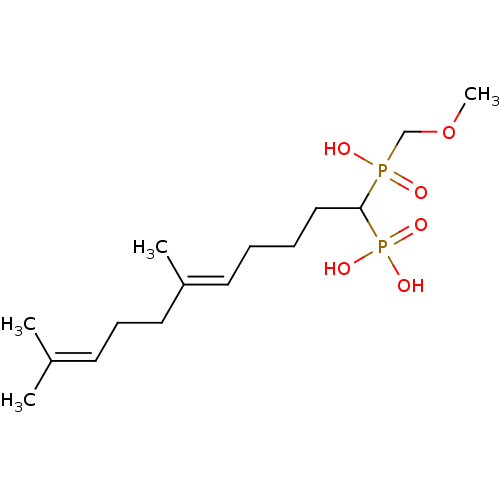 (CHEMBL87489 | [(E)-1-(Hydroxy-methoxymethyl-phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50153194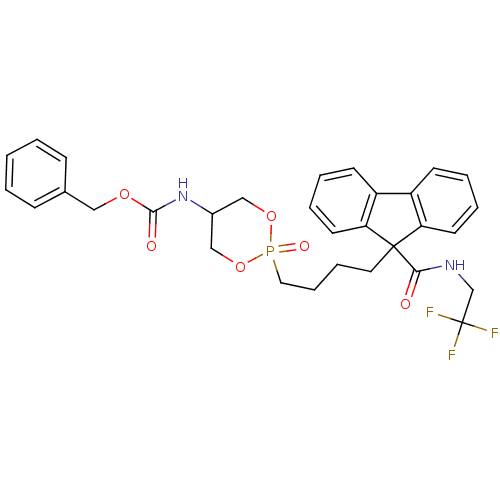 (CHEMBL187696 | Cis-(2-Oxo-2-{4-[9-(2,2,2-trifluoro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against human Microsomal triglyceride transfer protein | Bioorg Med Chem Lett 14: 5067-70 (2004) Article DOI: 10.1016/j.bmcl.2004.07.069 BindingDB Entry DOI: 10.7270/Q2H70F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Bos taurus) | BDBM50126188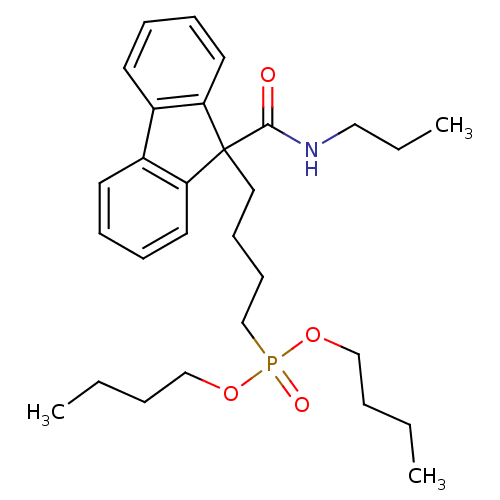 (CHEMBL28348 | [4-(9-Propylcarbamoyl-9H-fluoren-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against microsomal triglyceride transfer protein (MTP) | Bioorg Med Chem Lett 13: 1337-40 (2003) BindingDB Entry DOI: 10.7270/Q2NC60K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031840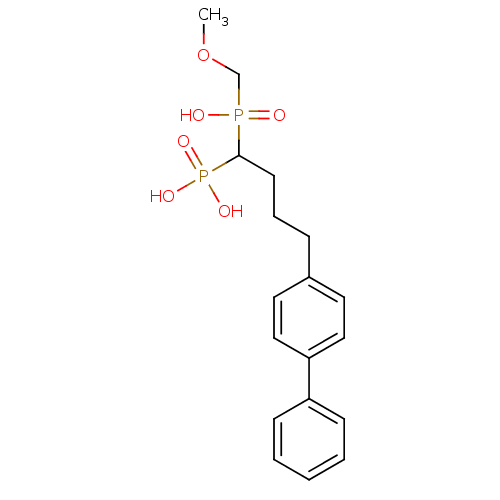 (CHEMBL84961 | [4-Biphenyl-4-yl-1-(hydroxy-methoxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049233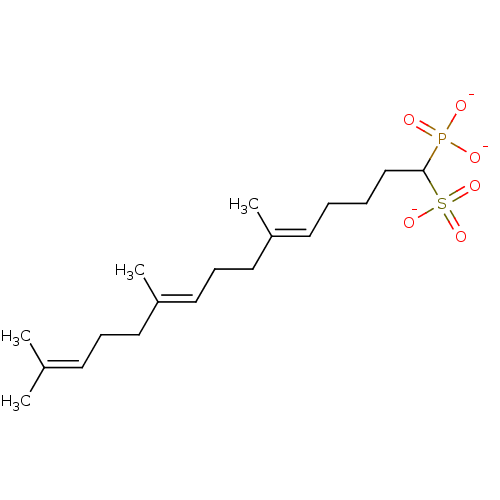 (CHEMBL160240 | Tripotassium salt of 4-Biphenyl-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatic triacylglycerol lipase (Homo sapiens (Human)) | BDBM50458647 (CHEMBL4212095) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human HL expressed in African green monkey COS7 cells using HDL as substrate pretreated for 10 mins followed by substrate a... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 164 total ) | Next | Last >> |