

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014354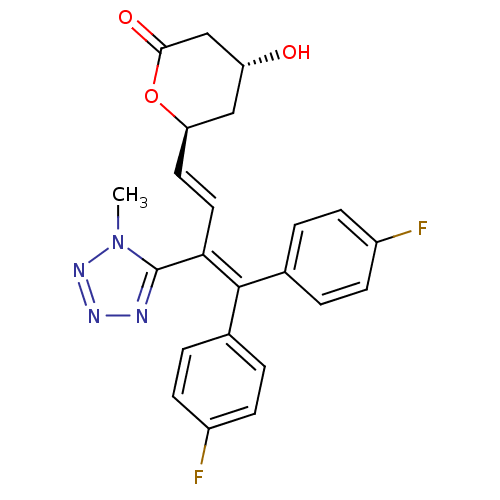 (6-[4,4-Bis-(4-fluoro-phenyl)-3-(1-methyl-1H-tetraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase using [2-14C]-acetate incorporation | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM34168 (LOVASTATIN | MLS000069585 | SMR000058779 | US91151...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014356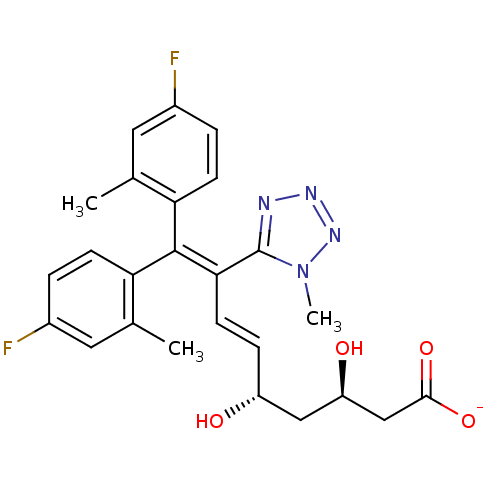 (CHEMBL320666 | Sodium; 9,9-bis-(4-fluoro-2-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM34168 (LOVASTATIN | MLS000069585 | SMR000058779 | US91151...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase using [2-14C]-acetate incorporation | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014345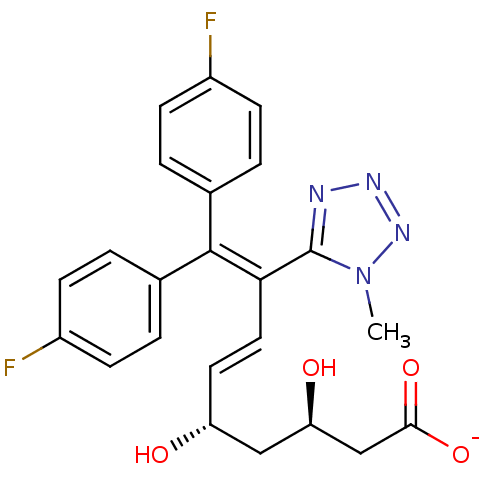 (CHEMBL11804 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014358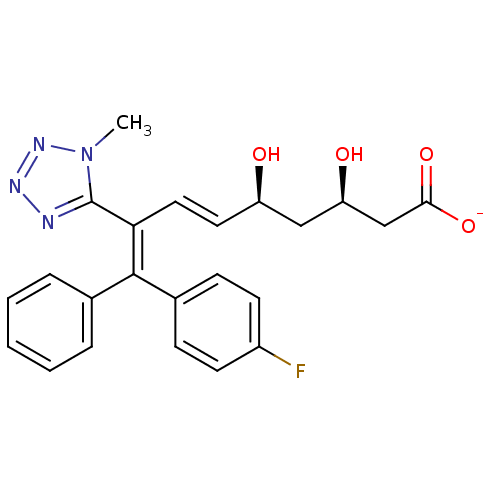 (CHEMBL103192 | Sodium; 9-(4-fluoro-phenyl)-3,5-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014355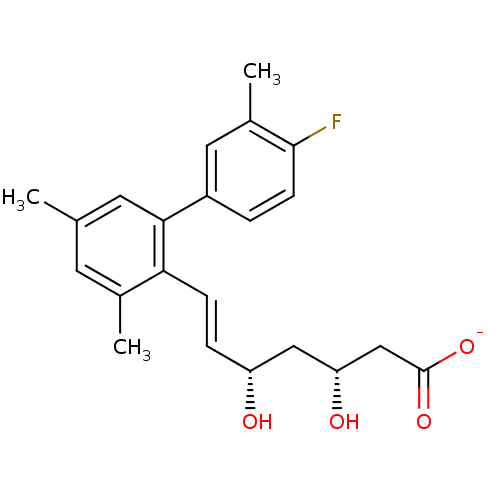 (CHEMBL103585 | Sodium; 7-(4'-fluoro-3,5,3'-trimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase using [2-14C]-acetate incorporation | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061130 ((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061124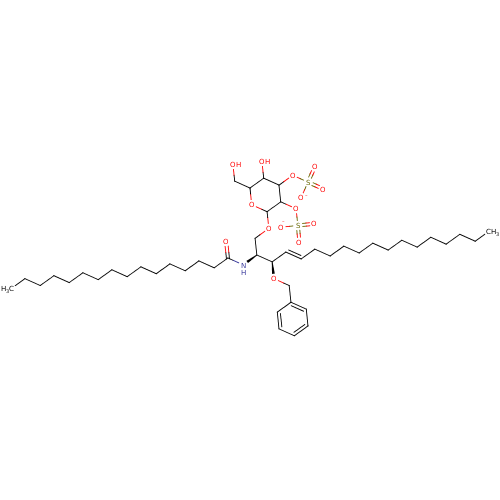 ((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061136 ((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50369315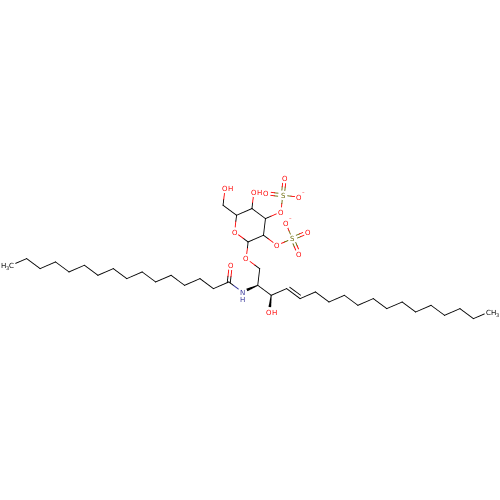 (CHEMBL1627019) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014365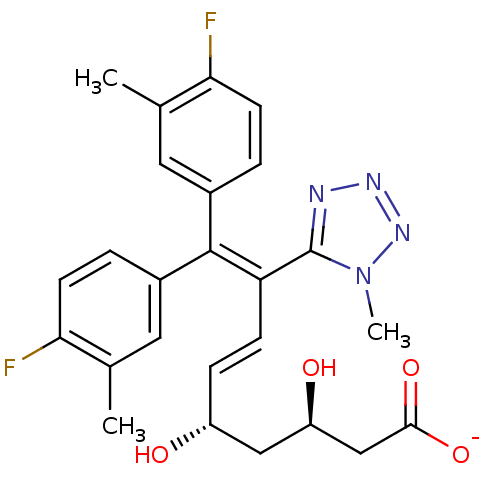 (CHEMBL103547 | Sodium; 9,9-bis-(4-fluoro-3-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014361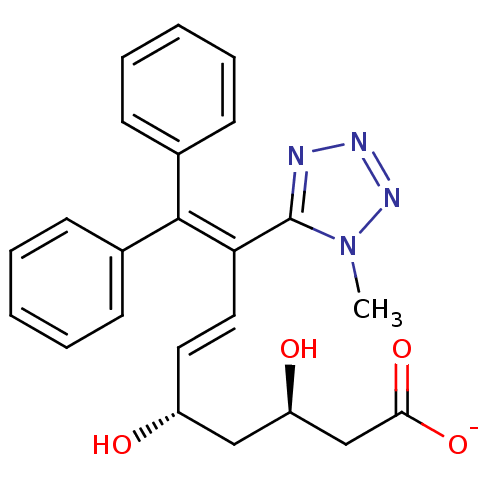 (CHEMBL318754 | Sodium; 3,5-dihydroxy-8-(1-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014364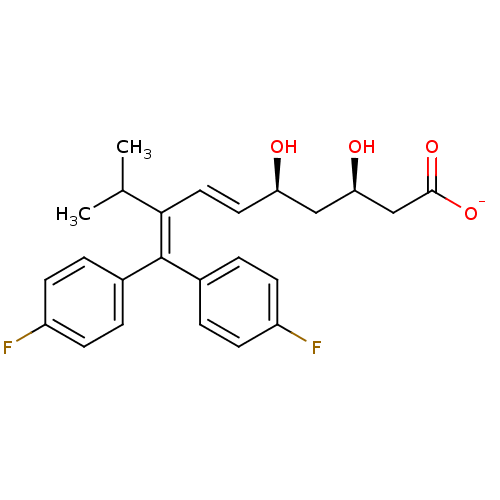 (CHEMBL318102 | Sodium; 8-[bis-(4-fluoro-phenyl)-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014363 (CHEMBL419542 | Sodium; 9,9-bis-(4-fluoro-phenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061125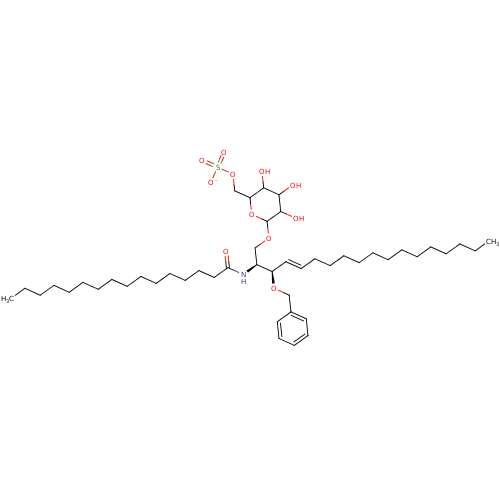 ((2S,3R,4E)-2-(hexadecanoylamino)-3-(benzoyloxy)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014349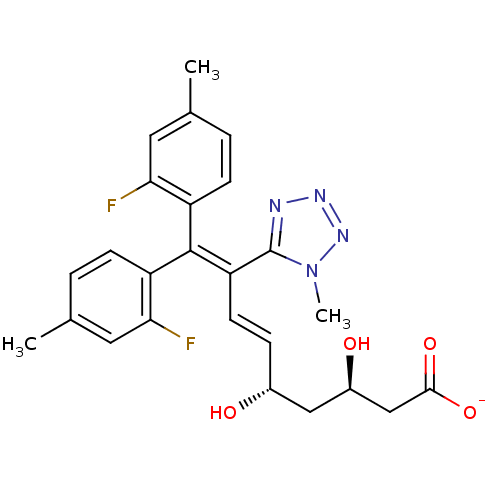 (CHEMBL328846 | Sodium; 9,9-bis-(2-fluoro-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061133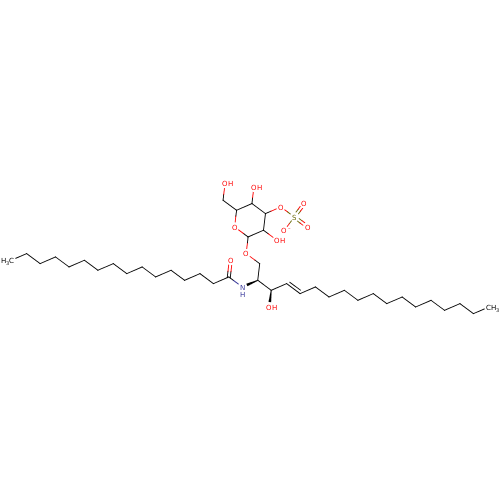 ((2S,3R,4E)-2-(Hexadecanoylamino)-3-hydroxy-1-[[3-O...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061128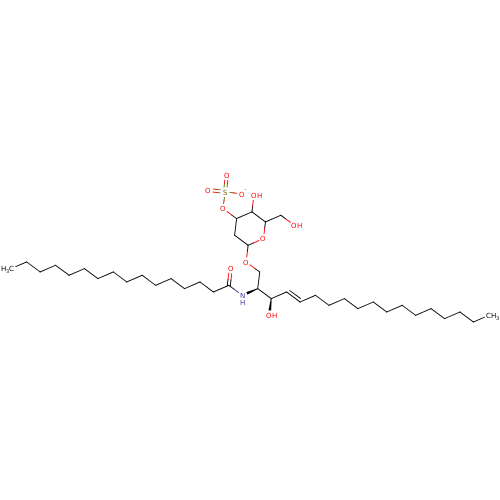 (1N-[2-hydroxy-1-(5-hydroxy-6-hydroxymethyltetrahyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061122 ((2S,3R,4E)-2-(Hexadecanoylamino)-3-(benzoyloxy)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014362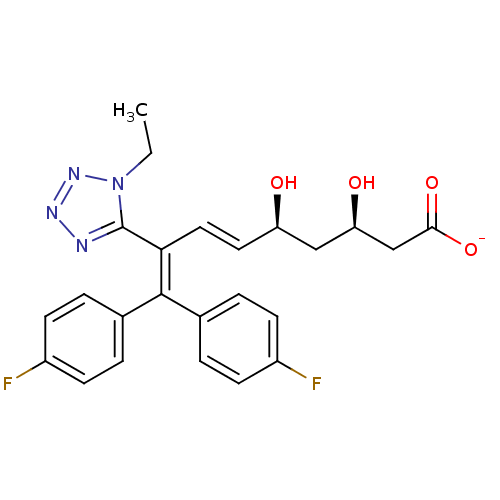 (CHEMBL103661 | Sodium; 8-(1-ethyl-1H-tetrazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061132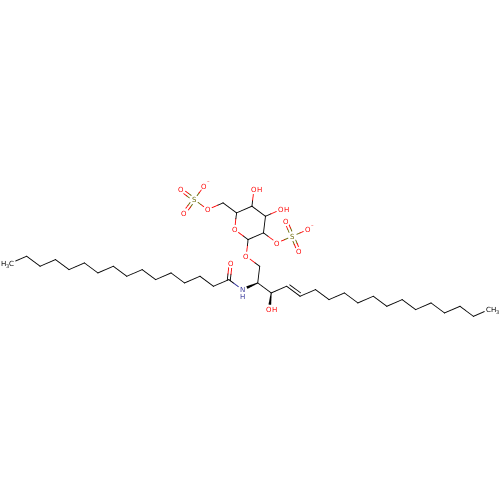 ((2S,3R,4E)-2-(Hexadecanoylamino)-3-hydroxy-1-[[2,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014346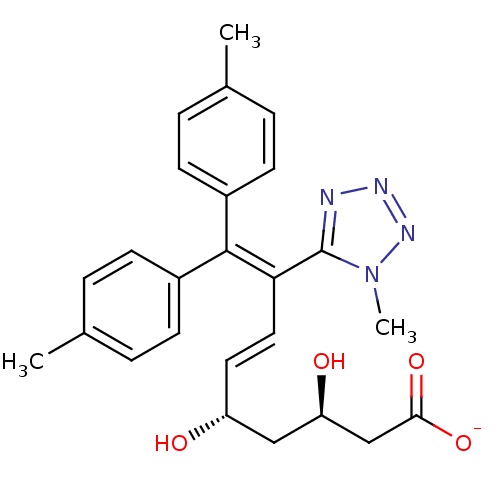 (CHEMBL441642 | Sodium; 3,5-dihydroxy-8-(1-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061135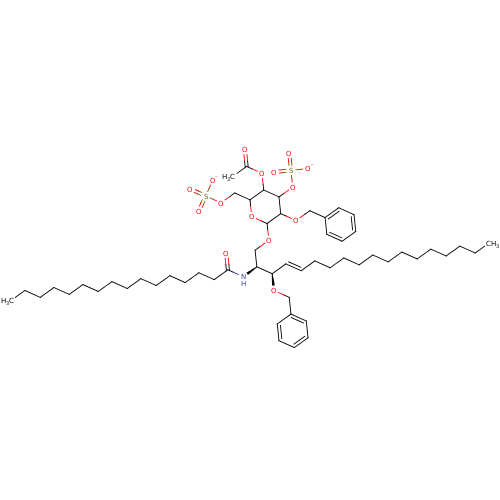 ((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014350 (CHEMBL323075 | Sodium; 3,5-dihydroxy-9,9-bis-(4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014348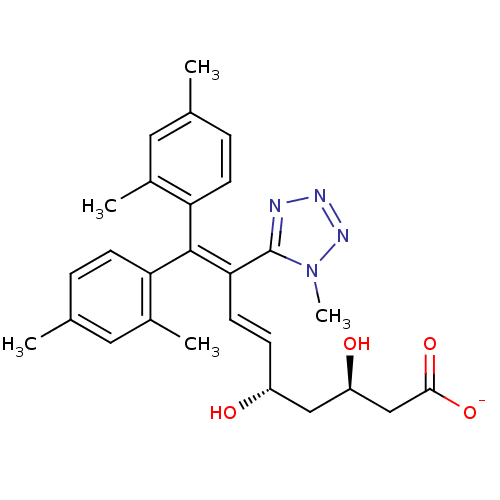 (CHEMBL100188 | Sodium; 9,9-bis-(2,4-dimethyl-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061137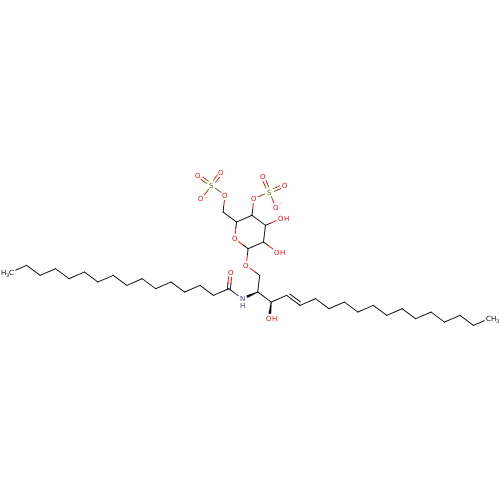 ((2S,3R,4E)-3-Hydroxy-2-(hexadecanoylamino)-1-[[4,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061131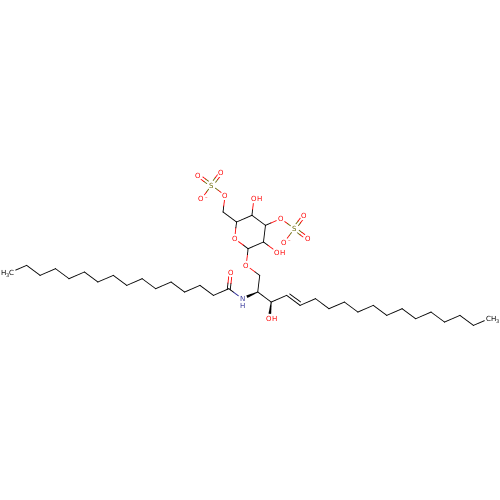 ((2S,3R,4E)-3-Hydroxy-2-(hexadecanoylamino)-1-[[3,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061127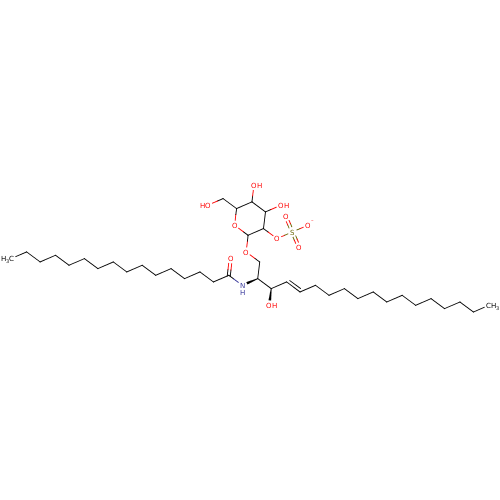 ((2S,3R,4E)-2-(Hexadecanoylamino)-3-hydroxy-1-[[2-O...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014351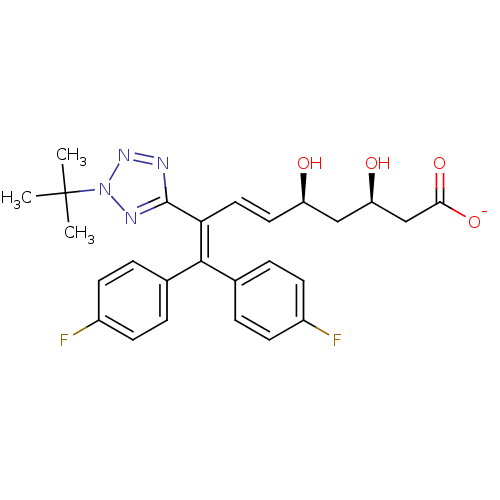 (CHEMBL323373 | Sodium; 8-(2-tert-butyl-2H-tetrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase using [2-14C]-acetate incorporation | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014351 (CHEMBL323373 | Sodium; 8-(2-tert-butyl-2H-tetrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061129 ((2S,3R,4E)-2-(hexadecanoylamino)-3-(benzoyloxy)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50421879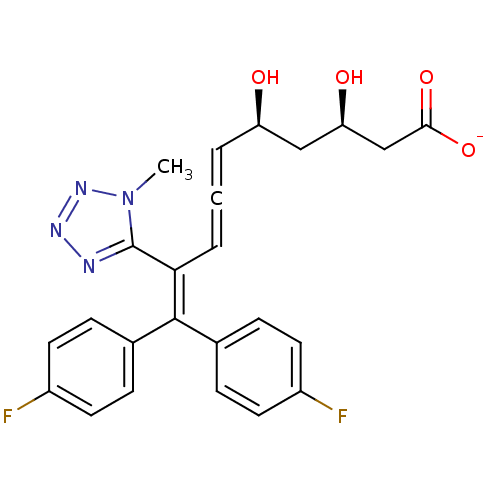 (CHEMBL2304072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014357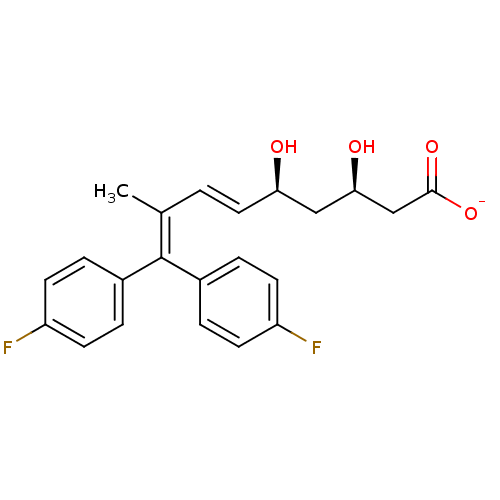 (CHEMBL318256 | Sodium; 9,9-bis-(4-fluoro-phenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014360 (CHEMBL322399 | Sodium; 8-cyano-9,9-bis-(4-fluoro-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061121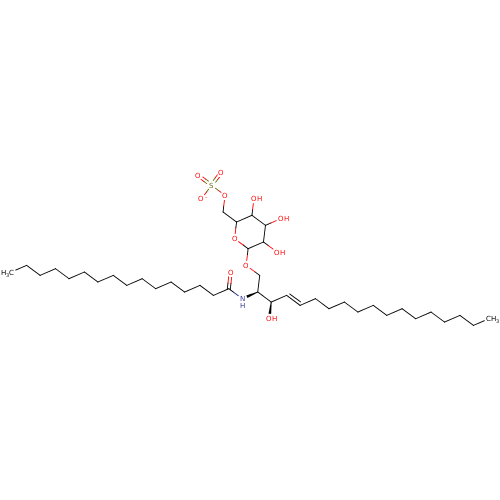 ((2S,3R,4E)-2-(Hexadecanoylamino)-1-[[6-O-(sodium o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061134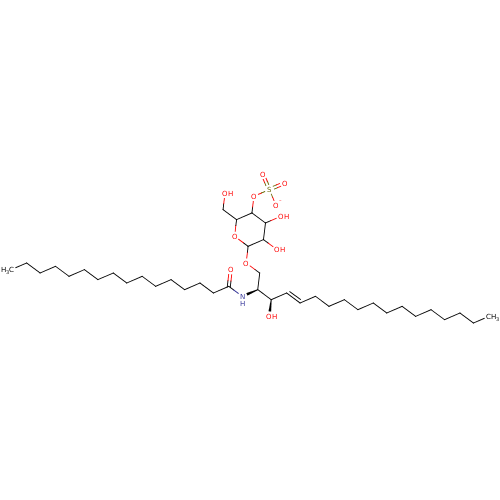 ((2S,3R,4E)-2-(Hexadecanoylamino)-3-hydroxy-1-[[4-O...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014353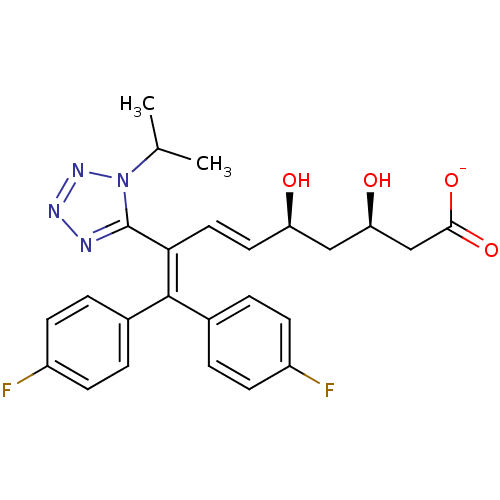 (CHEMBL319739 | Sodium; 9,9-bis-(4-fluoro-phenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014352 (CHEMBL102468 | Sodium; 9,9-bis-(4-fluoro-phenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061123 (((Z)-2-Hydroxy-1-hydroxymethyl-heptadec-3-enyl)-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014367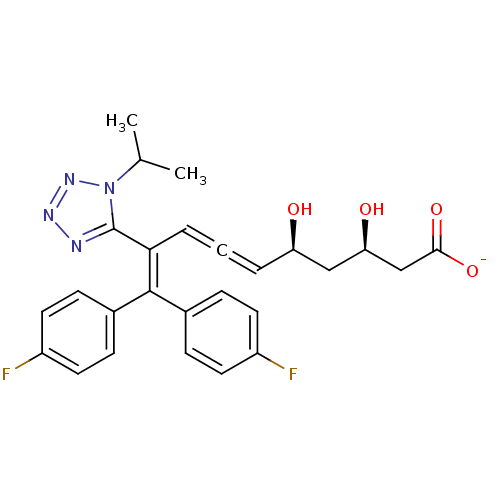 (CHEMBL103792 | Sodium; 10,10-bis-(4-fluoro-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014366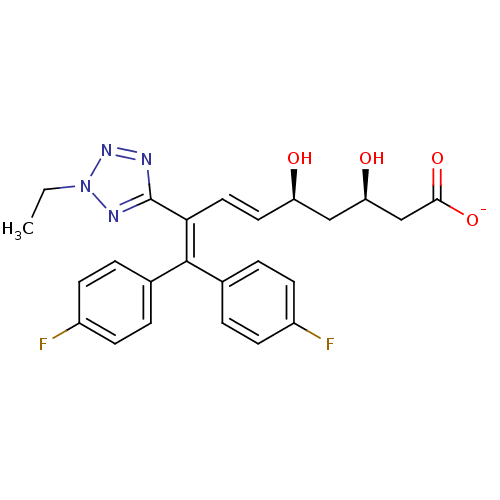 (CHEMBL102484 | Sodium; 8-(2-ethyl-2H-tetrazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014347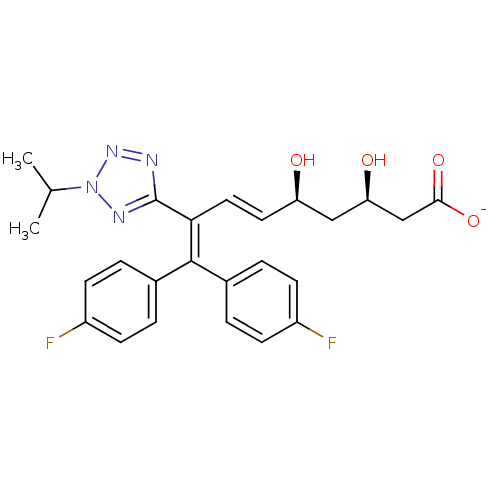 (CHEMBL321715 | Sodium; 9,9-bis-(4-fluoro-phenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014345 (CHEMBL11804 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro inhibitory activity was measured against rat liver HMG-CoA reductase | J Med Chem 33: 2982-99 (1990) BindingDB Entry DOI: 10.7270/Q26T0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||