

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50214707 (9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of human PNP in presence of 0.025 mM phosphate | Bioorg Med Chem Lett 17: 4173-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.054 BindingDB Entry DOI: 10.7270/Q29P31CS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50214707 (9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 0.02... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214707 (9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf PNP in presence of 0.025 mM phosphate | Bioorg Med Chem Lett 17: 4173-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.054 BindingDB Entry DOI: 10.7270/Q29P31CS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214707 (9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 0.025 mM i... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214705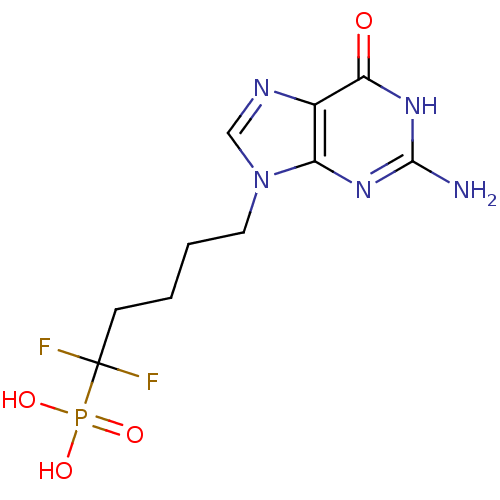 (5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 0.025 mM i... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214705 (5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf PNP in presence of 0.025 mM phosphate | Bioorg Med Chem Lett 17: 4173-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.054 BindingDB Entry DOI: 10.7270/Q29P31CS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214707 (9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM inorg... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50308521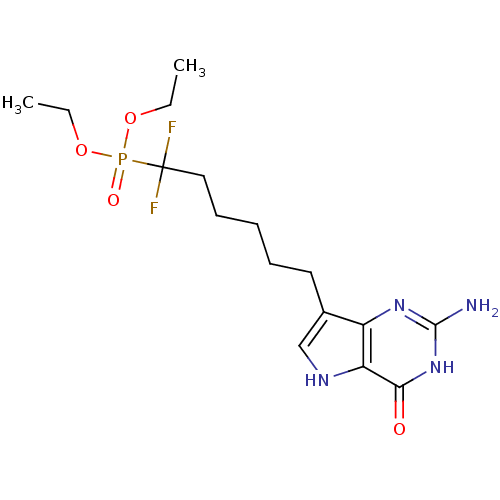 (9-[6',6'-Difluoro-6'-(diethylphosphono)hexyl]-9-de...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50415479 (CHEMBL601619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214708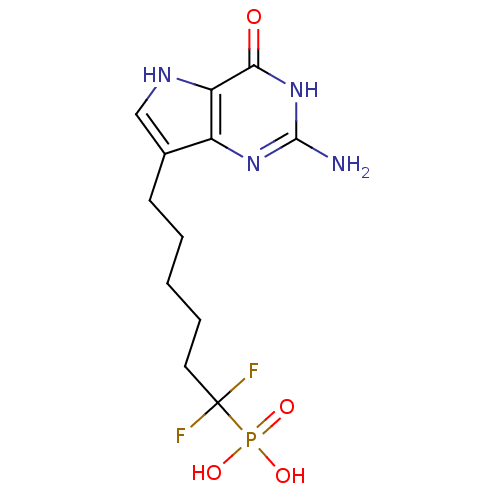 (6-(2-amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyri...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf PNP in presence of 1 mM phosphate | Bioorg Med Chem Lett 17: 4173-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.054 BindingDB Entry DOI: 10.7270/Q29P31CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50308521 (9-[6',6'-Difluoro-6'-(diethylphosphono)hexyl]-9-de...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM inorg... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50415479 (CHEMBL601619) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM inorg... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50415479 (CHEMBL601619) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM inorg... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214707 (9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw Curated by ChEMBL | Assay Description Inhibition of bovine PNP using 7-methylguanosine as substrate by spectrophotometric based coupled assay | Bioorg Med Chem 20: 6758-69 (2012) Article DOI: 10.1016/j.bmc.2012.08.045 BindingDB Entry DOI: 10.7270/Q23N24H7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214705 (5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrofluorimetric method in presence of 1... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50214707 (9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50214705 (5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50308522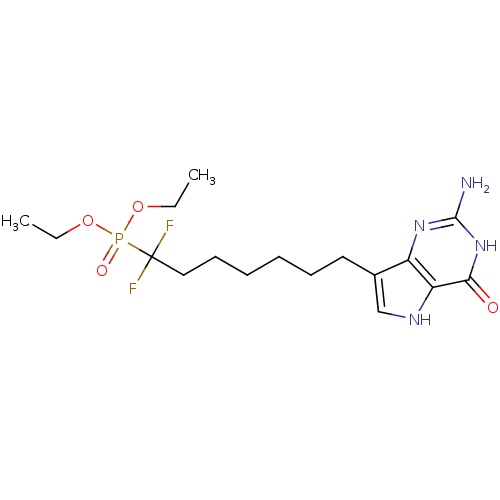 (9-[7',7'-Difluoro-7'-(diethylphosphono)heptyl]-9-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50308522 (9-[7',7'-Difluoro-7'-(diethylphosphono)heptyl]-9-d...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM inorg... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50195587 (1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 50 mM inor... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50394443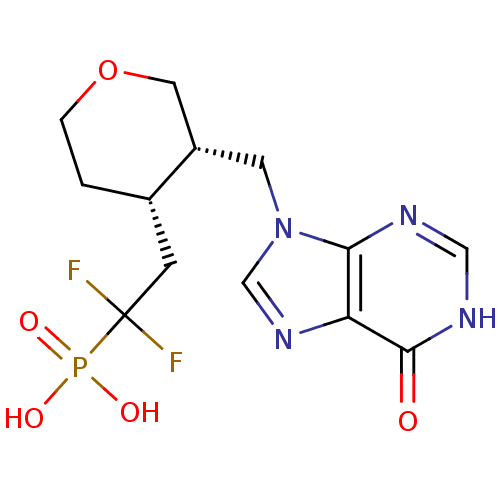 (CHEMBL2159650) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw Curated by ChEMBL | Assay Description Inhibition of bovine PNP using 7-methylguanosine as substrate by spectrophotometric based coupled assay | Bioorg Med Chem 20: 6758-69 (2012) Article DOI: 10.1016/j.bmc.2012.08.045 BindingDB Entry DOI: 10.7270/Q23N24H7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50322033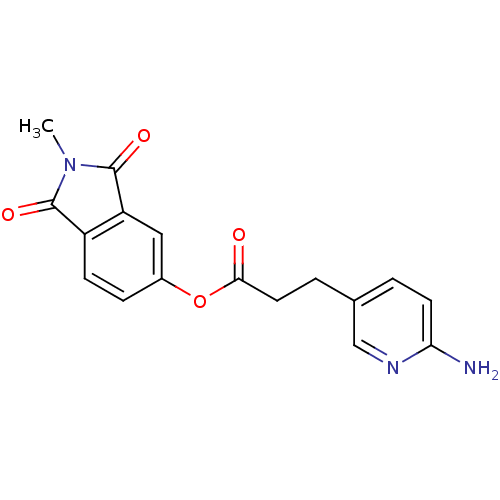 (2-Methylisoindole-1,3-dione-5-yl 3-(6-aminopyridin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Binding affinity to bovine thrombin after 3 mins | Bioorg Med Chem 18: 5323-38 (2010) Article DOI: 10.1016/j.bmc.2010.05.037 BindingDB Entry DOI: 10.7270/Q2Z0394Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50322034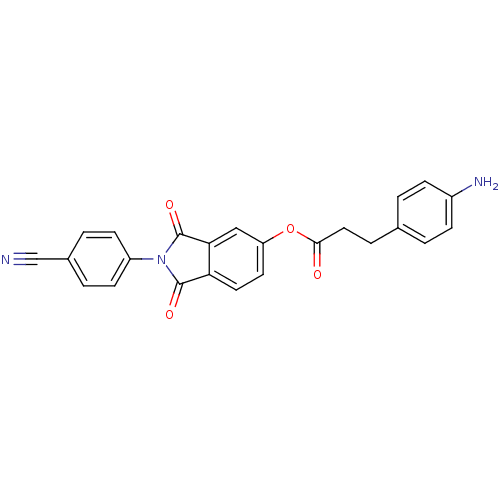 (2-(4-Cyanophenyl)isoindole-1,3-dione-5-yl(E)-3-(4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Binding affinity to bovine thrombin after 3 mins | Bioorg Med Chem 18: 5323-38 (2010) Article DOI: 10.1016/j.bmc.2010.05.037 BindingDB Entry DOI: 10.7270/Q2Z0394Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50322032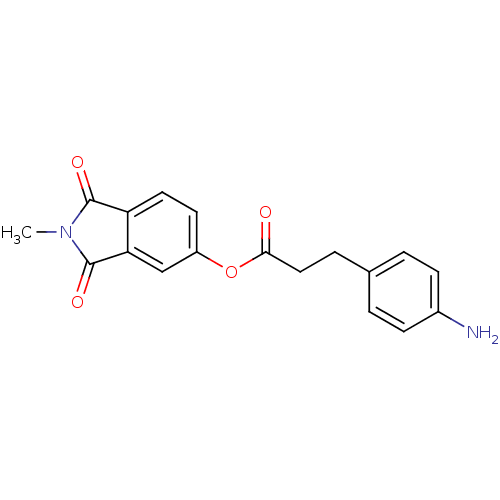 (2-Methylisoindole-1,3-dione-5-yl 3-(4-aminophenyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Binding affinity to bovine thrombin after 3 mins | Bioorg Med Chem 18: 5323-38 (2010) Article DOI: 10.1016/j.bmc.2010.05.037 BindingDB Entry DOI: 10.7270/Q2Z0394Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50322035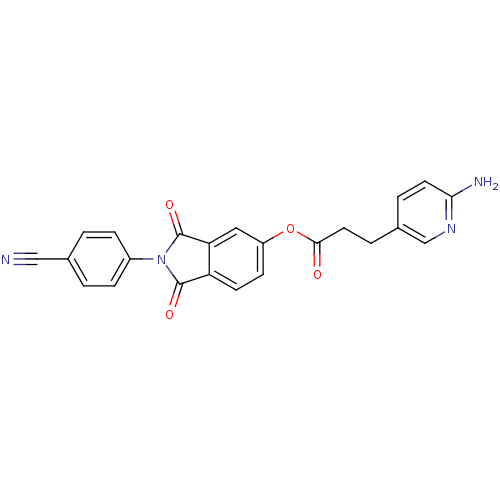 (2-(4-cyanophenyl)isoindole-1,3-dione-5-yl 3-(2-ami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Binding affinity to bovine thrombin after 3 mins | Bioorg Med Chem 18: 5323-38 (2010) Article DOI: 10.1016/j.bmc.2010.05.037 BindingDB Entry DOI: 10.7270/Q2Z0394Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoglucanase-5 (Humicola insolens) | BDBM50346888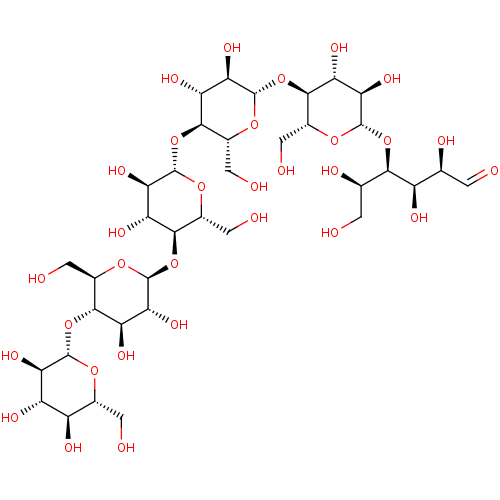 (CHEMBL1797815) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 4.0 | n/a |
Hirosaki University Curated by ChEMBL | Assay Description Binding affinity to Humicola insolens NCE5 assessed as dissociation constant at pH 4 | Bioorg Med Chem 19: 3812-30 (2011) Article DOI: 10.1016/j.bmc.2011.04.048 BindingDB Entry DOI: 10.7270/Q2057G8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoglucanase-5 (Humicola insolens) | BDBM50346887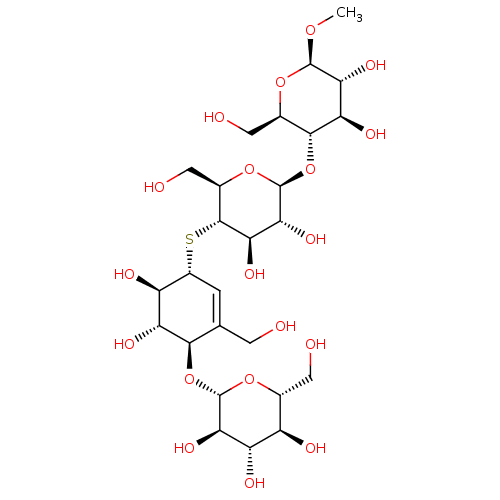 (CHEMBL1797814) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 3.0 | n/a |
Hirosaki University Curated by ChEMBL | Assay Description Binding affinity to Humicola insolens NCE5 assessed as dissociation constant at pH 3.0 and at 59 degC by differential scanning calorimetry | Bioorg Med Chem 19: 3812-30 (2011) Article DOI: 10.1016/j.bmc.2011.04.048 BindingDB Entry DOI: 10.7270/Q2057G8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoglucanase-5 (Humicola insolens) | BDBM50346884 (CHEMBL1797811) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 3.0 | n/a |
Hirosaki University Curated by ChEMBL | Assay Description Binding affinity to Humicola insolens NCE5 assessed as dissociation constant at pH 3.0 and at 59 degC by differential scanning calorimetry | Bioorg Med Chem 19: 3812-30 (2011) Article DOI: 10.1016/j.bmc.2011.04.048 BindingDB Entry DOI: 10.7270/Q2057G8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoglucanase-5 (Humicola insolens) | BDBM50346885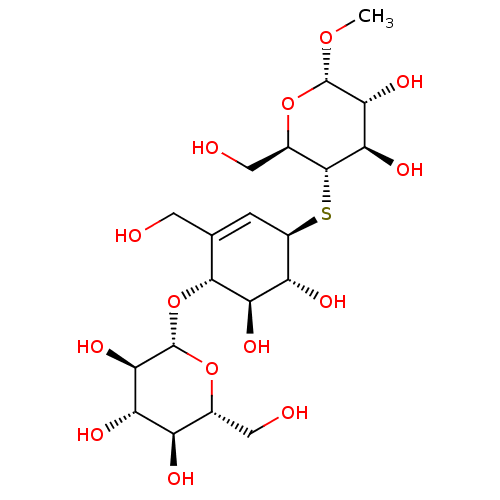 (CHEMBL1797812) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a | 3.0 | n/a |
Hirosaki University Curated by ChEMBL | Assay Description Binding affinity to Humicola insolens NCE5 assessed as dissociation constant at pH 3.0 and at 59 degC by differential scanning calorimetry | Bioorg Med Chem 19: 3812-30 (2011) Article DOI: 10.1016/j.bmc.2011.04.048 BindingDB Entry DOI: 10.7270/Q2057G8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoglucanase-5 (Humicola insolens) | BDBM50346886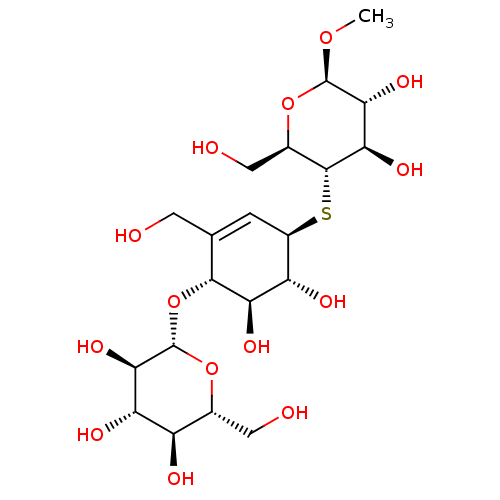 (CHEMBL1797813) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.90E+7 | n/a | n/a | n/a | n/a | n/a | n/a | 3.0 | n/a |
Hirosaki University Curated by ChEMBL | Assay Description Binding affinity to Humicola insolens NCE5 assessed as dissociation constant at pH 3.0 and at 59 degC by differential scanning calorimetry | Bioorg Med Chem 19: 3812-30 (2011) Article DOI: 10.1016/j.bmc.2011.04.048 BindingDB Entry DOI: 10.7270/Q2057G8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoglucanase-5 (Humicola insolens) | BDBM50346883 (CHEMBL1797810) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | >3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | 3.0 | n/a |
Hirosaki University Curated by ChEMBL | Assay Description Binding affinity to Humicola insolens NCE5 assessed as dissociation constant at pH 3.0 and at 59 degC by differential scanning calorimetry | Bioorg Med Chem 19: 3812-30 (2011) Article DOI: 10.1016/j.bmc.2011.04.048 BindingDB Entry DOI: 10.7270/Q2057G8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoglucanase-5 (Humicola insolens) | BDBM50346882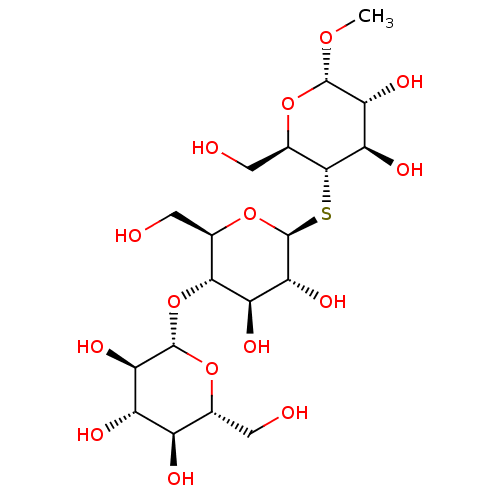 (CHEMBL1797809) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | >3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | 3.0 | n/a |
Hirosaki University Curated by ChEMBL | Assay Description Binding affinity to Humicola insolens NCE5 assessed as dissociation constant at pH 3.0 and at 59 degC by differential scanning calorimetry | Bioorg Med Chem 19: 3812-30 (2011) Article DOI: 10.1016/j.bmc.2011.04.048 BindingDB Entry DOI: 10.7270/Q2057G8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29995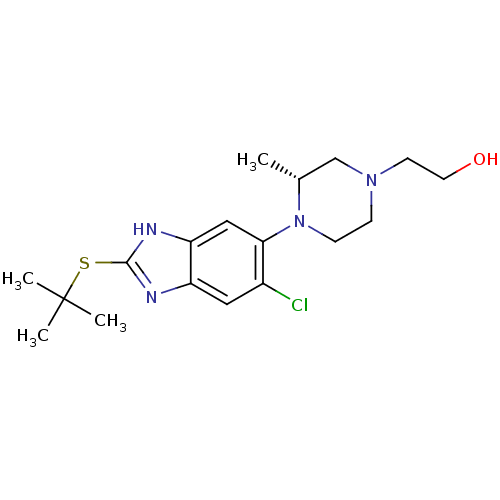 (CHEMBL494350 | benzimidazole-based antagonist, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239749 (2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor expressed in CHO cell membrane by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239749 (2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50260633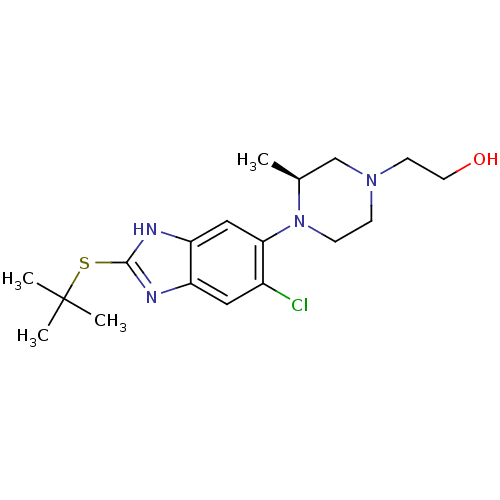 ((S)-2-(4-(2-(tert-butylthio)-6-chloro-3H-benzo[d]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC/OFQ from human ORL1 receptor | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239749 (2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC/OFQ from human ORL1 receptor | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239749 (2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC from human ORL1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50373360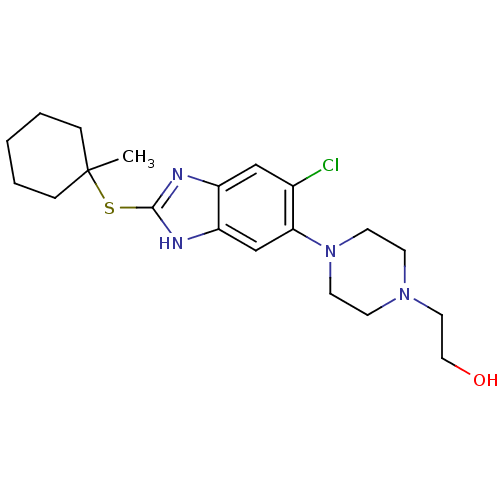 (CHEMBL263917) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor expressed in CHO cell membrane by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50373360 (CHEMBL263917) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC from human ORL1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50293856 (1-(2,2-dimethyl-1,3-dioxan-5-yl)-3-{[1S,3R,6S)-2,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human cloned ORL1 receptor expressed in CHO cells | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50373365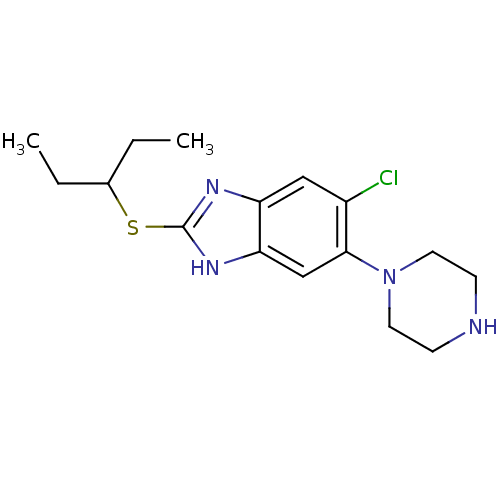 (CHEMBL258710) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC from human ORL1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50260633 ((S)-2-(4-(2-(tert-butylthio)-6-chloro-3H-benzo[d]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50293855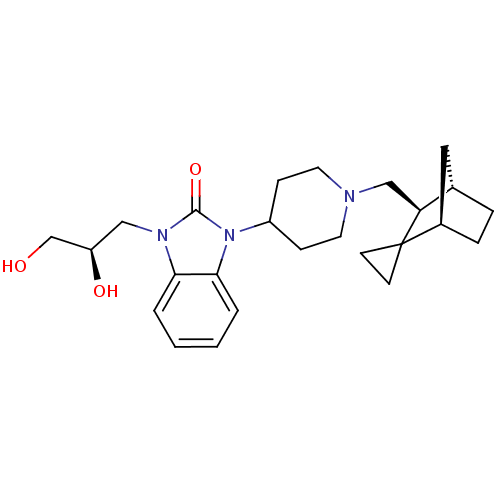 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human cloned ORL1 receptor expressed in CHO cells | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083230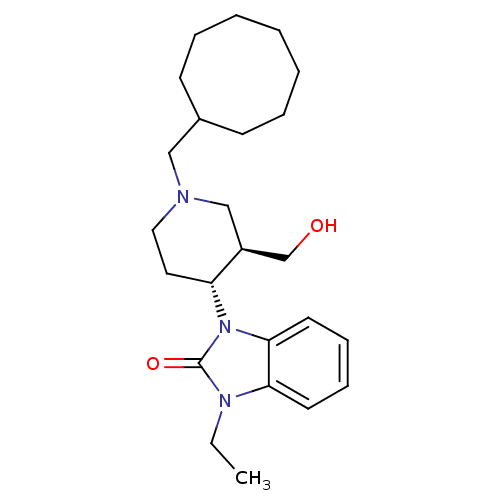 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human cloned ORL1 receptor expressed in CHO cells | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50293857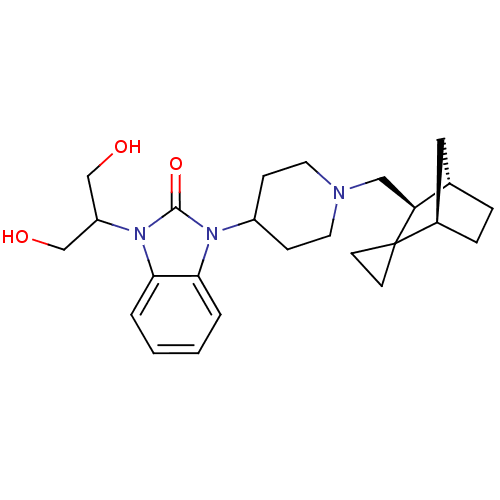 (1-(1,3-dihydroxypropan-2-yl)-3-(1-((1R,3S,4S)-spir...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human cloned ORL1 receptor expressed in CHO cells | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50373362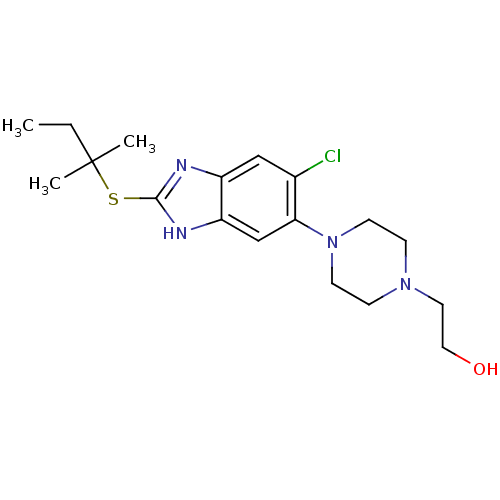 (CHEMBL263919) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor expressed in CHO cell membrane by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29995 (CHEMBL494350 | benzimidazole-based antagonist, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC/OFQ from human ORL1 receptor | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50368147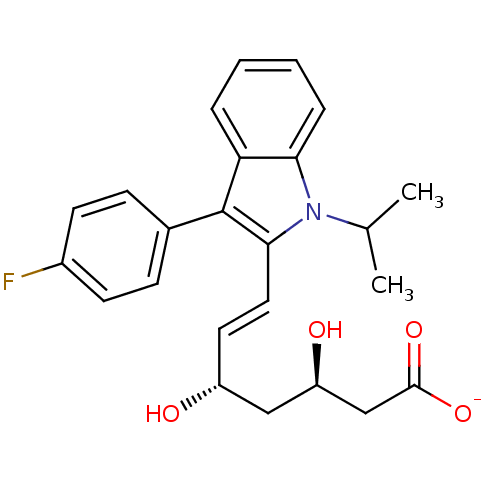 ((+)-(3R,5S)-fluvastatin | (3R,5S)-fluvastatin | (3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibition of rat HMG-CoA reductase using 0.37 MBq DL-[3-14C]HMG-CoA | Bioorg Med Chem Lett 19: 4228-31 (2009) Article DOI: 10.1016/j.bmcl.2009.05.100 BindingDB Entry DOI: 10.7270/Q22F7PCK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239745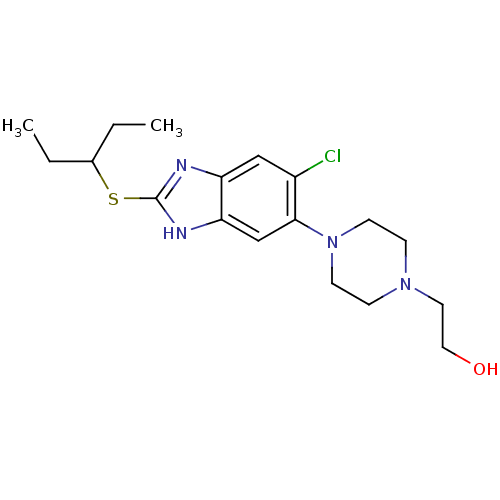 (2-(4-(6-chloro-2-(pentan-3-ylthio)-3H-benzo[d]imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 240 total ) | Next | Last >> |