

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA polymerase beta (Rattus norvegicus) | BDBM50241570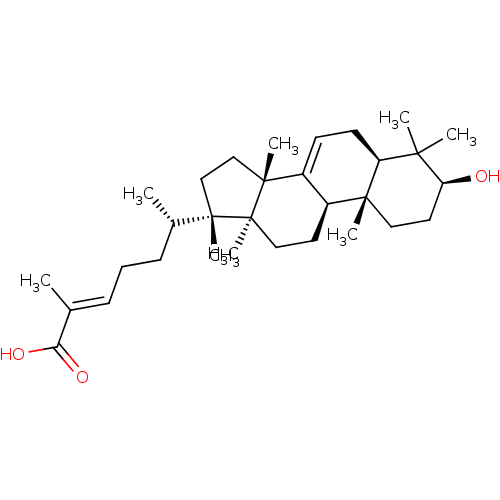 ((24E)-3beta-hydroxy-7,24-euphadien-26-oic acid | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat recombinant DNA polymerase beta after 20 mins by uncompetitive inhibition assay in presence of activated calf thymus DNA and 0.1 mg... | J Nat Prod 63: 1356-60 (2000) BindingDB Entry DOI: 10.7270/Q2X066S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50250426 ((+)-Myristinin A | CHEMBL465365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Activity of rat recombinant DNA polymerase beta using variable levels of DNA template-primer | J Nat Prod 68: 1625-8 (2005) Article DOI: 10.1021/np058064g BindingDB Entry DOI: 10.7270/Q2Q2400B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50250427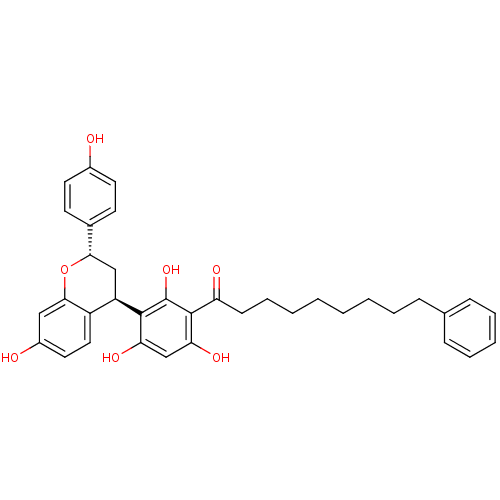 ((+)-Myristinin D | CHEMBL448072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Activity of rat recombinant DNA polymerase beta using variable levels of DNA template-primer | J Nat Prod 68: 1625-8 (2005) Article DOI: 10.1021/np058064g BindingDB Entry DOI: 10.7270/Q2Q2400B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50241570 ((24E)-3beta-hydroxy-7,24-euphadien-26-oic acid | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat recombinant DNA polymerase beta after 20 mins by noncompetitive inhibition assay in presence of activated calf thymus DNA and 0.1 m... | J Nat Prod 63: 1356-60 (2000) BindingDB Entry DOI: 10.7270/Q2X066S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50241570 ((24E)-3beta-hydroxy-7,24-euphadien-26-oic acid | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat recombinant DNA polymerase beta after 20 mins by uncompetitive inhibition assay in presence of [3H]dTTP and 0.1 mg/mL BSA | J Nat Prod 63: 1356-60 (2000) BindingDB Entry DOI: 10.7270/Q2X066S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50241570 ((24E)-3beta-hydroxy-7,24-euphadien-26-oic acid | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat recombinant DNA polymerase beta after 20 mins by noncompetitive inhibition assay in presence of [3H]dTTP and 0.1 mg/mL BSA | J Nat Prod 63: 1356-60 (2000) BindingDB Entry DOI: 10.7270/Q2X066S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50426389 (CHEMBL2321925) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of recombinant RSK2 (unknown origin) expressed in baculovirus infected Sf9 cells assessed as decrease in substrate phosphorylation using u... | ACS Med Chem Lett 4: 175-179 (2012) Article DOI: 10.1021/ml300298v BindingDB Entry DOI: 10.7270/Q2668FHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50250996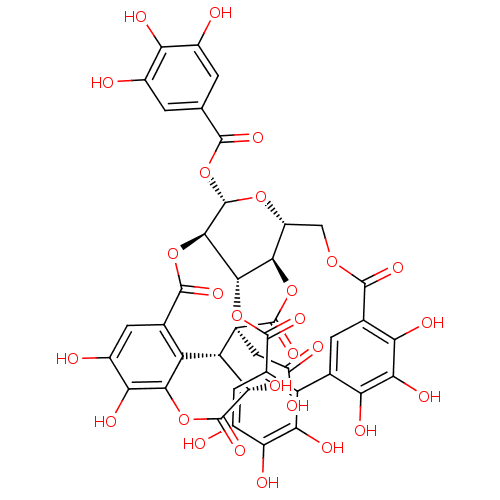 (CHEMBL525240 | TANNIN | chebulagic acid | chebulan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human COLO201 cellular topoisomerase-1 mediated plasmid DNA cleavage by electrophoresis in presence of 14 units of enzyme | J Nat Prod 55: 401-413 (1992) Article DOI: 10.1021/np50082a001 BindingDB Entry DOI: 10.7270/Q2542NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50250996 (CHEMBL525240 | TANNIN | chebulagic acid | chebulan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human COLO201 cellular topoisomerase-1 mediated plasmid DNA cleavage by electrophoresis in presence of 70 units of enzyme | J Nat Prod 55: 401-413 (1992) Article DOI: 10.1021/np50082a001 BindingDB Entry DOI: 10.7270/Q2542NNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50426392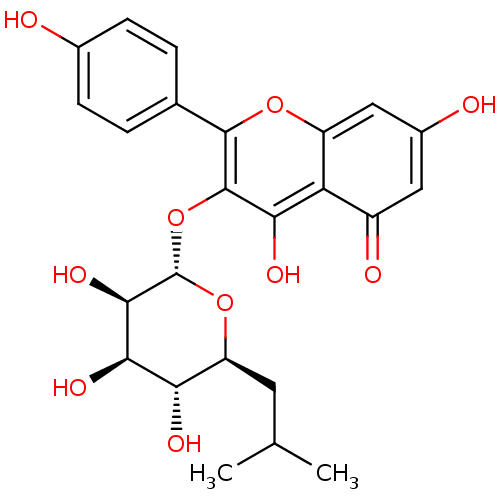 (CHEMBL2326706) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of recombinant RSK2 (unknown origin) expressed in baculovirus infected Sf9 cells assessed as decrease in substrate phosphorylation using u... | ACS Med Chem Lett 4: 175-179 (2012) Article DOI: 10.1021/ml300298v BindingDB Entry DOI: 10.7270/Q2668FHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50426390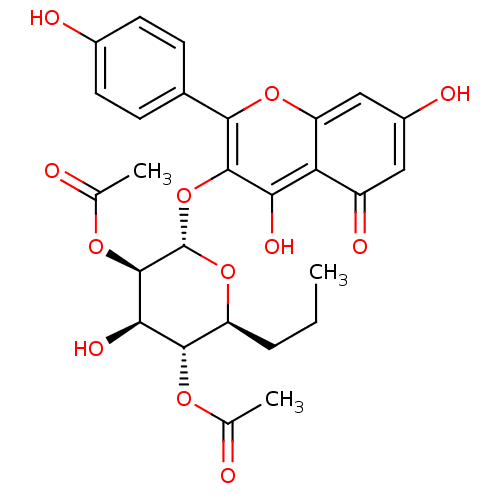 (CHEMBL2326708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of recombinant RSK2 (unknown origin) expressed in baculovirus infected Sf9 cells assessed as decrease in substrate phosphorylation using u... | ACS Med Chem Lett 4: 175-179 (2012) Article DOI: 10.1021/ml300298v BindingDB Entry DOI: 10.7270/Q2668FHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50426393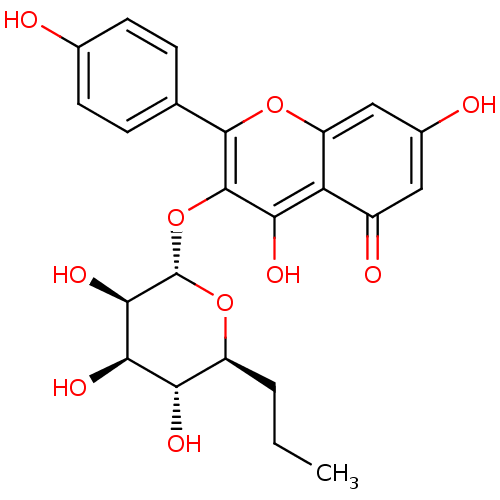 (CHEMBL2326705) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of recombinant RSK2 (unknown origin) expressed in baculovirus infected Sf9 cells assessed as decrease in substrate phosphorylation using u... | ACS Med Chem Lett 4: 175-179 (2012) Article DOI: 10.1021/ml300298v BindingDB Entry DOI: 10.7270/Q2668FHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50241294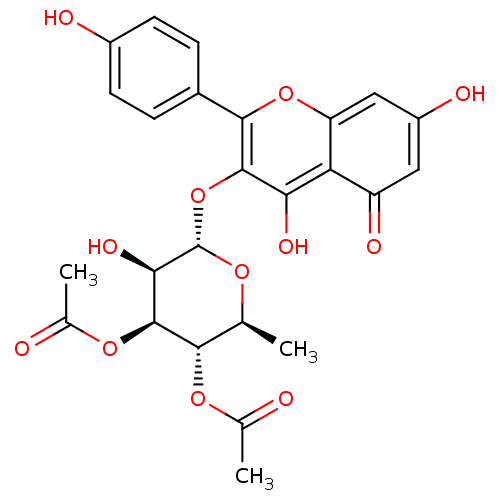 (CHEMBL240954 | SL-0101 | kaempferol 3-O-(3'',4''-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of recombinant RSK2 (unknown origin) expressed in baculovirus infected Sf9 cells assessed as decrease in substrate phosphorylation using u... | ACS Med Chem Lett 4: 175-179 (2012) Article DOI: 10.1021/ml300298v BindingDB Entry DOI: 10.7270/Q2668FHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50250427 ((+)-Myristinin D | CHEMBL448072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat recombinant DNA polymerase beta in absence of bovine serum albumin | J Nat Prod 68: 1625-8 (2005) Article DOI: 10.1021/np058064g BindingDB Entry DOI: 10.7270/Q2Q2400B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50426391 (CHEMBL2326707) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of recombinant RSK2 (unknown origin) expressed in baculovirus infected Sf9 cells assessed as decrease in substrate phosphorylation using u... | ACS Med Chem Lett 4: 175-179 (2012) Article DOI: 10.1021/ml300298v BindingDB Entry DOI: 10.7270/Q2668FHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50426394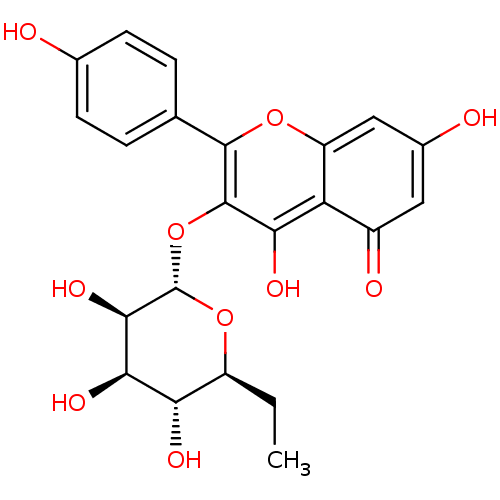 (CHEMBL2326704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of recombinant RSK2 (unknown origin) expressed in baculovirus infected Sf9 cells assessed as decrease in substrate phosphorylation using u... | ACS Med Chem Lett 4: 175-179 (2012) Article DOI: 10.1021/ml300298v BindingDB Entry DOI: 10.7270/Q2668FHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50426388 (CHEMBL2326709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of recombinant RSK2 (unknown origin) expressed in baculovirus infected Sf9 cells assessed as decrease in substrate phosphorylation using u... | ACS Med Chem Lett 4: 175-179 (2012) Article DOI: 10.1021/ml300298v BindingDB Entry DOI: 10.7270/Q2668FHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50259787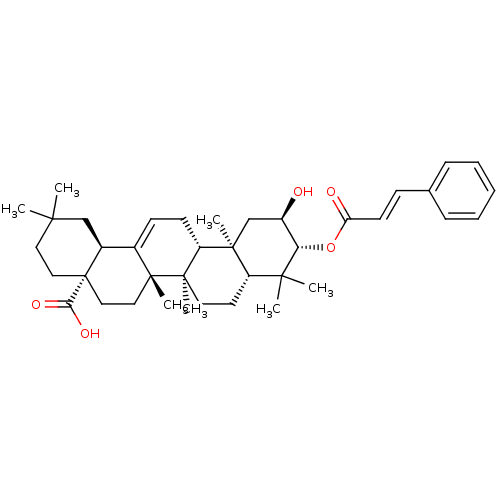 (3-trans-p-coumaroyl maslinic acid | CHEMBL453751) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in absence of BSA | J Nat Prod 62: 1660-3 (2000) BindingDB Entry DOI: 10.7270/Q2QF8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50259782 (3beta-hydroxyrus-18,20(30)-dien-28-oic acid | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in absence of BSA | J Nat Prod 62: 1624-6 (2000) BindingDB Entry DOI: 10.7270/Q2V69JBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50250426 ((+)-Myristinin A | CHEMBL465365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat recombinant DNA polymerase beta in absence of bovine serum albumin | J Nat Prod 68: 1625-8 (2005) Article DOI: 10.1021/np058064g BindingDB Entry DOI: 10.7270/Q2Q2400B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50250305 (3alpha-O-trans-p-coumaroyl-7-labden-15-oic acid | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in absence of BSA | J Nat Prod 62: 1000-2 (1999) Article DOI: 10.1021/np990099r BindingDB Entry DOI: 10.7270/Q2RF5TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50259781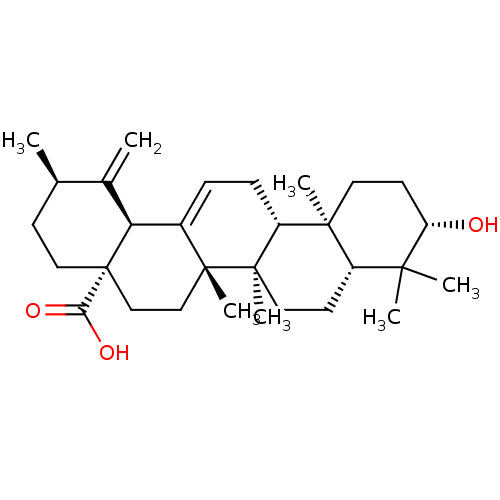 (3beta-hydroxyrus-12,19(29)-dien-28-oic acid | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in absence of BSA | J Nat Prod 62: 1624-6 (2000) BindingDB Entry DOI: 10.7270/Q2V69JBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50079577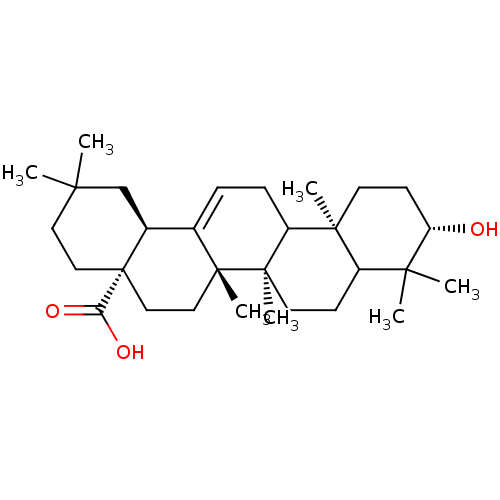 ((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in absence of BSA | J Nat Prod 62: 1624-6 (2000) BindingDB Entry DOI: 10.7270/Q2V69JBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50259787 (3-trans-p-coumaroyl maslinic acid | CHEMBL453751) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in presence of 0.1 mg/mL BSA | J Nat Prod 62: 1660-3 (2000) BindingDB Entry DOI: 10.7270/Q2QF8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50250427 ((+)-Myristinin D | CHEMBL448072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat recombinant DNA polymerase beta in presence of bovine serum albumin | J Nat Prod 68: 1625-8 (2005) Article DOI: 10.1021/np058064g BindingDB Entry DOI: 10.7270/Q2Q2400B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50250305 (3alpha-O-trans-p-coumaroyl-7-labden-15-oic acid | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in presence of BSA | J Nat Prod 62: 1000-2 (1999) Article DOI: 10.1021/np990099r BindingDB Entry DOI: 10.7270/Q2RF5TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in absence of BSA | J Nat Prod 62: 1624-6 (2000) BindingDB Entry DOI: 10.7270/Q2V69JBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50259781 (3beta-hydroxyrus-12,19(29)-dien-28-oic acid | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in presence of BSA | J Nat Prod 62: 1624-6 (2000) BindingDB Entry DOI: 10.7270/Q2V69JBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50259782 (3beta-hydroxyrus-18,20(30)-dien-28-oic acid | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in presence of BSA | J Nat Prod 62: 1624-6 (2000) BindingDB Entry DOI: 10.7270/Q2V69JBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50203241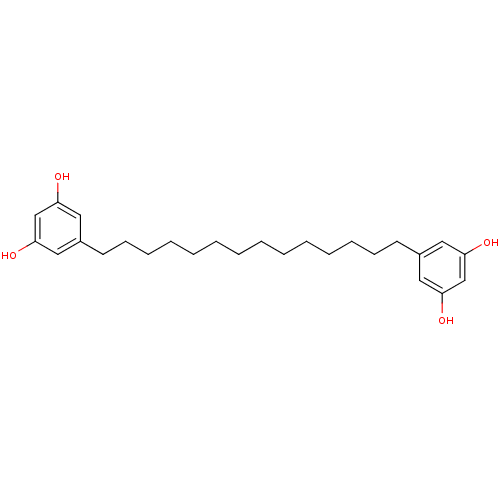 (1, 3-dihydroxy-5-[14'-(3' ',5' '-dihydroxyphenyl)t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta assessed as [3H]TTP incorporation after 60 mins by scintillation counting in presence of bovine serum albumin | J Nat Prod 62: 477-80 (1999) Article DOI: 10.1021/np980522g BindingDB Entry DOI: 10.7270/Q20Z731M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM23208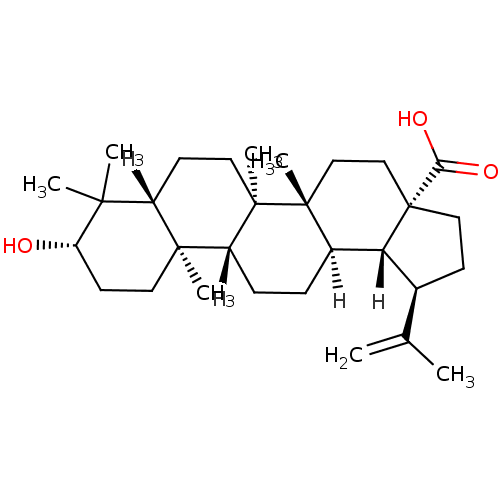 ((1R,2R,5S,8R,9R,10R,13R,14R,17S,19R)-17-hydroxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in absence of BSA | J Nat Prod 62: 1660-3 (2000) BindingDB Entry DOI: 10.7270/Q2QF8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50241611 (1, 3-dihydroxy-5-[14'-(3' ',5' '-dihydroxyphenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta assessed as [3H]TTP incorporation after 60 mins by scintillation counting in presence of bovine serum albumin | J Nat Prod 62: 477-80 (1999) Article DOI: 10.1021/np980522g BindingDB Entry DOI: 10.7270/Q20Z731M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50079577 ((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in presence of BSA | J Nat Prod 62: 1624-6 (2000) BindingDB Entry DOI: 10.7270/Q2V69JBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50259786 (3-cis-p-coumaroyl maslinic acid | CHEMBL463995) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in absence of BSA | J Nat Prod 62: 1660-3 (2000) BindingDB Entry DOI: 10.7270/Q2QF8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50241610 (1,3-dihydroxy-5-[14'-(3' ',5' '-dihydroxyphenyl)-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta assessed as [3H]TTP incorporation after 60 mins by scintillation counting in presence of bovine serum albumin | J Nat Prod 62: 477-80 (1999) Article DOI: 10.1021/np980522g BindingDB Entry DOI: 10.7270/Q20Z731M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in presence of BSA | J Nat Prod 62: 1624-6 (2000) BindingDB Entry DOI: 10.7270/Q2V69JBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta lyase activity by deoxyribose phosphate excision assay | J Nat Prod 66: 1463-5 (2003) Article DOI: 10.1021/np0301893 BindingDB Entry DOI: 10.7270/Q2K35VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50079577 ((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50241570 ((24E)-3beta-hydroxy-7,24-euphadien-26-oic acid | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat recombinant DNA polymerase beta after 60 mins | J Nat Prod 63: 1356-60 (2000) BindingDB Entry DOI: 10.7270/Q2X066S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50250426 ((+)-Myristinin A | CHEMBL465365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat recombinant DNA polymerase beta in presence of bovine serum albumin | J Nat Prod 68: 1625-8 (2005) Article DOI: 10.1021/np058064g BindingDB Entry DOI: 10.7270/Q2Q2400B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242105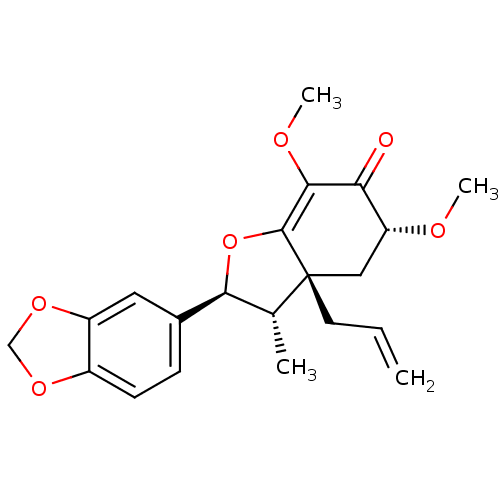 (CHEMBL470873 | armenin-B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50222205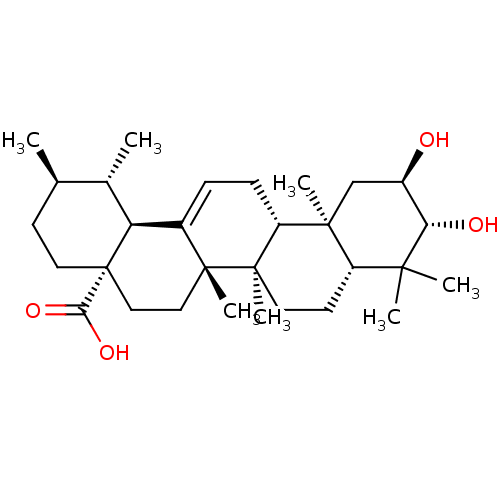 ((1S,2R,4aS,6aS,6bR,8aR,10R,11R,12aR,12bR,14bS)-10,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 899-901 (2004) Article DOI: 10.1021/np030531b BindingDB Entry DOI: 10.7270/Q2KH0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50250720 (3beta,16beta,23-triacetoxyolean-12-en-28-oic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta lyase activity by deoxyribose phosphate excision assay | J Nat Prod 66: 1463-5 (2003) Article DOI: 10.1021/np0301893 BindingDB Entry DOI: 10.7270/Q2K35VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM23208 ((1R,2R,5S,8R,9R,10R,13R,14R,17S,19R)-17-hydroxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in presence of 0.1 mg/mL BSA | J Nat Prod 62: 1660-3 (2000) BindingDB Entry DOI: 10.7270/Q2QF8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50259786 (3-cis-p-coumaroyl maslinic acid | CHEMBL463995) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta in presence of 0.1 mg/mL BSA | J Nat Prod 62: 1660-3 (2000) BindingDB Entry DOI: 10.7270/Q2QF8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242088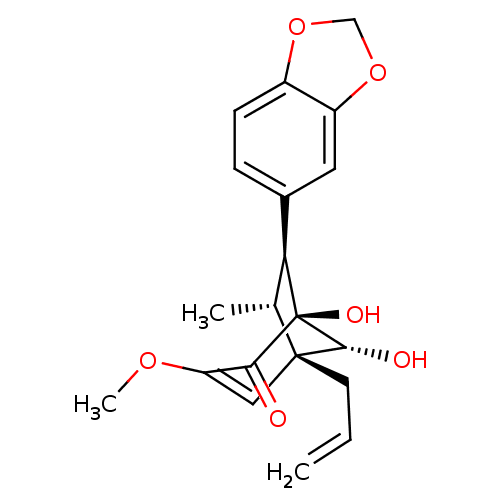 ((7S,8R,1'S,5'S,6'R)-delta 2',8'-5',6'-Dihydroxy-3'...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50250362 (3-Oximo-olean-12-en-29-oic acid | CHEMBL490347) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Charlottesville Curated by ChEMBL | Assay Description Inhibition of rat DNA polymerase beta | J Nat Prod 62: 1110-3 (1999) Article DOI: 10.1021/np990104r BindingDB Entry DOI: 10.7270/Q2C8293M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM23417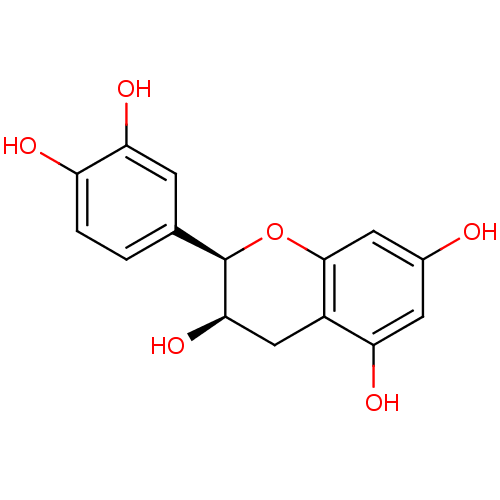 (α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of lyase activity of rat DNA polymerase beta after 30 mins by deoxyribose phosphate excision assay | J Nat Prod 67: 1744-7 (2004) Article DOI: 10.1021/np040057p BindingDB Entry DOI: 10.7270/Q2251K28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 899-901 (2004) Article DOI: 10.1021/np030531b BindingDB Entry DOI: 10.7270/Q2KH0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242090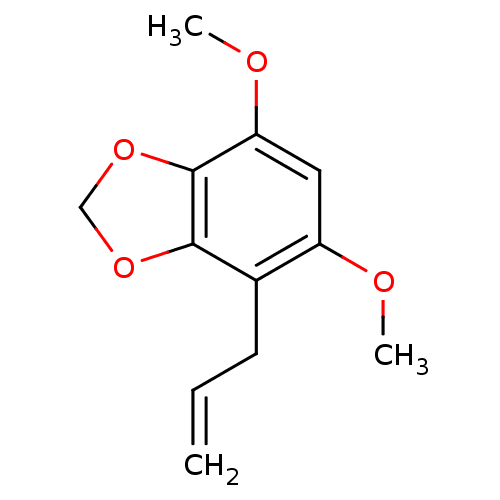 (2,4-Dimethoxy-5,6-methylenedioxy-1-(2-propenyl)ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 86 total ) | Next | Last >> |