 Found 3417 hits with Last Name = 'helal' and Initial = 'c'
Found 3417 hits with Last Name = 'helal' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398003
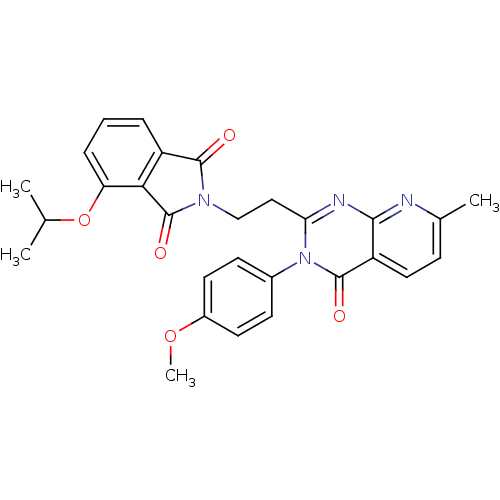
(CHEMBL2180402)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2nc(C)ccc2c1=O Show InChI InChI=1S/C28H26N4O5/c1-16(2)37-22-7-5-6-20-24(22)28(35)31(26(20)33)15-14-23-30-25-21(13-8-17(3)29-25)27(34)32(23)18-9-11-19(36-4)12-10-18/h5-13,16H,14-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398003

(CHEMBL2180402)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2nc(C)ccc2c1=O Show InChI InChI=1S/C28H26N4O5/c1-16(2)37-22-7-5-6-20-24(22)28(35)31(26(20)33)15-14-23-30-25-21(13-8-17(3)29-25)27(34)32(23)18-9-11-19(36-4)12-10-18/h5-13,16H,14-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398012
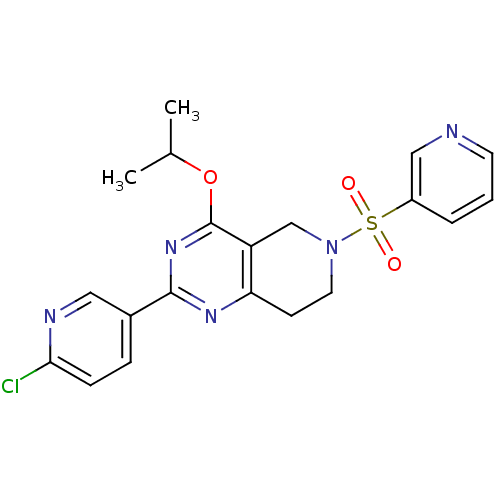
(CHEMBL2180422 | US8492392, 1-17)Show SMILES CC(C)Oc1nc(nc2CCN(Cc12)S(=O)(=O)c1cccnc1)-c1ccc(Cl)nc1 Show InChI InChI=1S/C20H20ClN5O3S/c1-13(2)29-20-16-12-26(30(27,28)15-4-3-8-22-11-15)9-7-17(16)24-19(25-20)14-5-6-18(21)23-10-14/h3-6,8,10-11,13H,7,9,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398013
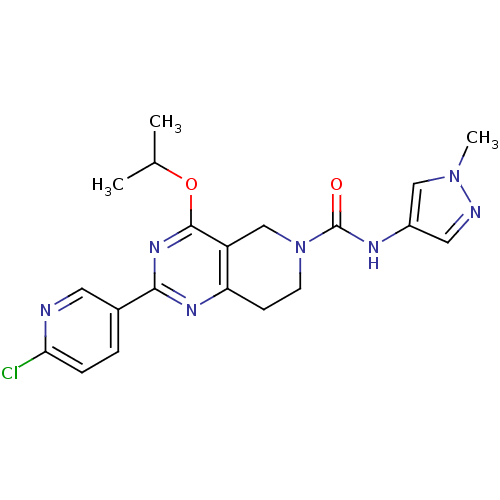
(CHEMBL2180421 | US8492392, K-2)Show SMILES CC(C)Oc1nc(nc2CCN(Cc12)C(=O)Nc1cnn(C)c1)-c1ccc(Cl)nc1 Show InChI InChI=1S/C20H22ClN7O2/c1-12(2)30-19-15-11-28(20(29)24-14-9-23-27(3)10-14)7-6-16(15)25-18(26-19)13-4-5-17(21)22-8-13/h4-5,8-10,12H,6-7,11H2,1-3H3,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398011
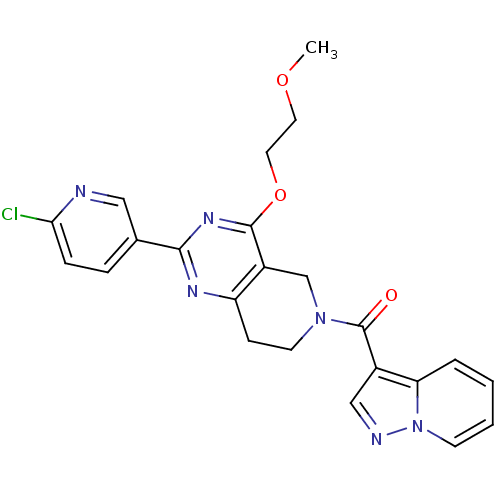
(CHEMBL2180423 | US8492392, O-2)Show SMILES COCCOc1nc(nc2CCN(Cc12)C(=O)c1cnn2ccccc12)-c1ccc(Cl)nc1 Show InChI InChI=1S/C23H21ClN6O3/c1-32-10-11-33-22-17-14-29(23(31)16-13-26-30-8-3-2-4-19(16)30)9-7-18(17)27-21(28-22)15-5-6-20(24)25-12-15/h2-6,8,12-13H,7,9-11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398004
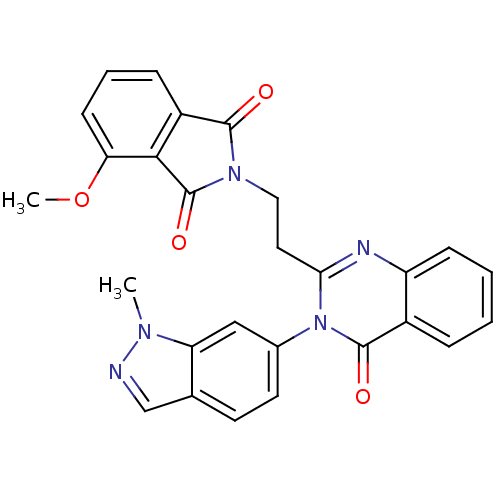
(CHEMBL2180401)Show SMILES COc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc4cnn(C)c4c3)C(=O)c12 Show InChI InChI=1S/C27H21N5O4/c1-30-21-14-17(11-10-16(21)15-28-30)32-23(29-20-8-4-3-6-18(20)26(32)34)12-13-31-25(33)19-7-5-9-22(36-2)24(19)27(31)35/h3-11,14-15H,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398008
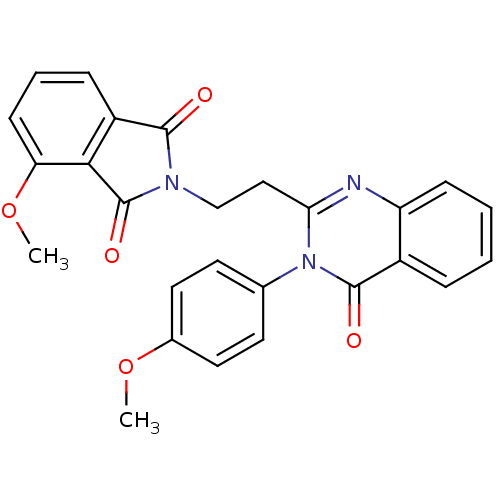
(CHEMBL2180426)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C26H21N3O5/c1-33-17-12-10-16(11-13-17)29-22(27-20-8-4-3-6-18(20)25(29)31)14-15-28-24(30)19-7-5-9-21(34-2)23(19)26(28)32/h3-13H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM119778
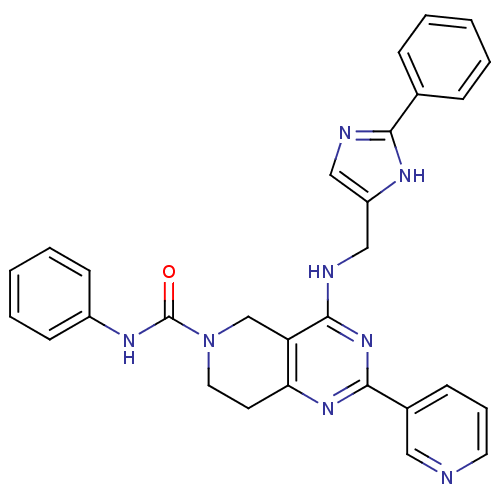
(CHEMBL2180429 | US8691827, I-9)Show SMILES O=C(Nc1ccccc1)N1CCc2nc(nc(NCc3cnc([nH]3)-c3ccccc3)c2C1)-c1cccnc1 Show InChI InChI=1S/C29H26N8O/c38-29(34-22-11-5-2-6-12-22)37-15-13-25-24(19-37)28(36-27(35-25)21-10-7-14-30-16-21)32-18-23-17-31-26(33-23)20-8-3-1-4-9-20/h1-12,14,16-17H,13,15,18-19H2,(H,31,33)(H,34,38)(H,32,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM107767
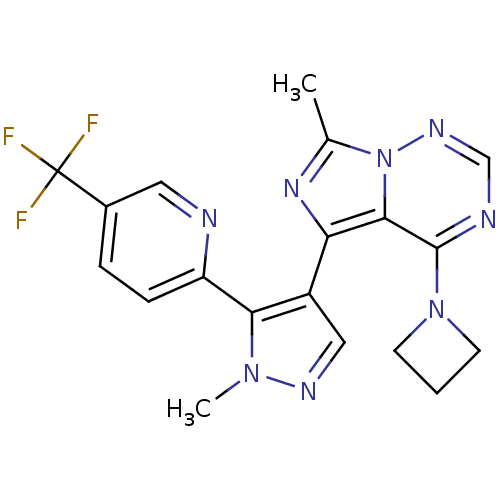
(US11419874, PF-05180999 | US8598155, 2)Show SMILES Cc1nc(-c2cnn(C)c2-c2ccc(cn2)C(F)(F)F)c2c(ncnn12)N1CCC1 Show InChI InChI=1S/C19H17F3N8/c1-11-27-15(17-18(29-6-3-7-29)24-10-26-30(11)17)13-9-25-28(2)16(13)14-5-4-12(8-23-14)19(20,21)22/h4-5,8-10H,3,6-7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled 4-(azetidin-1-yl)-3-[5-[4-(trifluoromethyl)phenyl]-1H-pyrazol-4-yl]-1-(tritritiomethyl)pyrazolo[3,4-d]pyrimidine from PD... |
J Med Chem 61: 1001-1018 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01466
BindingDB Entry DOI: 10.7270/Q2ZS2ZZ4 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50465935
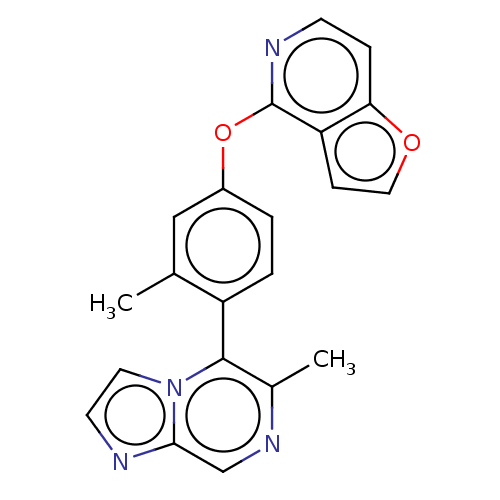
(CHEMBL4277264)Show SMILES Cc1cc(Oc2nccc3occc23)ccc1-c1c(C)ncc2nccn12 |(29.98,-5.28,;29.99,-6.82,;28.67,-7.59,;28.68,-9.13,;27.35,-9.9,;27.36,-11.44,;28.69,-12.2,;28.7,-13.75,;27.36,-14.52,;26.03,-13.75,;24.57,-14.23,;23.66,-13,;24.55,-11.74,;26.02,-12.21,;30.02,-9.9,;31.35,-9.12,;31.34,-7.58,;32.66,-6.8,;34,-7.57,;34.01,-9.11,;35.33,-6.79,;35.32,-5.25,;33.97,-4.49,;33.64,-2.99,;32.12,-2.84,;31.5,-4.24,;32.64,-5.27,)| Show InChI InChI=1S/C21H16N4O2/c1-13-11-15(27-21-17-6-10-26-18(17)5-7-23-21)3-4-16(13)20-14(2)24-12-19-22-8-9-25(19)20/h3-12H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D5 receptor (unknown origin) |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465945
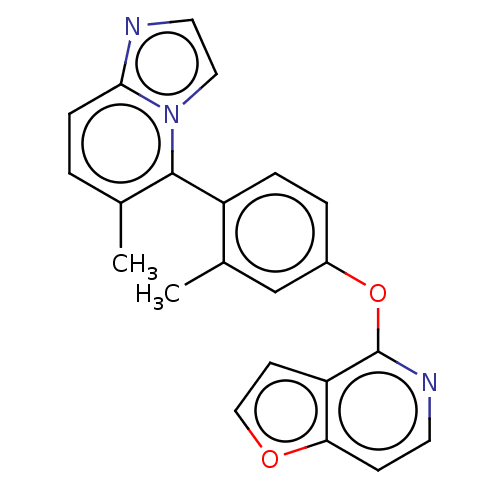
(CHEMBL4279267)Show SMILES Cc1ccc2nccn2c1-c1ccc(Oc2nccc3occc23)cc1C |(71.15,-35.83,;71.14,-34.29,;72.47,-33.52,;72.46,-31.97,;71.11,-31.22,;70.79,-29.72,;69.26,-29.56,;68.64,-30.97,;69.79,-31.99,;69.81,-33.53,;68.49,-34.3,;68.49,-35.85,;67.16,-36.62,;65.83,-35.85,;64.5,-36.63,;64.5,-38.17,;65.83,-38.93,;65.84,-40.47,;64.51,-41.24,;63.17,-40.47,;61.71,-40.95,;60.8,-39.72,;61.7,-38.47,;63.17,-38.94,;65.82,-34.31,;67.14,-33.54,;67.13,-32,)| Show InChI InChI=1S/C22H17N3O2/c1-14-3-6-20-23-10-11-25(20)21(14)17-5-4-16(13-15(17)2)27-22-18-8-12-26-19(18)7-9-24-22/h3-13H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465944
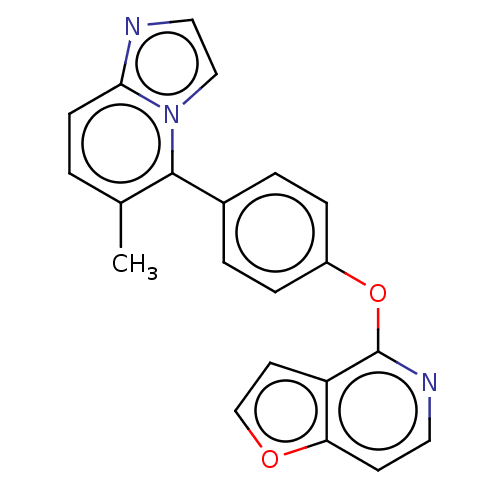
(CHEMBL4286177)Show InChI InChI=1S/C21H15N3O2/c1-14-2-7-19-22-11-12-24(19)20(14)15-3-5-16(6-4-15)26-21-17-9-13-25-18(17)8-10-23-21/h2-13H,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465935

(CHEMBL4277264)Show SMILES Cc1cc(Oc2nccc3occc23)ccc1-c1c(C)ncc2nccn12 |(29.98,-5.28,;29.99,-6.82,;28.67,-7.59,;28.68,-9.13,;27.35,-9.9,;27.36,-11.44,;28.69,-12.2,;28.7,-13.75,;27.36,-14.52,;26.03,-13.75,;24.57,-14.23,;23.66,-13,;24.55,-11.74,;26.02,-12.21,;30.02,-9.9,;31.35,-9.12,;31.34,-7.58,;32.66,-6.8,;34,-7.57,;34.01,-9.11,;35.33,-6.79,;35.32,-5.25,;33.97,-4.49,;33.64,-2.99,;32.12,-2.84,;31.5,-4.24,;32.64,-5.27,)| Show InChI InChI=1S/C21H16N4O2/c1-13-11-15(27-21-17-6-10-26-18(17)5-7-23-21)3-4-16(13)20-14(2)24-12-19-22-8-9-25(19)20/h3-12H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465935

(CHEMBL4277264)Show SMILES Cc1cc(Oc2nccc3occc23)ccc1-c1c(C)ncc2nccn12 |(29.98,-5.28,;29.99,-6.82,;28.67,-7.59,;28.68,-9.13,;27.35,-9.9,;27.36,-11.44,;28.69,-12.2,;28.7,-13.75,;27.36,-14.52,;26.03,-13.75,;24.57,-14.23,;23.66,-13,;24.55,-11.74,;26.02,-12.21,;30.02,-9.9,;31.35,-9.12,;31.34,-7.58,;32.66,-6.8,;34,-7.57,;34.01,-9.11,;35.33,-6.79,;35.32,-5.25,;33.97,-4.49,;33.64,-2.99,;32.12,-2.84,;31.5,-4.24,;32.64,-5.27,)| Show InChI InChI=1S/C21H16N4O2/c1-13-11-15(27-21-17-6-10-26-18(17)5-7-23-21)3-4-16(13)20-14(2)24-12-19-22-8-9-25(19)20/h3-12H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398005
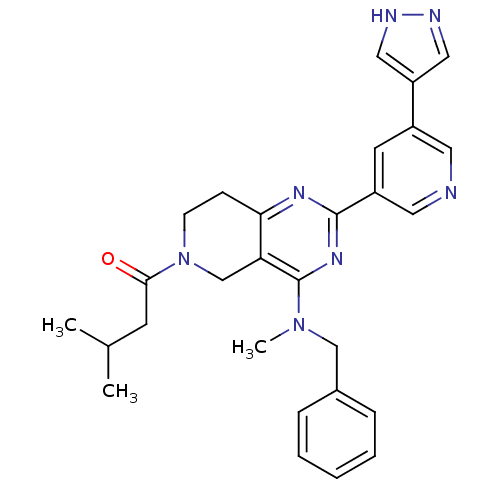
(CHEMBL2180430 | US8691827, I-8)Show SMILES CC(C)CC(=O)N1CCc2nc(nc(N(C)Cc3ccccc3)c2C1)-c1cncc(c1)-c1cn[nH]c1 Show InChI InChI=1S/C28H31N7O/c1-19(2)11-26(36)35-10-9-25-24(18-35)28(34(3)17-20-7-5-4-6-8-20)33-27(32-25)22-12-21(13-29-14-22)23-15-30-31-16-23/h4-8,12-16,19H,9-11,17-18H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465935

(CHEMBL4277264)Show SMILES Cc1cc(Oc2nccc3occc23)ccc1-c1c(C)ncc2nccn12 |(29.98,-5.28,;29.99,-6.82,;28.67,-7.59,;28.68,-9.13,;27.35,-9.9,;27.36,-11.44,;28.69,-12.2,;28.7,-13.75,;27.36,-14.52,;26.03,-13.75,;24.57,-14.23,;23.66,-13,;24.55,-11.74,;26.02,-12.21,;30.02,-9.9,;31.35,-9.12,;31.34,-7.58,;32.66,-6.8,;34,-7.57,;34.01,-9.11,;35.33,-6.79,;35.32,-5.25,;33.97,-4.49,;33.64,-2.99,;32.12,-2.84,;31.5,-4.24,;32.64,-5.27,)| Show InChI InChI=1S/C21H16N4O2/c1-13-11-15(27-21-17-6-10-26-18(17)5-7-23-21)3-4-16(13)20-14(2)24-12-19-22-8-9-25(19)20/h3-12H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 21.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465946

(CHEMBL4294397)Show InChI InChI=1S/C21H15N3O2/c1-14-13-15(26-21-17-8-12-25-19(17)7-9-23-21)5-6-16(14)18-3-2-4-20-22-10-11-24(18)20/h2-13H,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398007
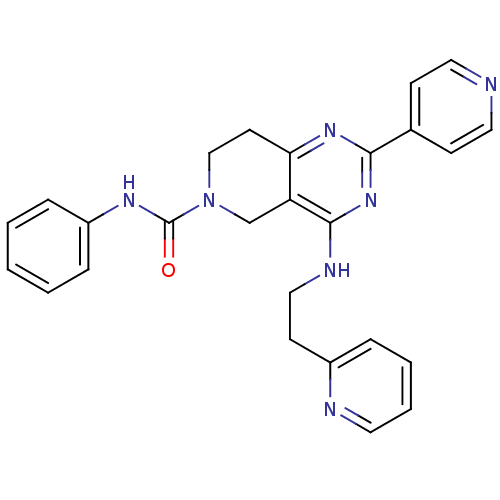
(CHEMBL2180428 | US8691827, I-1)Show SMILES O=C(Nc1ccccc1)N1CCc2nc(nc(NCCc3ccccn3)c2C1)-c1ccncc1 Show InChI InChI=1S/C26H25N7O/c34-26(30-21-7-2-1-3-8-21)33-17-12-23-22(18-33)25(29-16-11-20-6-4-5-13-28-20)32-24(31-23)19-9-14-27-15-10-19/h1-10,13-15H,11-12,16-18H2,(H,30,34)(H,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465943
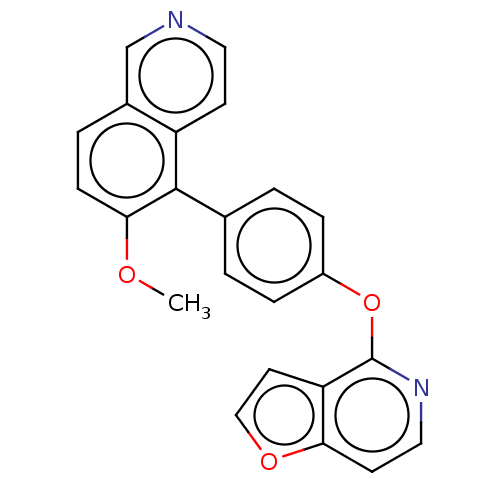
(CHEMBL4286110)Show InChI InChI=1S/C23H16N2O3/c1-26-21-7-4-16-14-24-11-8-18(16)22(21)15-2-5-17(6-3-15)28-23-19-10-13-27-20(19)9-12-25-23/h2-14H,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398010

(CHEMBL2180424)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3ccccc3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C25H19N3O4/c1-32-17-12-10-16(11-13-17)28-22(26-21-9-5-4-8-20(21)25(28)31)14-15-27-23(29)18-6-2-3-7-19(18)24(27)30/h2-13H,14-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465938

(CHEMBL4294009)Show InChI InChI=1S/C20H13N3O2/c1-2-17(23-12-11-21-19(23)3-1)14-4-6-15(7-5-14)25-20-16-9-13-24-18(16)8-10-22-20/h1-13H | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088503
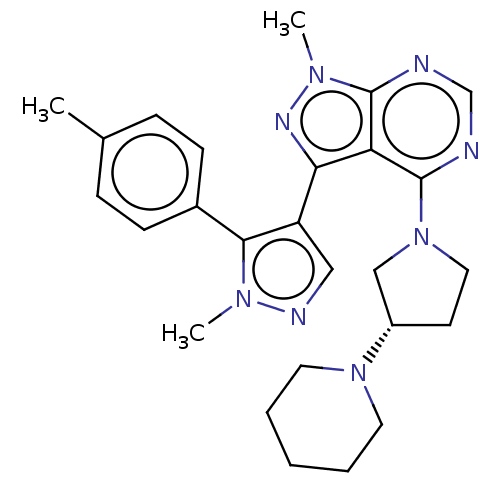
(CHEMBL3527048)Show SMILES Cc1ccc(cc1)-c1c(cnn1C)-c1nn(C)c2ncnc(N3CC[C@@H](C3)N3CCCCC3)c12 |r| Show InChI InChI=1S/C26H32N8/c1-18-7-9-19(10-8-18)24-21(15-29-31(24)2)23-22-25(32(3)30-23)27-17-28-26(22)34-14-11-20(16-34)33-12-5-4-6-13-33/h7-10,15,17,20H,4-6,11-14,16H2,1-3H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A4 harboring human P450 oxidoreductase and b5 assessed as decrease in enzyme... |
Drug Metab Dispos 40: 1686-97 (2012)
Article DOI: 10.1124/dmd.112.045302
BindingDB Entry DOI: 10.7270/Q2GT5PW2 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465934
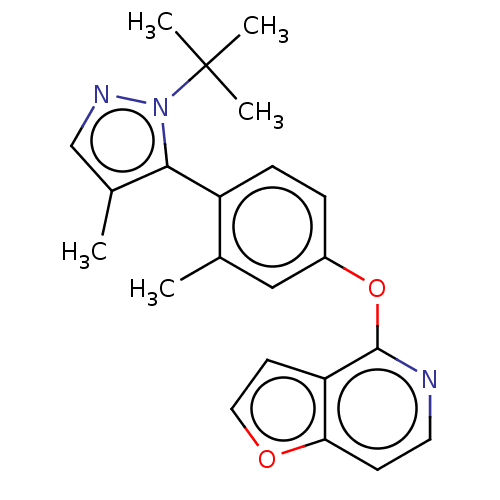
(CHEMBL4293757)Show SMILES Cc1cnn(c1-c1ccc(Oc2nccc3occc23)cc1C)C(C)(C)C |(32.4,-13.64,;33.56,-14.66,;35.05,-14.32,;35.84,-15.64,;34.83,-16.8,;33.41,-16.19,;32.09,-16.97,;32.1,-18.51,;30.77,-19.28,;29.43,-18.52,;28.1,-19.29,;28.11,-20.83,;29.44,-21.59,;29.45,-23.14,;28.11,-23.91,;26.78,-23.14,;25.32,-23.62,;24.41,-22.38,;25.3,-21.13,;26.78,-21.6,;29.42,-16.98,;30.75,-16.21,;30.74,-14.67,;35.16,-18.3,;36.64,-18.76,;34.03,-19.35,;35.6,-19.8,)| Show InChI InChI=1S/C22H23N3O2/c1-14-12-16(27-21-18-9-11-26-19(18)8-10-23-21)6-7-17(14)20-15(2)13-24-25(20)22(3,4)5/h6-13H,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088503

(CHEMBL3527048)Show SMILES Cc1ccc(cc1)-c1c(cnn1C)-c1nn(C)c2ncnc(N3CC[C@@H](C3)N3CCCCC3)c12 |r| Show InChI InChI=1S/C26H32N8/c1-18-7-9-19(10-8-18)24-21(15-29-31(24)2)23-22-25(32(3)30-23)27-17-28-26(22)34-14-11-20(16-34)33-12-5-4-6-13-33/h7-10,15,17,20H,4-6,11-14,16H2,1-3H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A4 in presence of human P450 oxidoreductase and b5 assessed as decreas... |
Drug Metab Dispos 40: 1686-97 (2012)
Article DOI: 10.1124/dmd.112.045302
BindingDB Entry DOI: 10.7270/Q2GT5PW2 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM55121
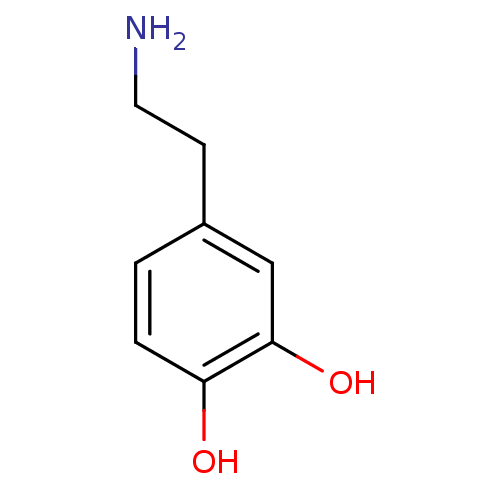
(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465942
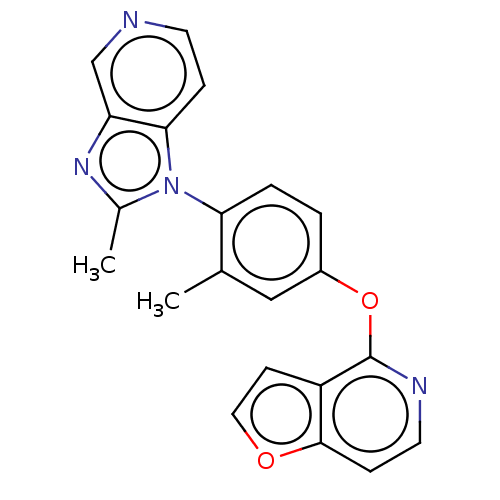
(CHEMBL4278861)Show SMILES Cc1nc2cnccc2n1-c1ccc(Oc2nccc3occc23)cc1C |(51.19,-1.16,;52.34,-2.18,;53.85,-1.85,;54.62,-3.18,;56.12,-3.49,;56.61,-4.94,;55.59,-6.09,;54.08,-5.78,;53.6,-4.33,;52.19,-3.72,;50.86,-4.5,;50.87,-6.05,;49.54,-6.82,;48.2,-6.05,;46.87,-6.83,;46.88,-8.37,;48.21,-9.13,;48.22,-10.67,;46.88,-11.45,;45.54,-10.68,;44.08,-11.16,;43.17,-9.92,;44.07,-8.67,;45.54,-9.14,;48.19,-4.51,;49.52,-3.74,;49.5,-2.2,)| Show InChI InChI=1S/C21H16N4O2/c1-13-11-15(27-21-16-7-10-26-20(16)6-9-23-21)3-4-18(13)25-14(2)24-17-12-22-8-5-19(17)25/h3-12H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465936
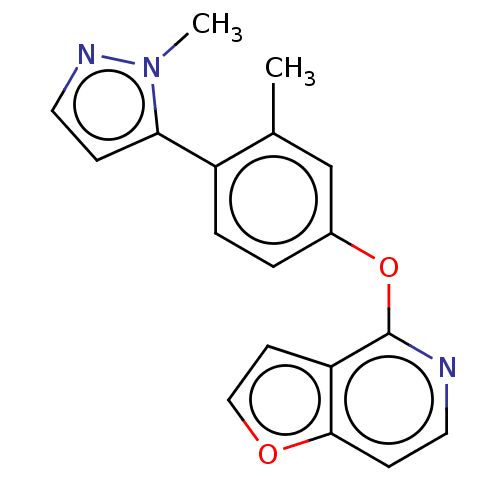
(CHEMBL4293356)Show InChI InChI=1S/C18H15N3O2/c1-12-11-13(3-4-14(12)16-5-9-20-21(16)2)23-18-15-7-10-22-17(15)6-8-19-18/h3-11H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 254 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50398789
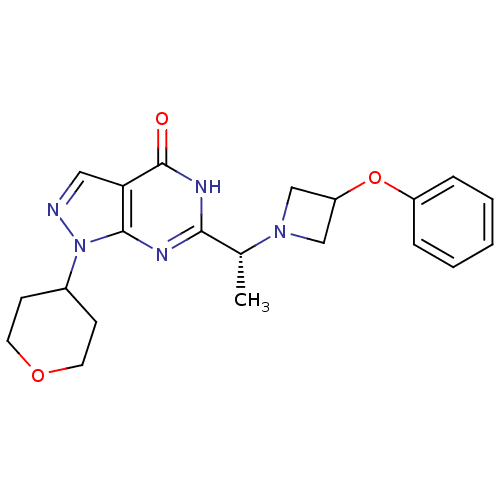
(CHEMBL2180073)Show SMILES C[C@@H](N1CC(C1)Oc1ccccc1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C21H25N5O3/c1-14(25-12-17(13-25)29-16-5-3-2-4-6-16)19-23-20-18(21(27)24-19)11-22-26(20)15-7-9-28-10-8-15/h2-6,11,14-15,17H,7-10,12-13H2,1H3,(H,23,24,27)/t14-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 293 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DAT |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465947
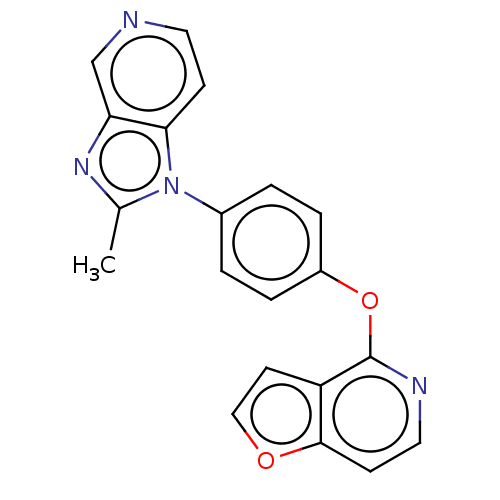
(CHEMBL4289538)Show InChI InChI=1S/C20H14N4O2/c1-13-23-17-12-21-9-6-18(17)24(13)14-2-4-15(5-3-14)26-20-16-8-11-25-19(16)7-10-22-20/h2-12H,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465941

(CHEMBL4283176)Show InChI InChI=1S/C21H21N3O2/c1-14-13-15(26-20-17-9-12-25-19(17)8-10-22-20)5-6-16(14)18-7-11-23-24(18)21(2,3)4/h5-13H,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 563 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50003985
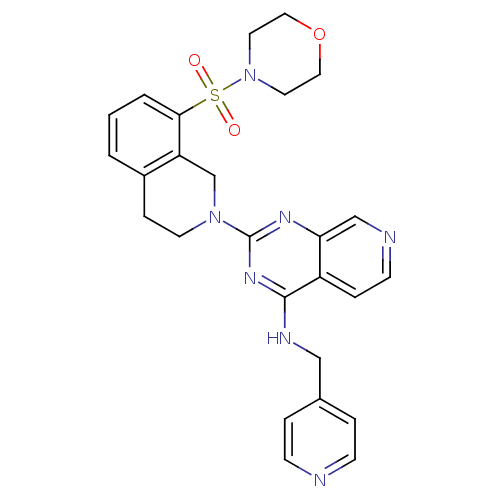
(CHEMBL2180436)Show SMILES O=S(=O)(N1CCOCC1)c1cccc2CCN(Cc12)c1nc(NCc2ccncc2)c2ccncc2n1 Show InChI InChI=1S/C26H27N7O3S/c34-37(35,33-12-14-36-15-13-33)24-3-1-2-20-7-11-32(18-22(20)24)26-30-23-17-28-10-6-21(23)25(31-26)29-16-19-4-8-27-9-5-19/h1-6,8-10,17H,7,11-16,18H2,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50003981
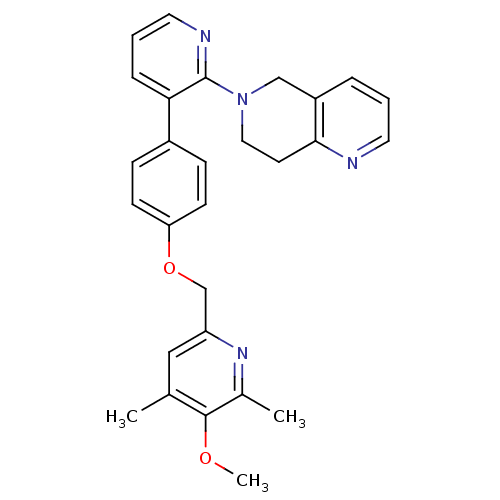
(CHEMBL2180431)Show SMILES COc1c(C)cc(COc2ccc(cc2)-c2cccnc2N2CCc3ncccc3C2)nc1C Show InChI InChI=1S/C28H28N4O2/c1-19-16-23(31-20(2)27(19)33-3)18-34-24-10-8-21(9-11-24)25-7-5-14-30-28(25)32-15-12-26-22(17-32)6-4-13-29-26/h4-11,13-14,16H,12,15,17-18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50003982
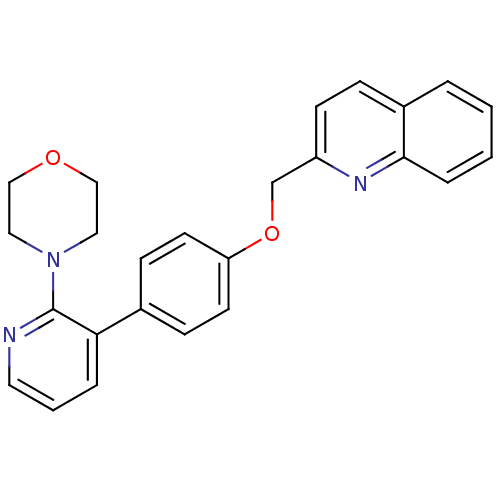
(CHEMBL2180432)Show SMILES C(Oc1ccc(cc1)-c1cccnc1N1CCOCC1)c1ccc2ccccc2n1 Show InChI InChI=1S/C25H23N3O2/c1-2-6-24-20(4-1)7-10-21(27-24)18-30-22-11-8-19(9-12-22)23-5-3-13-26-25(23)28-14-16-29-17-15-28/h1-13H,14-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50003979
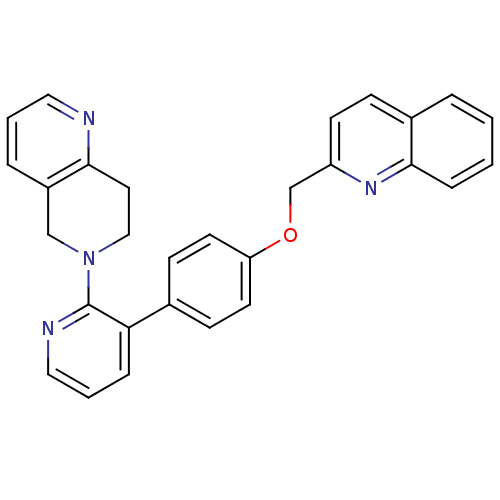
(CHEMBL2180433)Show SMILES C(Oc1ccc(cc1)-c1cccnc1N1CCc2ncccc2C1)c1ccc2ccccc2n1 Show InChI InChI=1S/C29H24N4O/c1-2-8-28-22(5-1)9-12-24(32-28)20-34-25-13-10-21(11-14-25)26-7-4-17-31-29(26)33-18-15-27-23(19-33)6-3-16-30-27/h1-14,16-17H,15,18-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50003980
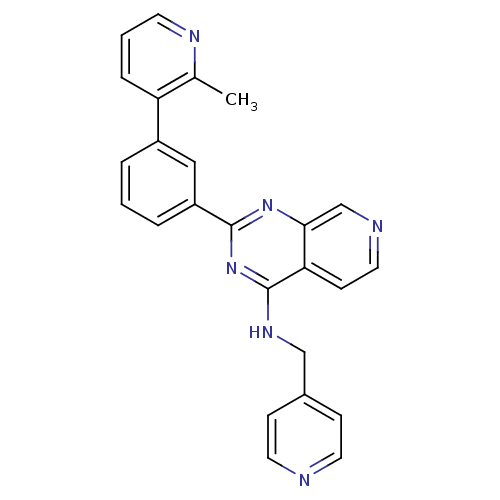
(CHEMBL2180434)Show SMILES Cc1ncccc1-c1cccc(c1)-c1nc(NCc2ccncc2)c2ccncc2n1 Show InChI InChI=1S/C25H20N6/c1-17-21(6-3-10-28-17)19-4-2-5-20(14-19)24-30-23-16-27-13-9-22(23)25(31-24)29-15-18-7-11-26-12-8-18/h2-14,16H,15H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50003984
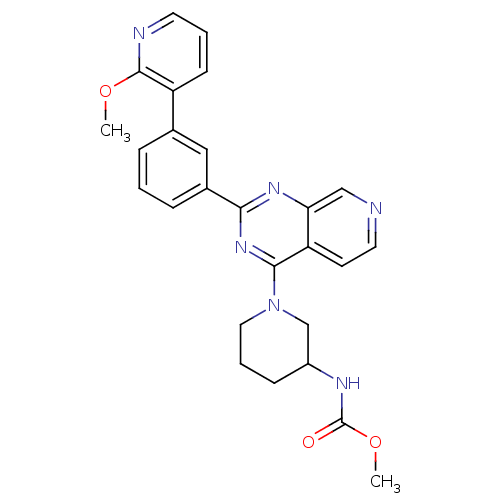
(CHEMBL2180437)Show SMILES COC(=O)NC1CCCN(C1)c1nc(nc2cnccc12)-c1cccc(c1)-c1cccnc1OC Show InChI InChI=1S/C26H26N6O3/c1-34-25-20(9-4-11-28-25)17-6-3-7-18(14-17)23-30-22-15-27-12-10-21(22)24(31-23)32-13-5-8-19(16-32)29-26(33)35-2/h3-4,6-7,9-12,14-15,19H,5,8,13,16H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50003983
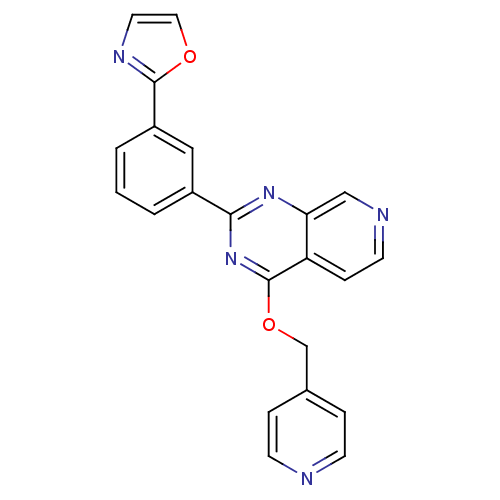
(CHEMBL2180435)Show SMILES C(Oc1nc(nc2cnccc12)-c1cccc(c1)-c1ncco1)c1ccncc1 Show InChI InChI=1S/C22H15N5O2/c1-2-16(12-17(3-1)21-25-10-11-28-21)20-26-19-13-24-9-6-18(19)22(27-20)29-14-15-4-7-23-8-5-15/h1-13H,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50398807
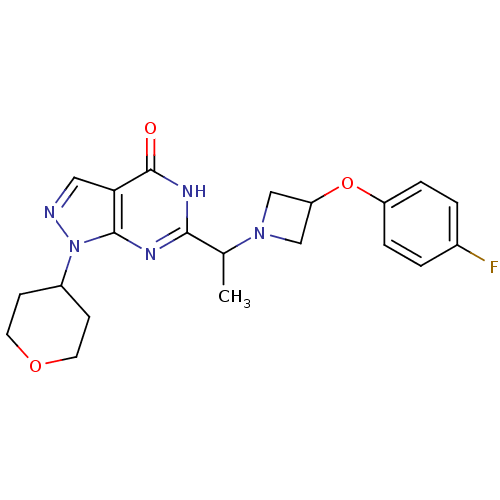
(CHEMBL2180074)Show SMILES CC(N1CC(C1)Oc1ccc(F)cc1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C21H24FN5O3/c1-13(26-11-17(12-26)30-16-4-2-14(22)3-5-16)19-24-20-18(21(28)25-19)10-23-27(20)15-6-8-29-9-7-15/h2-5,10,13,15,17H,6-9,11-12H2,1H3,(H,24,25,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DAT |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A5
(Homo sapiens (Human)) | BDBM50088503

(CHEMBL3527048)Show SMILES Cc1ccc(cc1)-c1c(cnn1C)-c1nn(C)c2ncnc(N3CC[C@@H](C3)N3CCCCC3)c12 |r| Show InChI InChI=1S/C26H32N8/c1-18-7-9-19(10-8-18)24-21(15-29-31(24)2)23-22-25(32(3)30-23)27-17-28-26(22)34-14-11-20(16-34)33-12-5-4-6-13-33/h7-10,15,17,20H,4-6,11-14,16H2,1-3H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A5 in presence of human P450 oxidoreductase and b5 assessed as decreas... |
Drug Metab Dispos 40: 1686-97 (2012)
Article DOI: 10.1124/dmd.112.045302
BindingDB Entry DOI: 10.7270/Q2GT5PW2 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465939
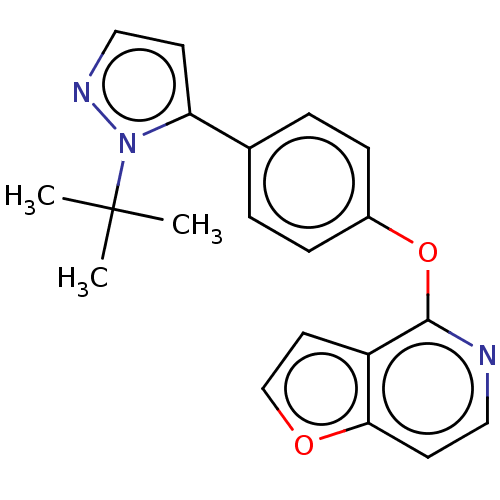
(CHEMBL4285528)Show InChI InChI=1S/C20H19N3O2/c1-20(2,3)23-17(8-12-22-23)14-4-6-15(7-5-14)25-19-16-10-13-24-18(16)9-11-21-19/h4-13H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398009

(CHEMBL2180425)Show SMILES COc1ccc(cc1)-n1c(CN2C(=O)c3ccccc3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C24H17N3O4/c1-31-16-12-10-15(11-13-16)27-21(25-20-9-5-4-8-19(20)24(27)30)14-26-22(28)17-6-2-3-7-18(17)23(26)29/h2-13H,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A by fluorescence polarization assay |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A5
(Homo sapiens (Human)) | BDBM50088503

(CHEMBL3527048)Show SMILES Cc1ccc(cc1)-c1c(cnn1C)-c1nn(C)c2ncnc(N3CC[C@@H](C3)N3CCCCC3)c12 |r| Show InChI InChI=1S/C26H32N8/c1-18-7-9-19(10-8-18)24-21(15-29-31(24)2)23-22-25(32(3)30-23)27-17-28-26(22)34-14-11-20(16-34)33-12-5-4-6-13-33/h7-10,15,17,20H,4-6,11-14,16H2,1-3H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A5 harboring human P450 oxidoreductase and b5 assessed as decrease in enzyme... |
Drug Metab Dispos 40: 1686-97 (2012)
Article DOI: 10.1124/dmd.112.045302
BindingDB Entry DOI: 10.7270/Q2GT5PW2 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465940
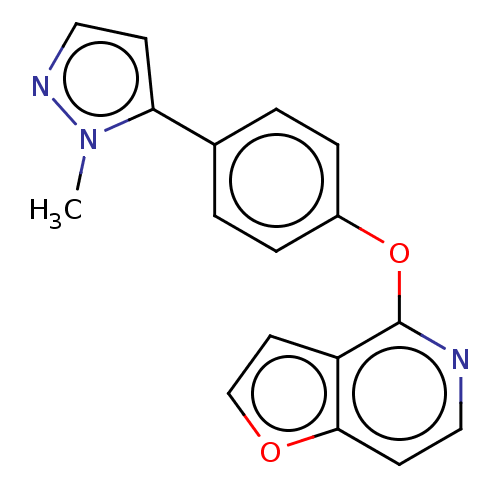
(CHEMBL4282096)Show InChI InChI=1S/C17H13N3O2/c1-20-15(6-10-19-20)12-2-4-13(5-3-12)22-17-14-8-11-21-16(14)7-9-18-17/h2-11H,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50465937

(CHEMBL4287192)Show InChI InChI=1S/C19H13N5O2/c1-12-8-13(26-19-15-5-7-25-17(15)4-6-21-19)2-3-14(12)16-9-20-10-18-22-11-23-24(16)18/h2-11H,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50398804
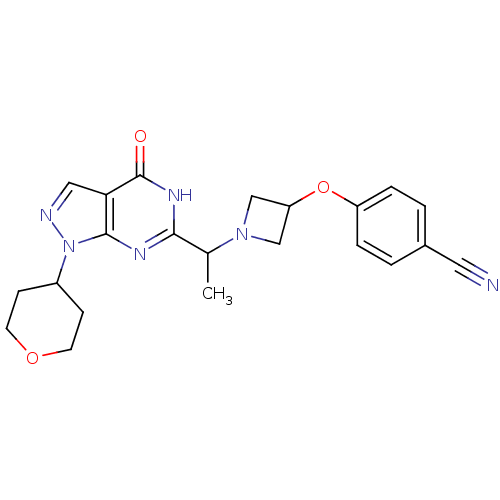
(CHEMBL2177497)Show SMILES CC(N1CC(C1)Oc1ccc(cc1)C#N)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C22H24N6O3/c1-14(27-12-18(13-27)31-17-4-2-15(10-23)3-5-17)20-25-21-19(22(29)26-20)11-24-28(21)16-6-8-30-9-7-16/h2-5,11,14,16,18H,6-9,12-13H2,1H3,(H,25,26,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DAT |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50398800
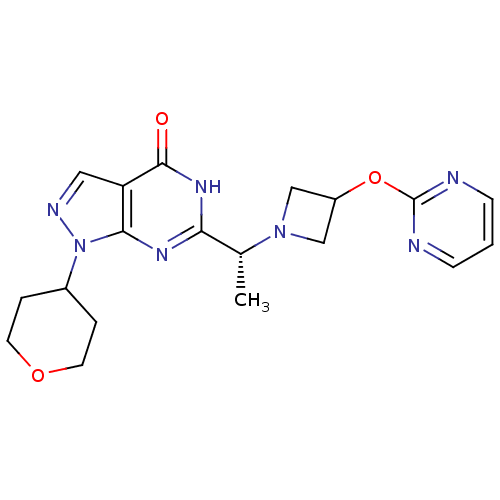
(CHEMBL2180069)Show SMILES C[C@@H](N1CC(C1)Oc1ncccn1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C19H23N7O3/c1-12(25-10-14(11-25)29-19-20-5-2-6-21-19)16-23-17-15(18(27)24-16)9-22-26(17)13-3-7-28-8-4-13/h2,5-6,9,12-14H,3-4,7-8,10-11H2,1H3,(H,23,24,27)/t12-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DAT |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50465935

(CHEMBL4277264)Show SMILES Cc1cc(Oc2nccc3occc23)ccc1-c1c(C)ncc2nccn12 |(29.98,-5.28,;29.99,-6.82,;28.67,-7.59,;28.68,-9.13,;27.35,-9.9,;27.36,-11.44,;28.69,-12.2,;28.7,-13.75,;27.36,-14.52,;26.03,-13.75,;24.57,-14.23,;23.66,-13,;24.55,-11.74,;26.02,-12.21,;30.02,-9.9,;31.35,-9.12,;31.34,-7.58,;32.66,-6.8,;34,-7.57,;34.01,-9.11,;35.33,-6.79,;35.32,-5.25,;33.97,-4.49,;33.64,-2.99,;32.12,-2.84,;31.5,-4.24,;32.64,-5.27,)| Show InChI InChI=1S/C21H16N4O2/c1-13-11-15(27-21-17-6-10-26-18(17)5-7-23-21)3-4-16(13)20-14(2)24-12-19-22-8-9-25(19)20/h3-12H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D2 receptor (unknown origin) |
J Med Chem 61: 11384-11397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01622
BindingDB Entry DOI: 10.7270/Q20G3NT6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50397973
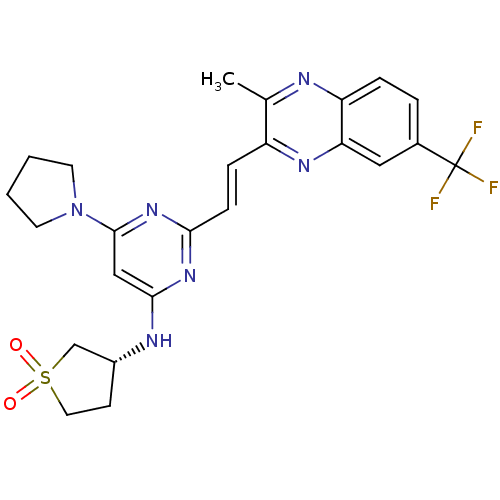
(CHEMBL2180441)Show SMILES Cc1nc2ccc(cc2nc1\C=C\c1nc(N[C@@H]2CCS(=O)(=O)C2)cc(n1)N1CCCC1)C(F)(F)F |r| Show InChI InChI=1S/C24H25F3N6O2S/c1-15-18(30-20-12-16(24(25,26)27)4-5-19(20)28-15)6-7-21-31-22(29-17-8-11-36(34,35)14-17)13-23(32-21)33-9-2-3-10-33/h4-7,12-13,17H,2-3,8-11,14H2,1H3,(H,29,31,32)/b7-6+/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A |
J Med Chem 55: 7299-331 (2012)
Article DOI: 10.1021/jm3004976
BindingDB Entry DOI: 10.7270/Q2C24XJK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM160666
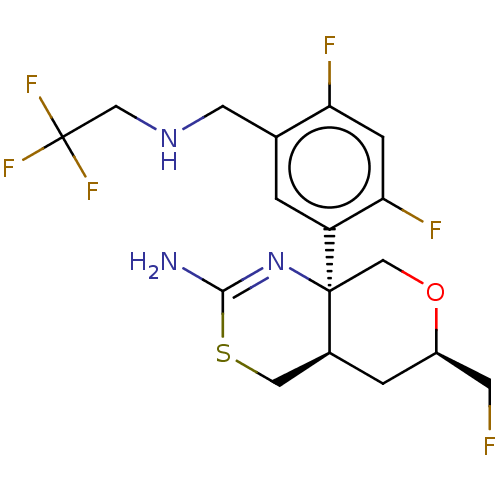
(US9045498, 8)Show SMILES NC1=N[C@]2(CO[C@@H](CF)C[C@H]2CS1)c1cc(CNCC(F)(F)F)c(F)cc1F |t:1| Show InChI InChI=1S/C17H19F6N3OS/c18-4-11-2-10-6-28-15(24)26-16(10,8-27-11)12-1-9(13(19)3-14(12)20)5-25-7-17(21,22)23/h1,3,10-11,25H,2,4-8H2,(H2,24,26)/t10-,11+,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA |
J Med Chem 60: 386-402 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01451
BindingDB Entry DOI: 10.7270/Q2154KHX |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50259962

(CHEMBL4088234)Show SMILES C[C@@H]1C[C@](C)(N=C(N)S1)c1cc(CNC2(CC2)C(F)(F)F)c(F)cc1F |r,t:5| Show InChI InChI=1S/C17H20F5N3S/c1-9-7-15(2,25-14(23)26-9)11-5-10(12(18)6-13(11)19)8-24-16(3-4-16)17(20,21)22/h5-6,9,24H,3-4,7-8H2,1-2H3,(H2,23,25)/t9-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA |
J Med Chem 60: 386-402 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01451
BindingDB Entry DOI: 10.7270/Q2154KHX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data