

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50156552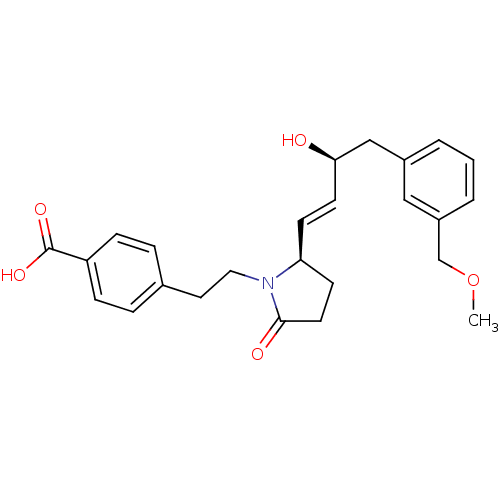 (4-(2-((R)-2-((S,E)-3-hydroxy-4-(3-(methoxymethyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50156551 (4-{2-[(2S)-2-[(3R)-3-[3-(4-chloro-2-methylphenyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50156553 (4-(2-((R)-2-((S,E)-5-cyclobutyl-3-hydroxypent-1-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50156547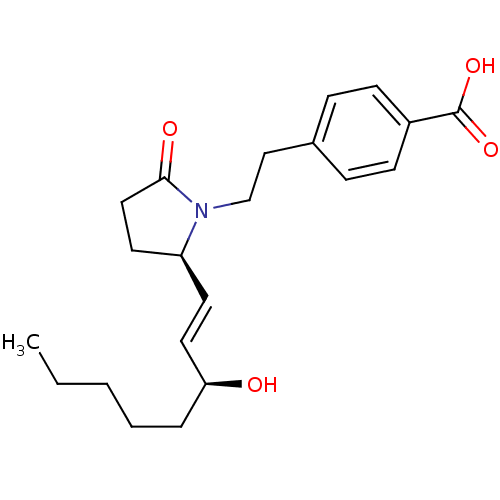 (4-(2-((R)-2-((S)-3-hydroxyoct-1-enyl)-5-oxopyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50156554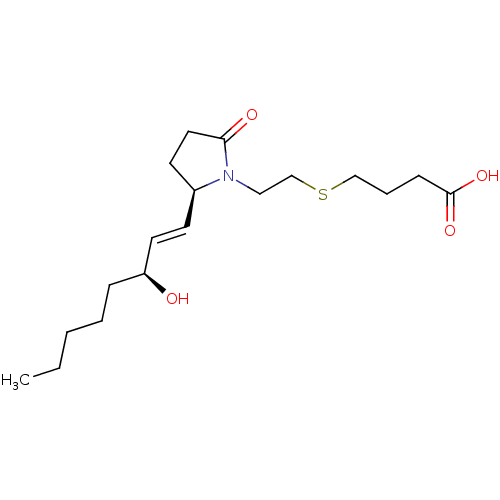 (4-(2-((R)-2-((S,E)-3-hydroxyoct-1-enyl)-5-oxopyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50142481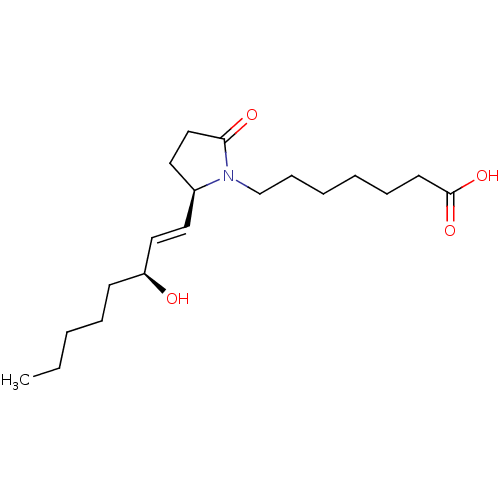 (7-[(R)-2-((E)-(S)-3-Hydroxy-oct-1-enyl)-5-oxo-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50156548 (5-(2-((R)-2-((S,E)-3-hydroxyoct-1-enyl)-5-oxopyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50156555 (2-(4-((R)-2-((S,E)-3-hydroxy-4-(3-(trifluoromethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50156549 (4-(2-((S)-2-((S)-3-hydroxyoctyl)-5-oxopyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50156546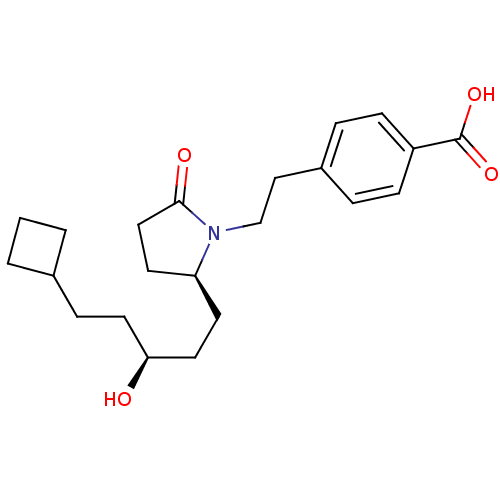 (4-(2-((S)-2-((S)-5-cyclobutyl-3-hydroxypentyl)-5-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156549 (4-(2-((S)-2-((S)-3-hydroxyoctyl)-5-oxopyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP2 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156553 (4-(2-((R)-2-((S,E)-5-cyclobutyl-3-hydroxypent-1-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP2 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156547 (4-(2-((R)-2-((S)-3-hydroxyoct-1-enyl)-5-oxopyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP2 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156546 (4-(2-((S)-2-((S)-5-cyclobutyl-3-hydroxypentyl)-5-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP2 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50156556 (2-(2-((R)-2-((S,E)-3-hydroxyoct-1-enyl)-5-oxopyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50156550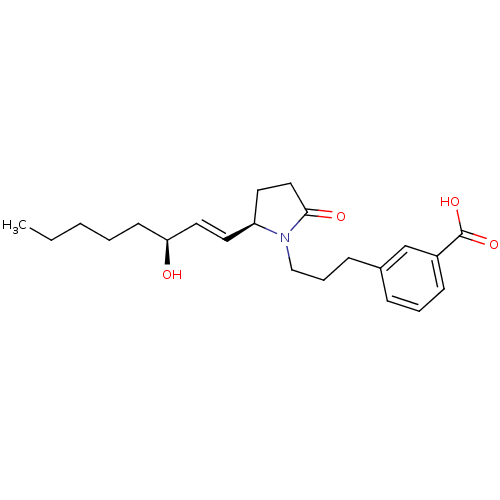 (3-(3-((R)-2-((S,E)-3-hydroxyoct-1-enyl)-5-oxopyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50156554 (4-(2-((R)-2-((S,E)-3-hydroxyoct-1-enyl)-5-oxopyrro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP3 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156552 (4-(2-((R)-2-((S,E)-3-hydroxy-4-(3-(methoxymethyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP2 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156548 (5-(2-((R)-2-((S,E)-3-hydroxyoct-1-enyl)-5-oxopyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP2 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156554 (4-(2-((R)-2-((S,E)-3-hydroxyoct-1-enyl)-5-oxopyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP2 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156551 (4-{2-[(2S)-2-[(3R)-3-[3-(4-chloro-2-methylphenyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP2 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50156547 (4-(2-((R)-2-((S)-3-hydroxyoct-1-enyl)-5-oxopyrroli...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP3 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50156551 (4-{2-[(2S)-2-[(3R)-3-[3-(4-chloro-2-methylphenyl)p...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP1 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50156551 (4-{2-[(2S)-2-[(3R)-3-[3-(4-chloro-2-methylphenyl)p...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP3 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50156546 (4-(2-((S)-2-((S)-5-cyclobutyl-3-hydroxypentyl)-5-o...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP3 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50156553 (4-(2-((R)-2-((S,E)-5-cyclobutyl-3-hydroxypent-1-en...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP3 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50156549 (4-(2-((S)-2-((S)-3-hydroxyoctyl)-5-oxopyrrolidin-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP3 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50156548 (5-(2-((R)-2-((S,E)-3-hydroxyoct-1-enyl)-5-oxopyrro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP3 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50156552 (4-(2-((R)-2-((S,E)-3-hydroxy-4-(3-(methoxymethyl)p...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP3 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50156547 (4-(2-((R)-2-((S)-3-hydroxyoct-1-enyl)-5-oxopyrroli...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity to human EP1 receptor | J Med Chem 47: 6124-7 (2004) Article DOI: 10.1021/jm049290a BindingDB Entry DOI: 10.7270/Q2P84BBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552303 (US11312727, Compound 123A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552356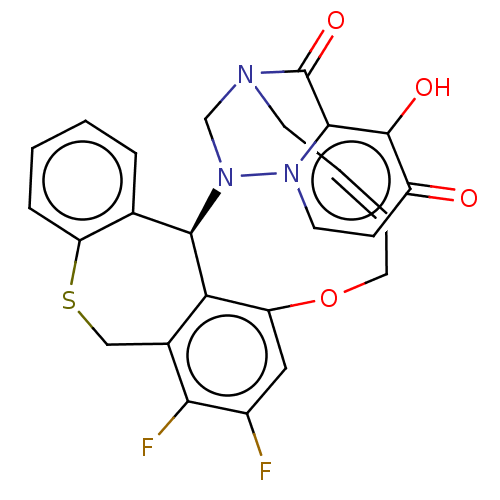 ((17a*R,*E)-24,25-difluoro-12-hydroxy-2,6,9,17a-tet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552337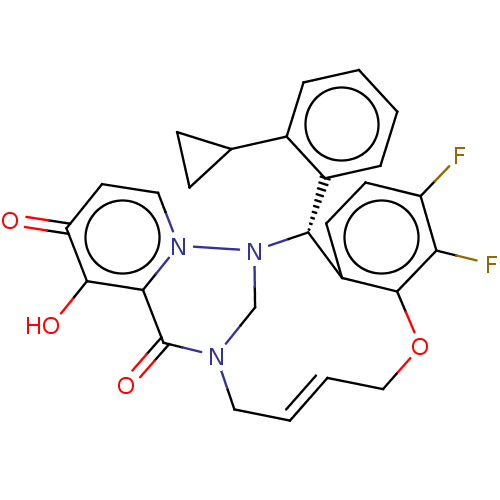 (US11312727, Compound 136A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300504 (CHEMBL574455 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552377 (US11312727, Compound 152BA) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552440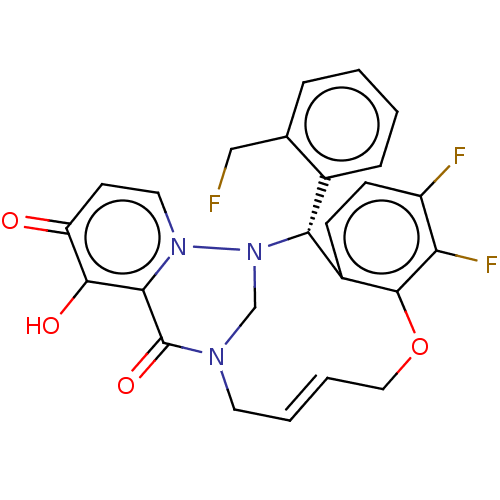 (US11312727, Compound 175A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552278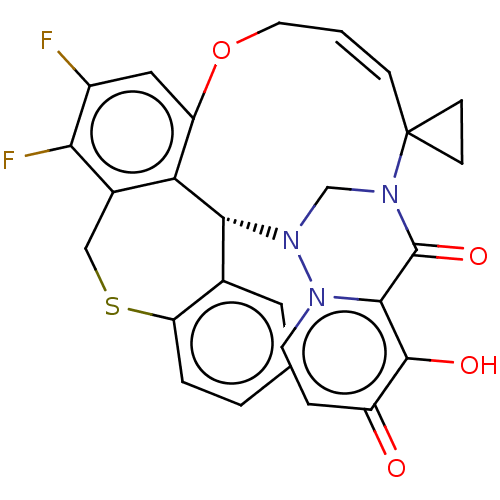 ((17a′*R,*E)-24′,25′-difluoro-12&...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552406 ((9*R,17a*R,*E)-24,25-difluoro-12-hydroxy-9-methyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552297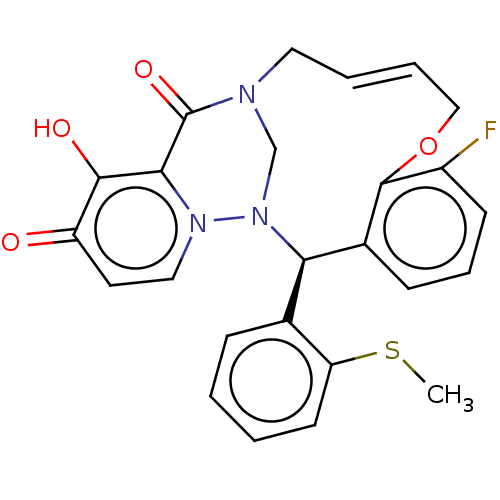 ((18*R,*Z)-4-fluoro-12-hydroxy-18-(2-(methylthio)ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301911 (CHEMBL583269 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552314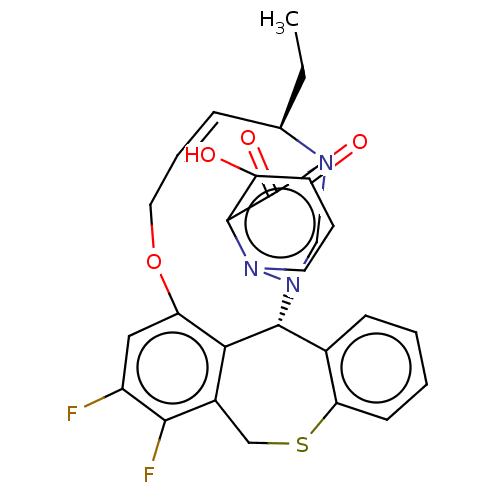 ((13*S,21a*R,Z)-13-ethyl-24,25-difluoro-16-hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552388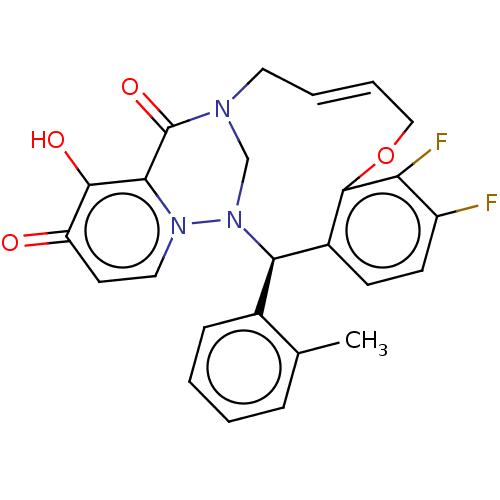 (US11312727, Compound 155A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552255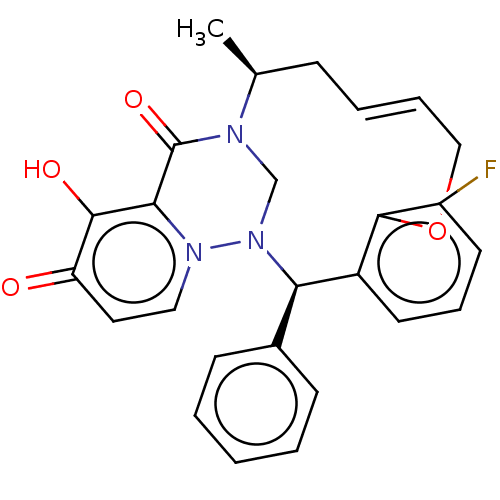 (US11312727, Compound 100AA) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300497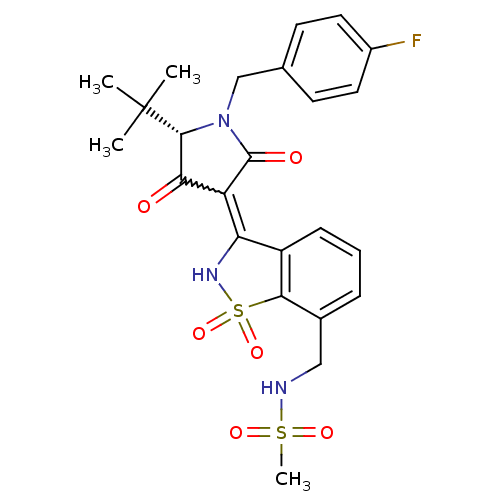 (CHEMBL578433 | N-({3-[(5S)-5-tert-butyl-1-(4-fluor...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50284330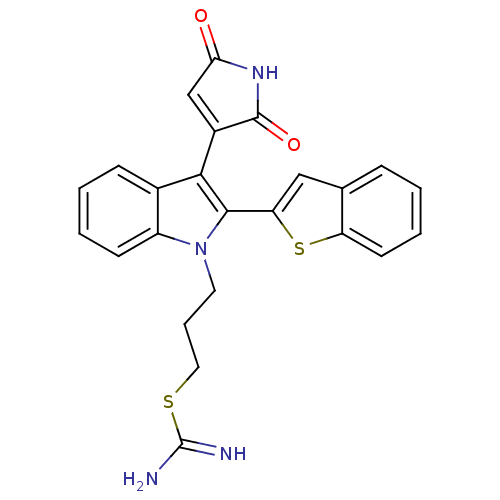 (2-{3-[2-Benzo[b]thiophen-2-yl-3-(2,5-dioxo-2,5-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of protein kinase C beta | Bioorg Med Chem Lett 5: 67-72 (1995) Article DOI: 10.1016/0960-894X(94)00460-W BindingDB Entry DOI: 10.7270/Q2ZG6S72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552147 (US11312727, Compound 46B) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552336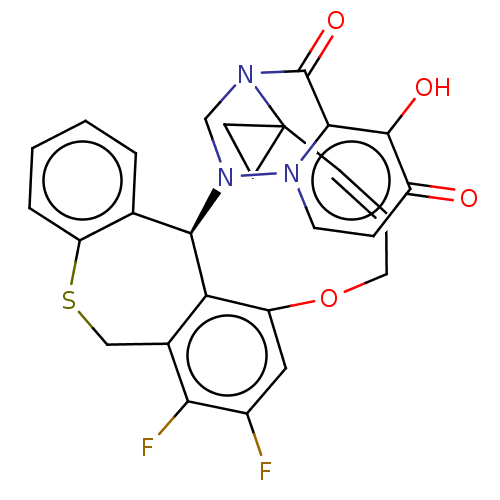 (US11312727, Compound 108D) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552344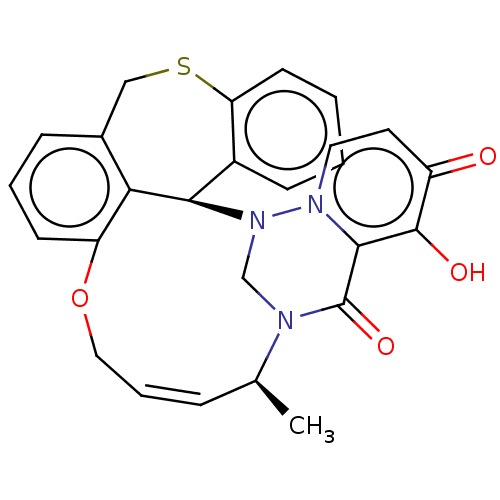 (US11312727, Compound 138D) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase basic protein 2 (Influenza A virus (strain A/WS/1933 H1N1)) | BDBM552412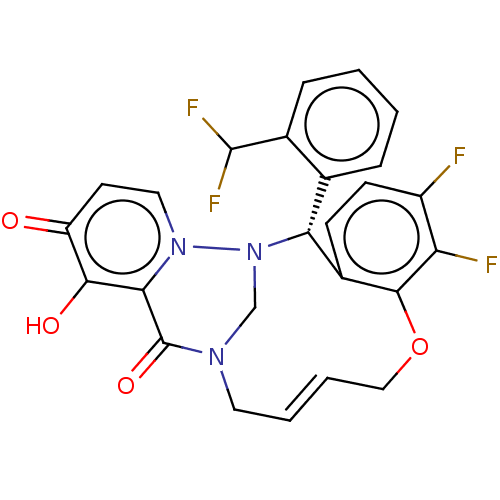 (US11312727, Compound 165A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description EN PA FRET inhibition assay was performed using a 19 nucleotide synthetic oligoribonucleotide substrate: 5′-FAM-AUUUUGUUUUUAAUAUUUC-BHQ-3′... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P272BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301902 (CHEMBL571825 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 825 total ) | Next | Last >> |