 Found 422 hits with Last Name = 'higa' and Initial = 't'
Found 422 hits with Last Name = 'higa' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4227
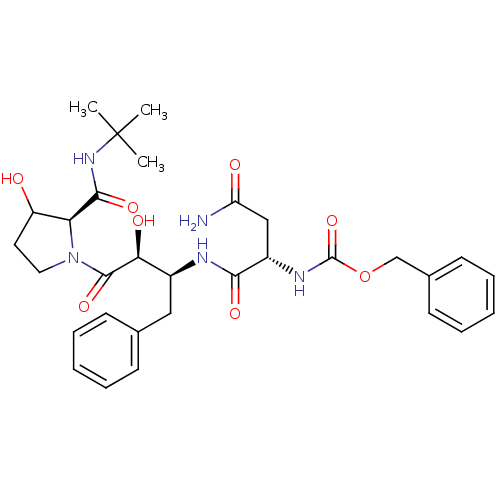
(AHPBA 35a | Z-Asn.(2S,3S)-AHPBA-[3(R)-hydroxy]Pro ...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C(O)CCN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C31H41N5O8/c1-31(2,3)35-28(41)25-23(37)14-15-36(25)29(42)26(39)21(16-19-10-6-4-7-11-19)33-27(40)22(17-24(32)38)34-30(43)44-18-20-12-8-5-9-13-20/h4-13,21-23,25-26,37,39H,14-18H2,1-3H3,(H2,32,38)(H,33,40)(H,34,43)(H,35,41)/t21-,22-,23?,25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4224

(AHPBA 32a | Z.Asn.( 2S,3S).AHPBA. [ 4( S)-morpholi...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CC(CN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)OCc1ccccc1)N1CCOCC1 |r| Show InChI InChI=1S/C35H48N6O8/c1-35(2,3)39-32(45)28-19-25(40-14-16-48-17-15-40)21-41(28)33(46)30(43)26(18-23-10-6-4-7-11-23)37-31(44)27(20-29(36)42)38-34(47)49-22-24-12-8-5-9-13-24/h4-13,25-28,30,43H,14-22H2,1-3H3,(H2,36,42)(H,37,44)(H,38,47)(H,39,45)/t25?,26-,27-,28-,30-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -53.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4216

(AHPBA 24a | Z.Asn-(2S,3S)-AHPBA-[4(S)-hydroxy]Pro ...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CC(O)CN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C31H41N5O8/c1-31(2,3)35-28(41)24-15-21(37)17-36(24)29(42)26(39)22(14-19-10-6-4-7-11-19)33-27(40)23(16-25(32)38)34-30(43)44-18-20-12-8-5-9-13-20/h4-13,21-24,26,37,39H,14-18H2,1-3H3,(H2,32,38)(H,33,40)(H,34,43)(H,35,41)/t21?,22-,23-,24-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -53.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50143890
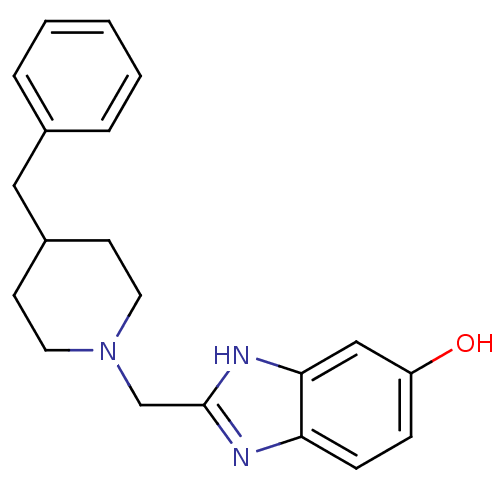
(2-(4-Benzyl-piperidin-1-ylmethyl)-3H-benzoimidazol...)Show InChI InChI=1S/C20H23N3O/c24-17-6-7-18-19(13-17)22-20(21-18)14-23-10-8-16(9-11-23)12-15-4-2-1-3-5-15/h1-7,13,16,24H,8-12,14H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamamatsu University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(E)-N1-(2-methoxybenzyl)cinnamamidine from human NR1a/NR2b receptor expressed in mouse Ltk cells |
Bioorg Med Chem 18: 7497-506 (2010)
Article DOI: 10.1016/j.bmc.2010.08.053
BindingDB Entry DOI: 10.7270/Q2MS3T06 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50143890

(2-(4-Benzyl-piperidin-1-ylmethyl)-3H-benzoimidazol...)Show InChI InChI=1S/C20H23N3O/c24-17-6-7-18-19(13-17)22-20(21-18)14-23-10-8-16(9-11-23)12-15-4-2-1-3-5-15/h1-7,13,16,24H,8-12,14H2,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamamatsu University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]ifenprodil from NR2B receptor in rat cortical synaptic membranes |
Bioorg Med Chem 18: 7497-506 (2010)
Article DOI: 10.1016/j.bmc.2010.08.053
BindingDB Entry DOI: 10.7270/Q2MS3T06 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589709

(CHEMBL5173504)Show SMILES O[C@H]1CCCC[C@@H]1N1CCN(CCCc2ccc(Br)cc2)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4236
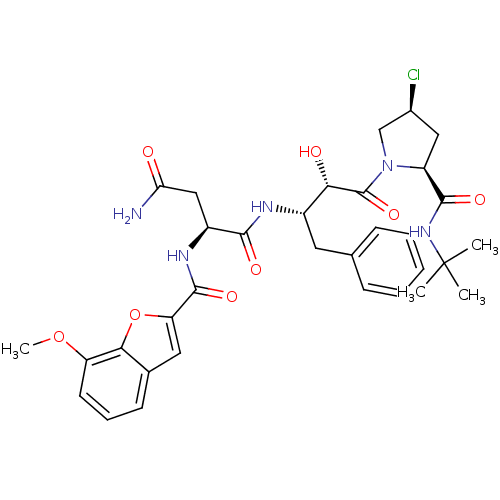
((2S)-N-[(2S,3S)-4-[(2S,4S)-2-(tert-butylcarbamoyl)...)Show SMILES COc1cccc2cc(oc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1C[C@@H](Cl)C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C33H40ClN5O8/c1-33(2,3)38-30(43)23-15-20(34)17-39(23)32(45)27(41)21(13-18-9-6-5-7-10-18)36-29(42)22(16-26(35)40)37-31(44)25-14-19-11-8-12-24(46-4)28(19)47-25/h5-12,14,20-23,27,41H,13,15-17H2,1-4H3,(H2,35,40)(H,36,42)(H,37,44)(H,38,43)/t20-,21-,22-,23-,27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | -49.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4230
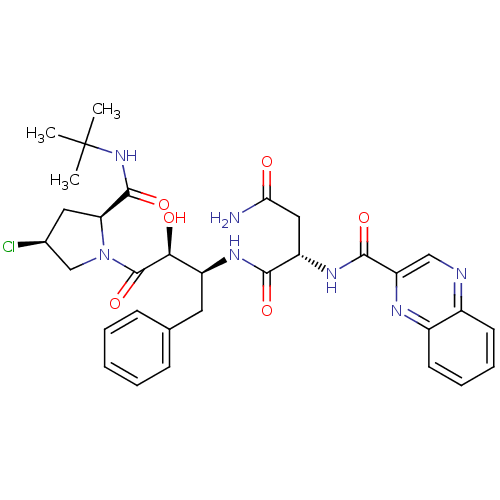
((2S)-N-[(2S,3S)-4-[(2S,4S)-2-(tert-butylcarbamoyl)...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@H](Cl)CN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1cnc2ccccc2n1 |r| Show InChI InChI=1S/C32H38ClN7O6/c1-32(2,3)39-30(45)25-14-19(33)17-40(25)31(46)27(42)22(13-18-9-5-4-6-10-18)37-28(43)23(15-26(34)41)38-29(44)24-16-35-20-11-7-8-12-21(20)36-24/h4-12,16,19,22-23,25,27,42H,13-15,17H2,1-3H3,(H2,34,41)(H,37,43)(H,38,44)(H,39,45)/t19-,22-,23-,25-,27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | -49.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589713

(CHEMBL5194064)Show SMILES O[C@H]1CCC[C@@H]1N1CCN(CCCc2cccc(Br)c2)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50329501
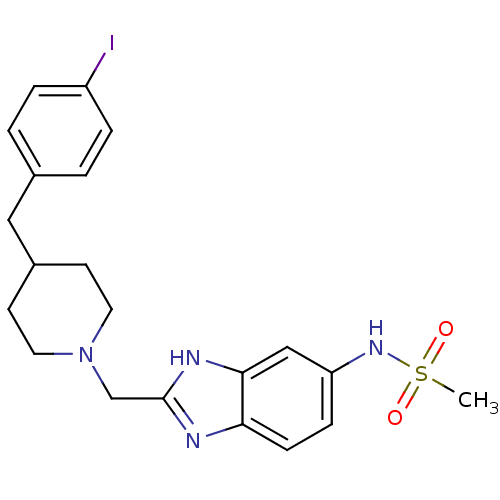
(CHEMBL1270332 | N-{2-[4-(4-Iodobenzyl)-piperidin-1...)Show SMILES CS(=O)(=O)Nc1ccc2nc(CN3CCC(Cc4ccc(I)cc4)CC3)[nH]c2c1 Show InChI InChI=1S/C21H25IN4O2S/c1-29(27,28)25-18-6-7-19-20(13-18)24-21(23-19)14-26-10-8-16(9-11-26)12-15-2-4-17(22)5-3-15/h2-7,13,16,25H,8-12,14H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamamatsu University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]ifenprodil from NR2B receptor in rat cortical synaptic membranes |
Bioorg Med Chem 18: 7497-506 (2010)
Article DOI: 10.1016/j.bmc.2010.08.053
BindingDB Entry DOI: 10.7270/Q2MS3T06 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50329502
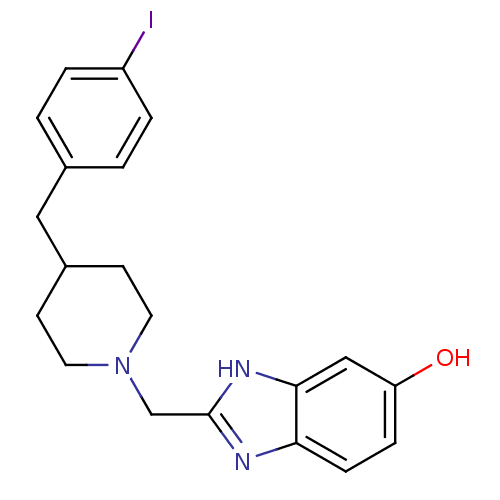
(2-{[4-(4-Iodobenzyl)piperidin-1-yl]methyl}benzimid...)Show InChI InChI=1S/C20H22IN3O/c21-16-3-1-14(2-4-16)11-15-7-9-24(10-8-15)13-20-22-18-6-5-17(25)12-19(18)23-20/h1-6,12,15,25H,7-11,13H2,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamamatsu University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]ifenprodil from NR2B receptor in rat cortical synaptic membranes |
Bioorg Med Chem 18: 7497-506 (2010)
Article DOI: 10.1016/j.bmc.2010.08.053
BindingDB Entry DOI: 10.7270/Q2MS3T06 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589707

(CHEMBL5171607)Show SMILES O[C@H]1CCCC[C@@H]1N1CCN(CCCc2cccc(Br)c2)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589716

(CHEMBL5173926)Show SMILES O[C@H]1CCC[C@@H]1N1CCN(CCc2cccc(I)c2)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4218
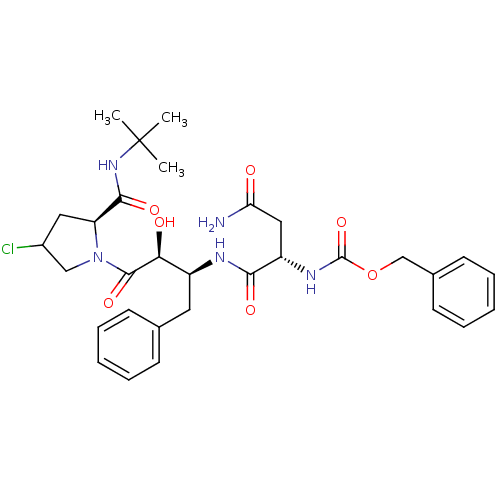
(AHPBA 26a | Z.Asn-(2S,3S).AHPBA.[4(S).chloro]Pro t...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CC(Cl)CN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C31H40ClN5O7/c1-31(2,3)36-28(41)24-15-21(32)17-37(24)29(42)26(39)22(14-19-10-6-4-7-11-19)34-27(40)23(16-25(33)38)35-30(43)44-18-20-12-8-5-9-13-20/h4-13,21-24,26,39H,14-18H2,1-3H3,(H2,33,38)(H,34,40)(H,35,43)(H,36,41)/t21?,22-,23-,24-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | -48.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50122787
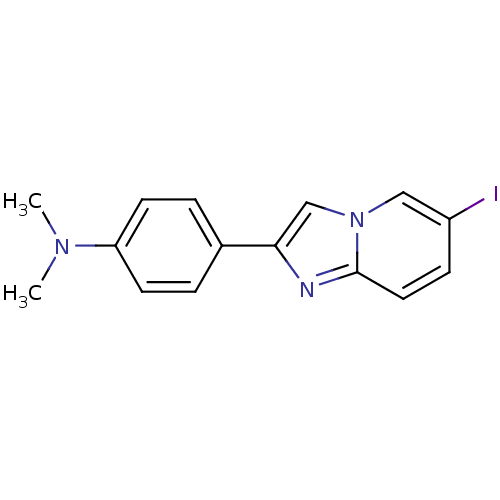
(2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyr...)Show InChI InChI=1S/C15H14IN3/c1-18(2)13-6-3-11(4-7-13)14-10-19-9-12(16)5-8-15(19)17-14/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting |
Eur J Med Chem 60: 469-78 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.020
BindingDB Entry DOI: 10.7270/Q2GF0XD0 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589715
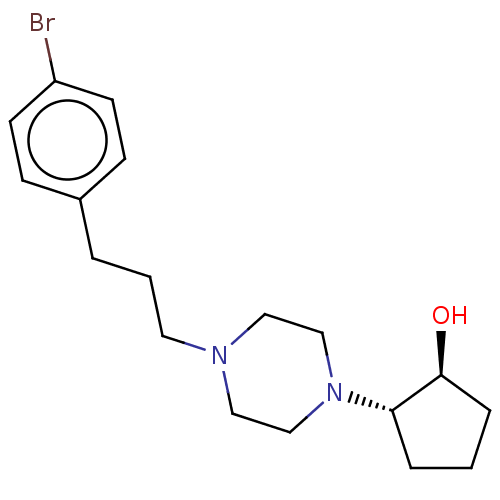
(CHEMBL5198312)Show SMILES O[C@H]1CCC[C@@H]1N1CCN(CCCc2ccc(Br)cc2)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50589713

(CHEMBL5194064)Show SMILES O[C@H]1CCC[C@@H]1N1CCN(CCCc2cccc(Br)c2)CC1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589708
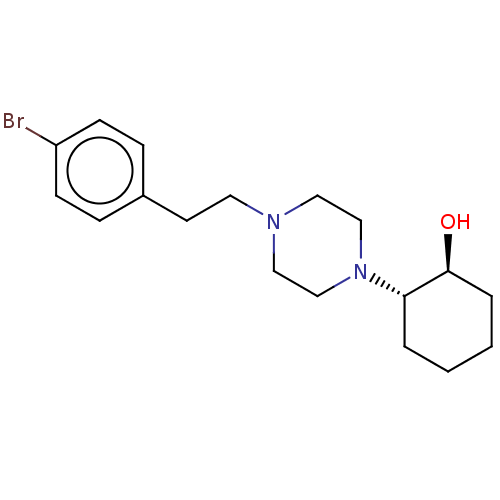
(CHEMBL5186452)Show SMILES O[C@H]1CCCC[C@@H]1N1CCN(CCc2ccc(Br)cc2)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589714

(CHEMBL5203770)Show SMILES O[C@H]1CCC[C@@H]1N1CCN(CCc2ccc(Br)cc2)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589704
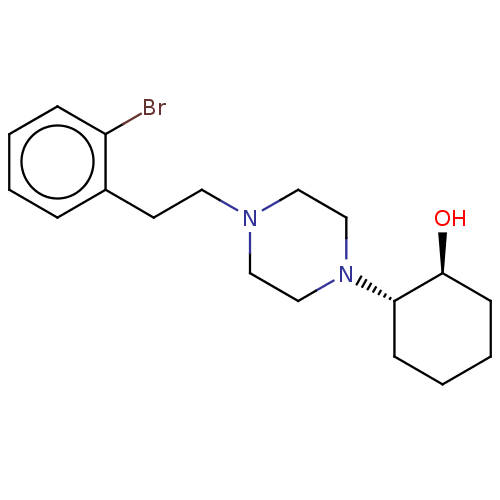
(CHEMBL5171303)Show SMILES O[C@H]1CCCC[C@@H]1N1CCN(CCc2ccccc2Br)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589712

(CHEMBL5196435)Show SMILES O[C@H]1CCC[C@@H]1N1CCN(CCc2cccc(Br)c2)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4225
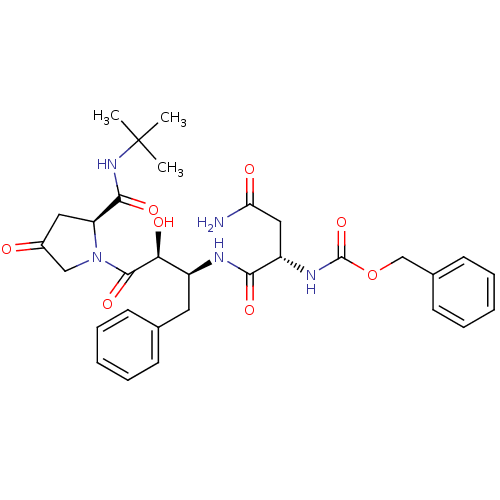
(AHPBA 33a | Z-Asn.(2S,3S)-AHPBA.(4.oxo)Pro tert-bu...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CC(=O)CN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C31H39N5O8/c1-31(2,3)35-28(41)24-15-21(37)17-36(24)29(42)26(39)22(14-19-10-6-4-7-11-19)33-27(40)23(16-25(32)38)34-30(43)44-18-20-12-8-5-9-13-20/h4-13,22-24,26,39H,14-18H2,1-3H3,(H2,32,38)(H,33,40)(H,34,43)(H,35,41)/t22-,23-,24-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.5 | -46.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4231
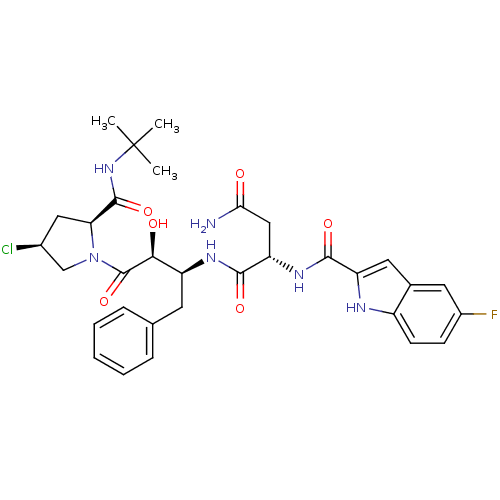
((2S)-N-[(2S,3S)-4-[(2S,4S)-2-(tert-butylcarbamoyl)...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@H](Cl)CN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1cc2cc(F)ccc2[nH]1 |r| Show InChI InChI=1S/C32H38ClFN6O6/c1-32(2,3)39-30(45)25-14-19(33)16-40(25)31(46)27(42)22(11-17-7-5-4-6-8-17)37-29(44)24(15-26(35)41)38-28(43)23-13-18-12-20(34)9-10-21(18)36-23/h4-10,12-13,19,22,24-25,27,36,42H,11,14-16H2,1-3H3,(H2,35,41)(H,37,44)(H,38,43)(H,39,45)/t19-,22-,24-,25-,27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.5 | -46.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50589715

(CHEMBL5198312)Show SMILES O[C@H]1CCC[C@@H]1N1CCN(CCCc2ccc(Br)cc2)CC1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50490496

(CHEMBL2326891)Show InChI InChI=1S/C15H13IN2O/c1-17-15-11-5-3-9(16)7-14(11)18-13-6-4-10(19-2)8-12(13)15/h3-8H,1-2H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting |
Eur J Med Chem 60: 469-78 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.020
BindingDB Entry DOI: 10.7270/Q2GF0XD0 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4229

(AHPBA 37a | Z-Asn.(2S,3S)-AHPBA.[3(S)-chloro]Pro t...)Show SMILES CC(C)(C)NC(=O)[C@@H]1[C@@H](Cl)CCN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C31H40ClN5O7/c1-31(2,3)36-28(41)25-21(32)14-15-37(25)29(42)26(39)22(16-19-10-6-4-7-11-19)34-27(40)23(17-24(33)38)35-30(43)44-18-20-12-8-5-9-13-20/h4-13,21-23,25-26,39H,14-18H2,1-3H3,(H2,33,38)(H,34,40)(H,35,43)(H,36,41)/t21-,22-,23-,25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.5 | -46.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589717
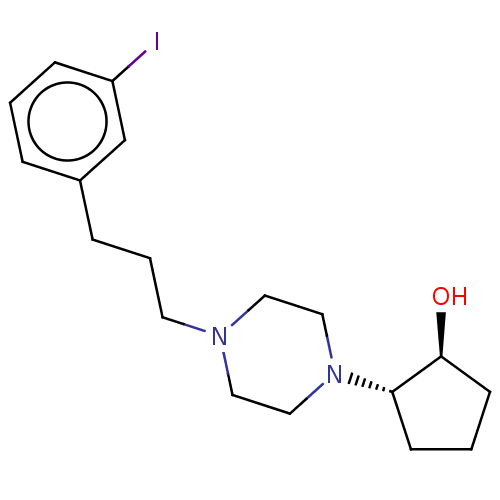
(CHEMBL5176294)Show SMILES O[C@H]1CCC[C@@H]1N1CCN(CCCc2cccc(I)c2)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4234

((1-Methylindazole-3-carbonyl)-Asn-(2S,3S)-AHPBA-[4...)Show SMILES Cn1nc(C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N2C[C@@H](Cl)C[C@H]2C(=O)NC(C)(C)C)c2ccccc12 |r| Show InChI InChI=1S/C32H40ClN7O6/c1-32(2,3)37-29(44)24-15-19(33)17-40(24)31(46)27(42)21(14-18-10-6-5-7-11-18)35-28(43)22(16-25(34)41)36-30(45)26-20-12-8-9-13-23(20)39(4)38-26/h5-13,19,21-22,24,27,42H,14-17H2,1-4H3,(H2,34,41)(H,35,43)(H,36,45)(H,37,44)/t19-,21-,22-,24-,27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | -46.3 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589706

(CHEMBL5172461)Show SMILES O[C@H]1CCCC[C@@H]1N1CCN(CCc2cccc(Br)c2)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50589717

(CHEMBL5176294)Show SMILES O[C@H]1CCC[C@@H]1N1CCN(CCCc2cccc(I)c2)CC1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4221
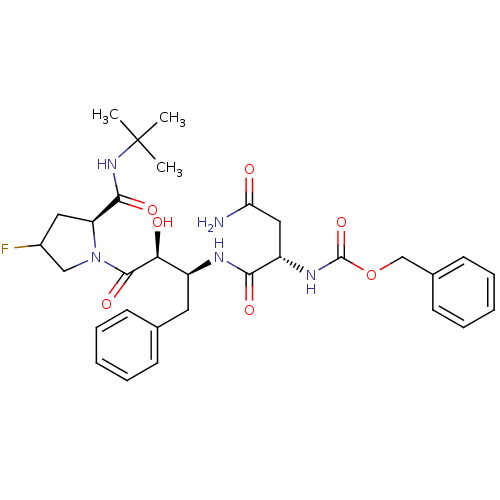
(AHPBA 29a | Z-Asn-(2S,3S)-AHPBA- [4(S)-fluoro] Pro...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CC(F)CN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C31H40FN5O7/c1-31(2,3)36-28(41)24-15-21(32)17-37(24)29(42)26(39)22(14-19-10-6-4-7-11-19)34-27(40)23(16-25(33)38)35-30(43)44-18-20-12-8-5-9-13-20/h4-13,21-24,26,39H,14-18H2,1-3H3,(H2,33,38)(H,34,40)(H,35,43)(H,36,41)/t21?,22-,23-,24-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | -46.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589705
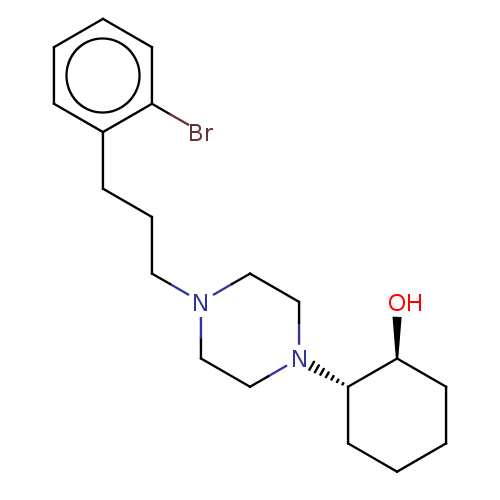
(CHEMBL5191856)Show SMILES O[C@H]1CCCC[C@@H]1N1CCN(CCCc2ccccc2Br)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4233

((1-Methylindole- 3 -carbonyl)- Asn - (2S,3S)-AHPBA...)Show SMILES Cn1cc(C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N2C[C@@H](Cl)C[C@H]2C(=O)NC(C)(C)C)c2ccccc12 |r| Show InChI InChI=1S/C33H41ClN6O6/c1-33(2,3)38-31(45)26-15-20(34)17-40(26)32(46)28(42)23(14-19-10-6-5-7-11-19)36-30(44)24(16-27(35)41)37-29(43)22-18-39(4)25-13-9-8-12-21(22)25/h5-13,18,20,23-24,26,28,42H,14-17H2,1-4H3,(H2,35,41)(H,36,44)(H,37,43)(H,38,45)/t20-,23-,24-,26-,28-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50007674

((+)-erythro 4-[2-(4-Benzyl-piperidin-1-yl)-1-hydro...)Show SMILES C[C@@H]([C@H](O)c1ccc(O)cc1)N1CCC(Cc2ccccc2)CC1 Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3/t16-,21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamamatsu University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]ifenprodil from NR2B receptor in rat cortical synaptic membranes |
Bioorg Med Chem 18: 7497-506 (2010)
Article DOI: 10.1016/j.bmc.2010.08.053
BindingDB Entry DOI: 10.7270/Q2MS3T06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4232
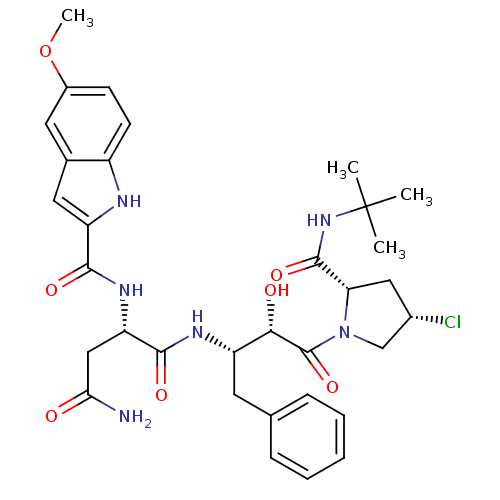
((2S)-N-[(2S,3S)-4-[(2S,4S)-2-(tert-butylcarbamoyl)...)Show SMILES COc1ccc2[nH]c(cc2c1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1C[C@@H](Cl)C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C33H41ClN6O7/c1-33(2,3)39-31(45)26-15-20(34)17-40(26)32(46)28(42)23(12-18-8-6-5-7-9-18)37-30(44)25(16-27(35)41)38-29(43)24-14-19-13-21(47-4)10-11-22(19)36-24/h5-11,13-14,20,23,25-26,28,36,42H,12,15-17H2,1-4H3,(H2,35,41)(H,37,44)(H,38,43)(H,39,45)/t20-,23-,25-,26-,28-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21.5 | -45.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50589707

(CHEMBL5171607)Show SMILES O[C@H]1CCCC[C@@H]1N1CCN(CCCc2cccc(Br)c2)CC1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589710

(CHEMBL5195262)Show SMILES O[C@H]1CCC[C@@H]1N1CCN(CCc2ccccc2Br)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4220
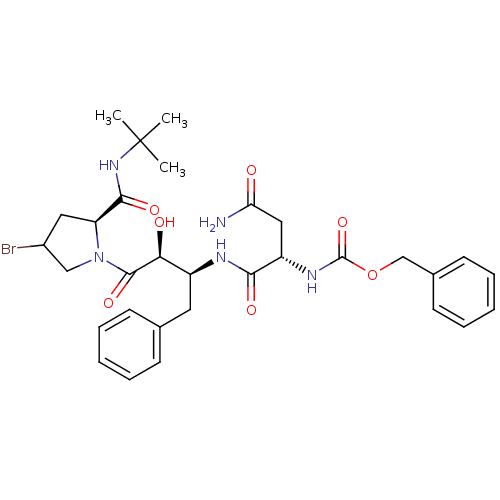
(AHPBA 28a | Z-Asn-(2S,3S)-AHPBA.[4(S)-bromo]Pro te...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CC(Br)CN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C31H40BrN5O7/c1-31(2,3)36-28(41)24-15-21(32)17-37(24)29(42)26(39)22(14-19-10-6-4-7-11-19)34-27(40)23(16-25(33)38)35-30(43)44-18-20-12-8-5-9-13-20/h4-13,21-24,26,39H,14-18H2,1-3H3,(H2,33,38)(H,34,40)(H,35,43)(H,36,41)/t21?,22-,23-,24-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22.5 | -45.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50589706

(CHEMBL5172461)Show SMILES O[C@H]1CCCC[C@@H]1N1CCN(CCc2cccc(Br)c2)CC1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50589709

(CHEMBL5173504)Show SMILES O[C@H]1CCCC[C@@H]1N1CCN(CCCc2ccc(Br)cc2)CC1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
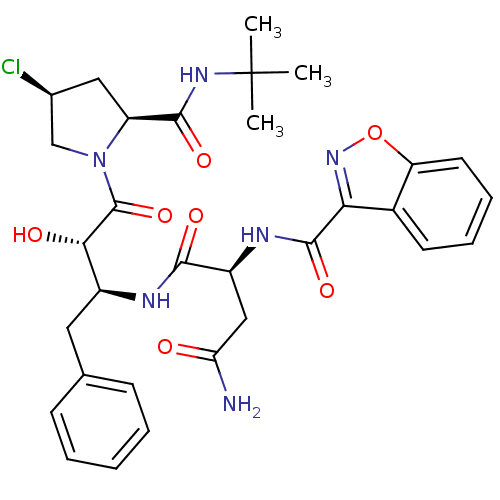
(Human immunodeficiency virus type 1) | BDBM4235
((2S)-2-(1,2-benzoxazol-3-ylformamido)-N-[(2S,3S)-4...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@H](Cl)CN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1noc2ccccc12 |r| Show InChI InChI=1S/C31H37ClN6O7/c1-31(2,3)36-28(42)22-14-18(32)16-38(22)30(44)26(40)20(13-17-9-5-4-6-10-17)34-27(41)21(15-24(33)39)35-29(43)25-19-11-7-8-12-23(19)45-37-25/h4-12,18,20-22,26,40H,13-16H2,1-3H3,(H2,33,39)(H,34,41)(H,35,43)(H,36,42)/t18-,20-,21-,22-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | -44.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50490499
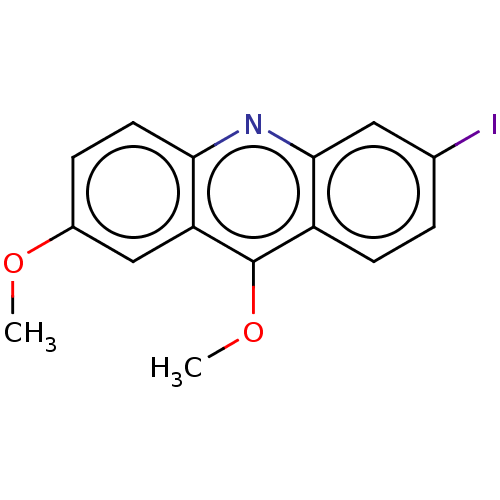
(CHEMBL2326889)Show InChI InChI=1S/C15H12INO2/c1-18-10-4-6-13-12(8-10)15(19-2)11-5-3-9(16)7-14(11)17-13/h3-8H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting |
Eur J Med Chem 60: 469-78 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.020
BindingDB Entry DOI: 10.7270/Q2GF0XD0 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4228
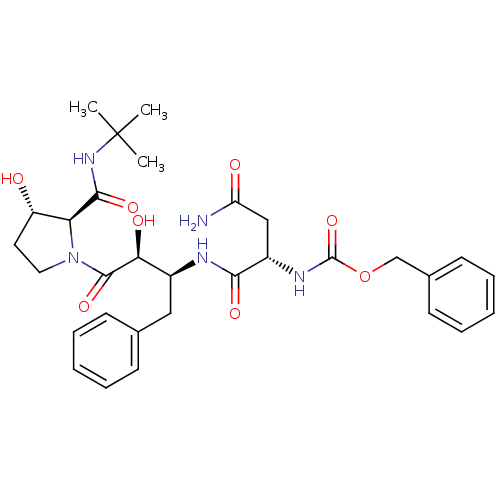
(AHPBA 36a | Z.Asn-(2S,3S).AHPBA.[3(S)-hydroxy]Pro ...)Show SMILES CC(C)(C)NC(=O)[C@@H]1[C@@H](O)CCN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C31H41N5O8/c1-31(2,3)35-28(41)25-23(37)14-15-36(25)29(42)26(39)21(16-19-10-6-4-7-11-19)33-27(40)22(17-24(32)38)34-30(43)44-18-20-12-8-5-9-13-20/h4-13,21-23,25-26,37,39H,14-18H2,1-3H3,(H2,32,38)(H,33,40)(H,34,43)(H,35,41)/t21-,22-,23-,25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | -44.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50329503

(2-{[4-(4-Iodophenoxy)piperidin-1-yl]methyl}benzimi...)Show InChI InChI=1S/C19H20IN3O2/c20-13-1-4-15(5-2-13)25-16-7-9-23(10-8-16)12-19-21-17-6-3-14(24)11-18(17)22-19/h1-6,11,16,24H,7-10,12H2,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 32.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamamatsu University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]ifenprodil from NR2B receptor in rat cortical synaptic membranes |
Bioorg Med Chem 18: 7497-506 (2010)
Article DOI: 10.1016/j.bmc.2010.08.053
BindingDB Entry DOI: 10.7270/Q2MS3T06 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50589705

(CHEMBL5191856)Show SMILES O[C@H]1CCCC[C@@H]1N1CCN(CCCc2ccccc2Br)CC1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM4222

(AHPBA 30a | Z.Asn-(2S,3S)-AHPBA.(4,4-difluoro)Pro ...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CC(F)(F)CN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C31H39F2N5O7/c1-30(2,3)37-27(42)23-16-31(32,33)18-38(23)28(43)25(40)21(14-19-10-6-4-7-11-19)35-26(41)22(15-24(34)39)36-29(44)45-17-20-12-8-5-9-13-20/h4-13,21-23,25,40H,14-18H2,1-3H3,(H2,34,39)(H,35,41)(H,36,44)(H,37,42)/t21-,22-,23-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | -44.3 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd.
| Assay Description
The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... |
Bioorg Med Chem 4: 1365-77 (1996)
Article DOI: 10.1016/0968-0896(96)00130-7
BindingDB Entry DOI: 10.7270/Q2V9868K |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50589711

(CHEMBL5183643)Show SMILES O[C@H]1CCC[C@@H]1N1CCN(CCCc2ccccc2Br)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50490493
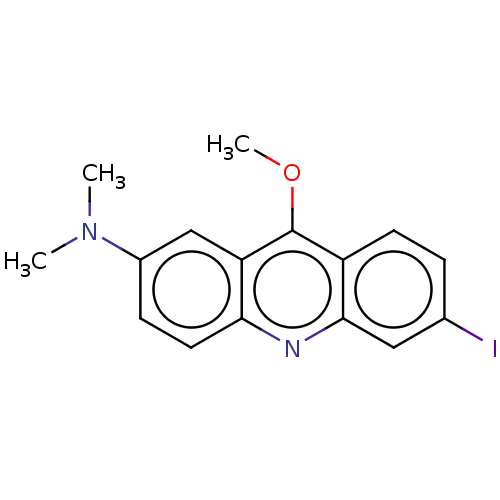
(CHEMBL2326888)Show InChI InChI=1S/C16H15IN2O/c1-19(2)11-5-7-14-13(9-11)16(20-3)12-6-4-10(17)8-15(12)18-14/h4-9H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting |
Eur J Med Chem 60: 469-78 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.020
BindingDB Entry DOI: 10.7270/Q2GF0XD0 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50589704

(CHEMBL5171303)Show SMILES O[C@H]1CCCC[C@@H]1N1CCN(CCc2ccccc2Br)CC1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00099g
BindingDB Entry DOI: 10.7270/Q22B930B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data