 Found 56 hits with Last Name = 'hilgendorf' and Initial = 'c'
Found 56 hits with Last Name = 'hilgendorf' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasminogen
(Homo sapiens (Human)) | BDBM50020035

(CHEMBL3287849)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](Cc2ccccc2)C1 |r| Show InChI InChI=1S/C15H18N2O2/c18-15-10-14(19-17-15)12-6-7-16-13(9-12)8-11-4-2-1-3-5-11/h1-5,10,12-13,16H,6-9H2,(H,17,18)/t12-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasminogen
(Homo sapiens (Human)) | BDBM50020042
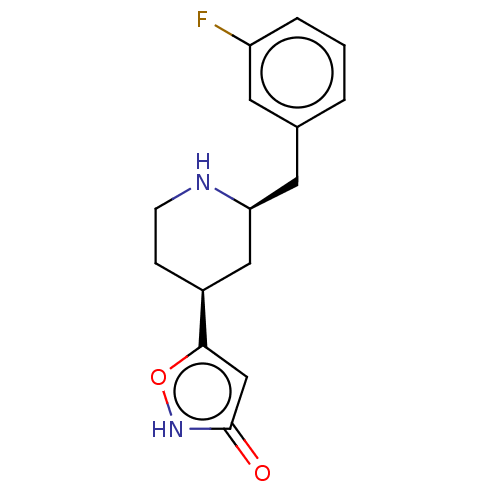
(CHEMBL3287856)Show SMILES Fc1cccc(C[C@H]2C[C@H](CCN2)c2cc(=O)[nH]o2)c1 |r| Show InChI InChI=1S/C15H17FN2O2/c16-12-3-1-2-10(6-12)7-13-8-11(4-5-17-13)14-9-15(19)18-20-14/h1-3,6,9,11,13,17H,4-5,7-8H2,(H,18,19)/t11-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020037
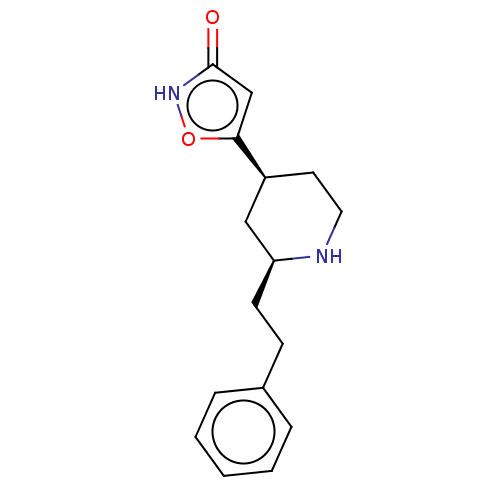
(CHEMBL3287854)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](CCc2ccccc2)C1 |r| Show InChI InChI=1S/C16H20N2O2/c19-16-11-15(20-18-16)13-8-9-17-14(10-13)7-6-12-4-2-1-3-5-12/h1-5,11,13-14,17H,6-10H2,(H,18,19)/t13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020036
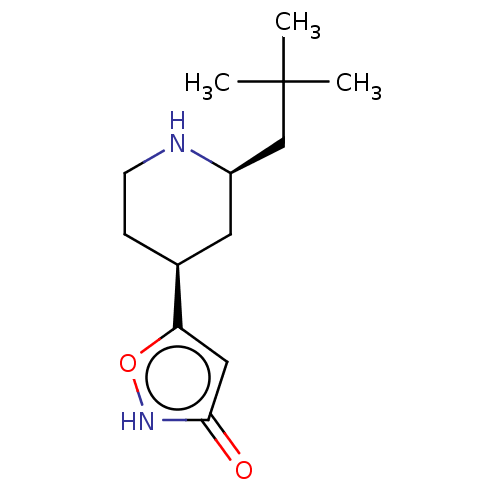
(CHEMBL3287851)Show SMILES CC(C)(C)C[C@H]1C[C@H](CCN1)c1cc(=O)[nH]o1 |r| Show InChI InChI=1S/C13H22N2O2/c1-13(2,3)8-10-6-9(4-5-14-10)11-7-12(16)15-17-11/h7,9-10,14H,4-6,8H2,1-3H3,(H,15,16)/t9-,10+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020036

(CHEMBL3287851)Show SMILES CC(C)(C)C[C@H]1C[C@H](CCN1)c1cc(=O)[nH]o1 |r| Show InChI InChI=1S/C13H22N2O2/c1-13(2,3)8-10-6-9(4-5-14-10)11-7-12(16)15-17-11/h7,9-10,14H,4-6,8H2,1-3H3,(H,15,16)/t9-,10+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020043
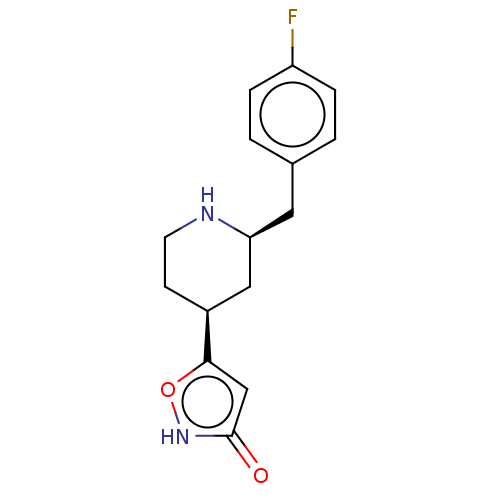
(CHEMBL3287857)Show SMILES Fc1ccc(C[C@H]2C[C@H](CCN2)c2cc(=O)[nH]o2)cc1 |r| Show InChI InChI=1S/C15H17FN2O2/c16-12-3-1-10(2-4-12)7-13-8-11(5-6-17-13)14-9-15(19)18-20-14/h1-4,9,11,13,17H,5-8H2,(H,18,19)/t11-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020033
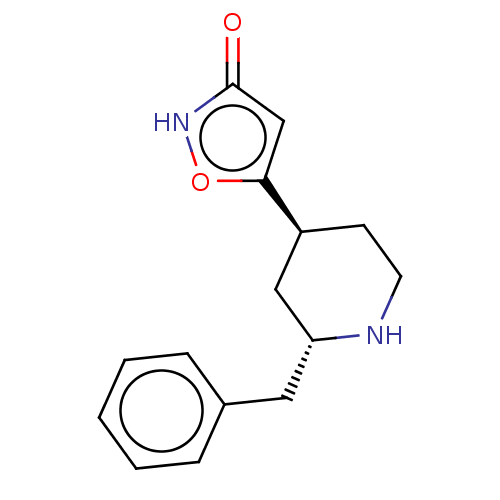
(CHEMBL3287847)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@H](Cc2ccccc2)C1 |r| Show InChI InChI=1S/C15H18N2O2/c18-15-10-14(19-17-15)12-6-7-16-13(9-12)8-11-4-2-1-3-5-11/h1-5,10,12-13,16H,6-9H2,(H,17,18)/t12-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020039
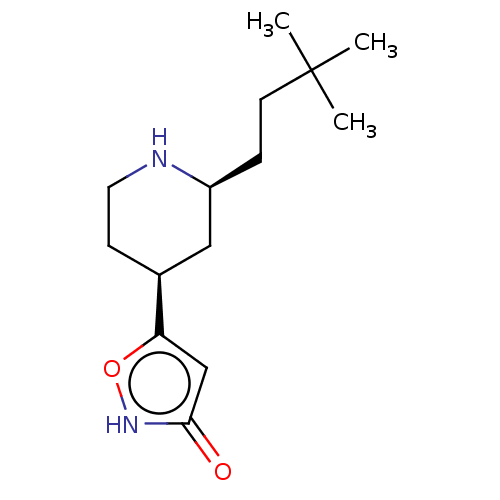
(CHEMBL3287852)Show SMILES CC(C)(C)CC[C@H]1C[C@H](CCN1)c1cc(=O)[nH]o1 |r| Show InChI InChI=1S/C14H24N2O2/c1-14(2,3)6-4-11-8-10(5-7-15-11)12-9-13(17)16-18-12/h9-11,15H,4-8H2,1-3H3,(H,16,17)/t10-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50032954
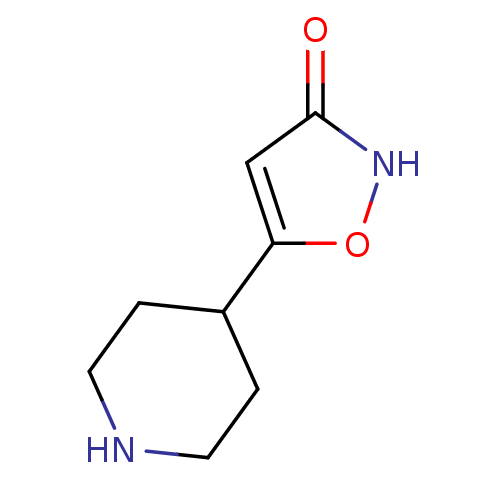
(5-(piperidin-4-yl)isoxazol-3-ol | 5-Piperidin-4-yl...)Show InChI InChI=1S/C8H12N2O2/c11-8-5-7(12-10-8)6-1-3-9-4-2-6/h5-6,9H,1-4H2,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020035

(CHEMBL3287849)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](Cc2ccccc2)C1 |r| Show InChI InChI=1S/C15H18N2O2/c18-15-10-14(19-17-15)12-6-7-16-13(9-12)8-11-4-2-1-3-5-11/h1-5,10,12-13,16H,6-9H2,(H,17,18)/t12-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Glu-plasminogen in buffer assessed as inhibition of interaction with fibrin preincubated for 15 mins followed b... |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasminogen
(Homo sapiens (Human)) | BDBM50020038
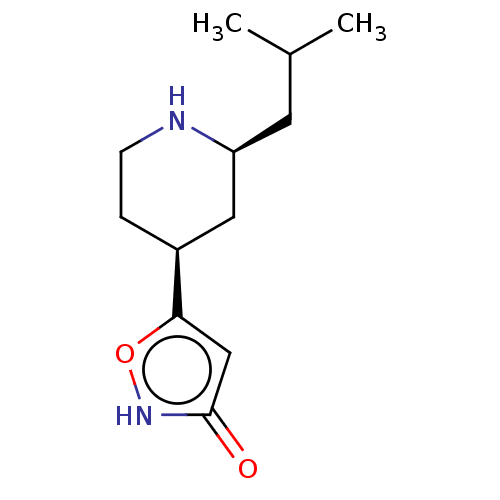
(CHEMBL3287850)Show InChI InChI=1S/C12H20N2O2/c1-8(2)5-10-6-9(3-4-13-10)11-7-12(15)14-16-11/h7-10,13H,3-6H2,1-2H3,(H,14,15)/t9-,10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020040

(CHEMBL3287853)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](CC2CCCCC2)C1 |r| Show InChI InChI=1S/C15H24N2O2/c18-15-10-14(19-17-15)12-6-7-16-13(9-12)8-11-4-2-1-3-5-11/h10-13,16H,1-9H2,(H,17,18)/t12-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020041
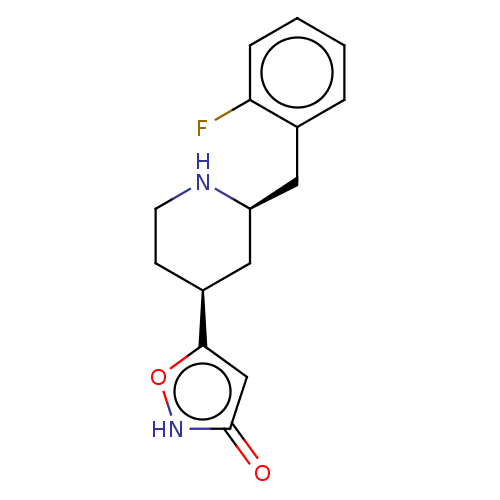
(CHEMBL3287855)Show SMILES Fc1ccccc1C[C@H]1C[C@H](CCN1)c1cc(=O)[nH]o1 |r| Show InChI InChI=1S/C15H17FN2O2/c16-13-4-2-1-3-10(13)7-12-8-11(5-6-17-12)14-9-15(19)18-20-14/h1-4,9,11-12,17H,5-8H2,(H,18,19)/t11-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020029
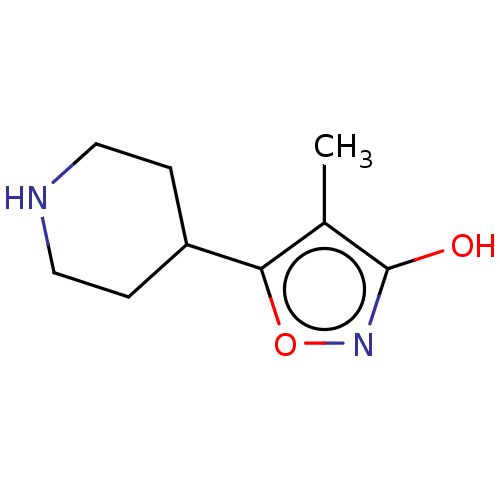
(CHEMBL144416)Show InChI InChI=1S/C9H14N2O2/c1-6-8(13-11-9(6)12)7-2-4-10-5-3-7/h7,10H,2-5H2,1H3,(H,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020031
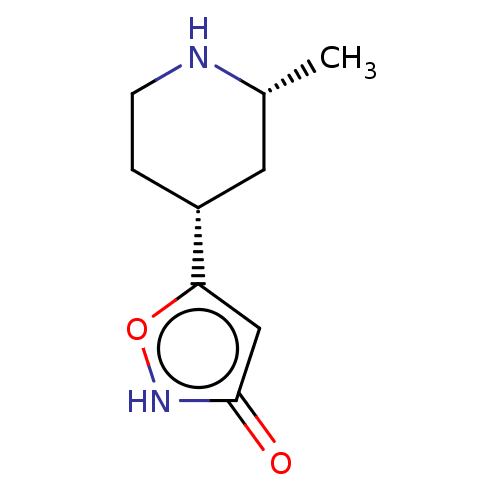
(CHEMBL3287844)Show InChI InChI=1S/C9H14N2O2/c1-6-4-7(2-3-10-6)8-5-9(12)11-13-8/h5-7,10H,2-4H2,1H3,(H,11,12)/t6-,7-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50032954

(5-(piperidin-4-yl)isoxazol-3-ol | 5-Piperidin-4-yl...)Show InChI InChI=1S/C8H12N2O2/c11-8-5-7(12-10-8)6-1-3-9-4-2-6/h5-6,9H,1-4H2,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Glu-plasminogen in buffer assessed as inhibition of interaction with fibrin preincubated for 15 mins followed b... |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020033

(CHEMBL3287847)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@H](Cc2ccccc2)C1 |r| Show InChI InChI=1S/C15H18N2O2/c18-15-10-14(19-17-15)12-6-7-16-13(9-12)8-11-4-2-1-3-5-11/h1-5,10,12-13,16H,6-9H2,(H,17,18)/t12-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Glu-plasminogen in buffer assessed as inhibition of interaction with fibrin preincubated for 15 mins followed b... |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50428067

(CL-65336 | Cyklokapron | Lysteda | TRANEXAMIC ACID...)Show SMILES NC[C@H]1CC[C@@H](CC1)C(O)=O |r,wU:5.8,wD:2.1,(1.2,4.21,;-.14,3.44,;-.14,1.9,;-1.47,1.13,;-1.47,-.41,;-.14,-1.18,;1.2,-.41,;1.2,1.13,;-.14,-2.72,;-1.47,-3.49,;1.2,-3.49,)| Show InChI InChI=1S/C8H15NO2/c9-5-6-1-3-7(4-2-6)8(10)11/h6-7H,1-5,9H2,(H,10,11)/t6-,7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasminogen
(Homo sapiens (Human)) | BDBM50020029

(CHEMBL144416)Show InChI InChI=1S/C9H14N2O2/c1-6-8(13-11-9(6)12)7-2-4-10-5-3-7/h7,10H,2-5H2,1H3,(H,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Glu-plasminogen in buffer assessed as inhibition of interaction with fibrin preincubated for 15 mins followed b... |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020031

(CHEMBL3287844)Show InChI InChI=1S/C9H14N2O2/c1-6-4-7(2-3-10-6)8-5-9(12)11-13-8/h5-7,10H,2-4H2,1H3,(H,11,12)/t6-,7-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Glu-plasminogen in buffer assessed as inhibition of interaction with fibrin preincubated for 15 mins followed b... |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50428067

(CL-65336 | Cyklokapron | Lysteda | TRANEXAMIC ACID...)Show SMILES NC[C@H]1CC[C@@H](CC1)C(O)=O |r,wU:5.8,wD:2.1,(1.2,4.21,;-.14,3.44,;-.14,1.9,;-1.47,1.13,;-1.47,-.41,;-.14,-1.18,;1.2,-.41,;1.2,1.13,;-.14,-2.72,;-1.47,-3.49,;1.2,-3.49,)| Show InChI InChI=1S/C8H15NO2/c9-5-6-1-3-7(4-2-6)8(10)11/h6-7H,1-5,9H2,(H,10,11)/t6-,7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Glu-plasminogen in buffer assessed as inhibition of interaction with fibrin preincubated for 15 mins followed b... |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasminogen
(Homo sapiens (Human)) | BDBM50032956

(3-Piperidin-4-yl-isoxazol-5-ol | CHEMBL110210)Show InChI InChI=1S/C8H12N2O2/c11-8-5-7(10-12-8)6-1-3-9-4-2-6/h5-6,9-10H,1-4H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Glu-plasminogen in buffer assessed as inhibition of interaction with fibrin preincubated for 15 mins followed b... |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020028
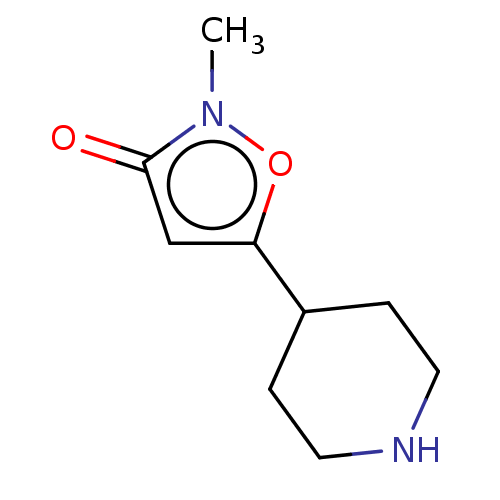
(CHEMBL3287843)Show InChI InChI=1S/C9H14N2O2/c1-11-9(12)6-8(13-11)7-2-4-10-5-3-7/h6-7,10H,2-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Glu-plasminogen in buffer assessed as inhibition of interaction with fibrin preincubated for 15 mins followed b... |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50357211
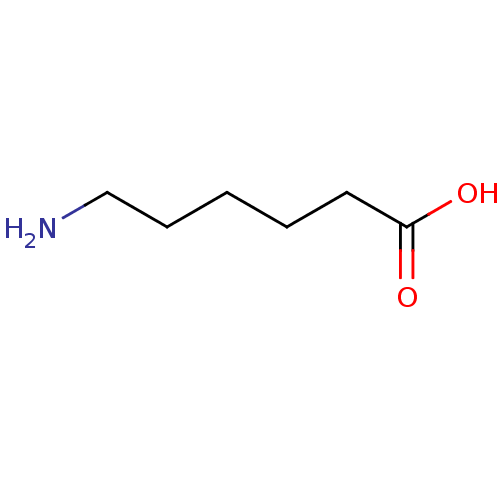
(177 J.D. | AMINOCAPROIC ACID | Amicar | Aminocapro...)Show InChI InChI=1S/C6H13NO2/c7-5-3-1-2-4-6(8)9/h1-5,7H2,(H,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasminogen
(Homo sapiens (Human)) | BDBM50020028

(CHEMBL3287843)Show InChI InChI=1S/C9H14N2O2/c1-11-9(12)6-8(13-11)7-2-4-10-5-3-7/h6-7,10H,2-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020027
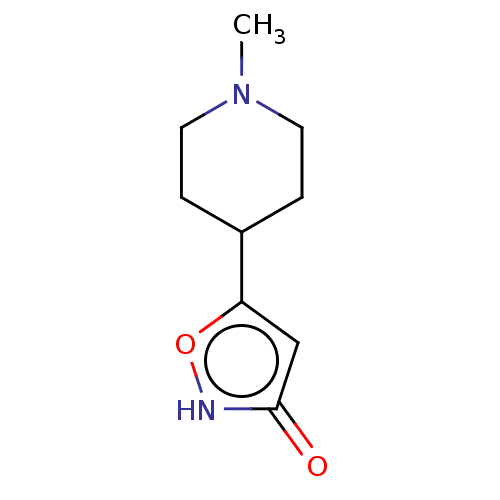
(CHEMBL3287842)Show InChI InChI=1S/C9H14N2O2/c1-11-4-2-7(3-5-11)8-6-9(12)10-13-8/h6-7H,2-5H2,1H3,(H,10,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to full length Glu-plasminogen in human plasma assessed as inhibition of interaction with fibrin in presence of tPA |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020027

(CHEMBL3287842)Show InChI InChI=1S/C9H14N2O2/c1-11-4-2-7(3-5-11)8-6-9(12)10-13-8/h6-7H,2-5H2,1H3,(H,10,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Glu-plasminogen in buffer assessed as inhibition of interaction with fibrin preincubated for 15 mins followed b... |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50357211

(177 J.D. | AMINOCAPROIC ACID | Amicar | Aminocapro...)Show InChI InChI=1S/C6H13NO2/c7-5-3-1-2-4-6(8)9/h1-5,7H2,(H,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Glu-plasminogen in buffer assessed as inhibition of interaction with fibrin preincubated for 15 mins followed b... |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasminogen
(Homo sapiens (Human)) | BDBM50020030
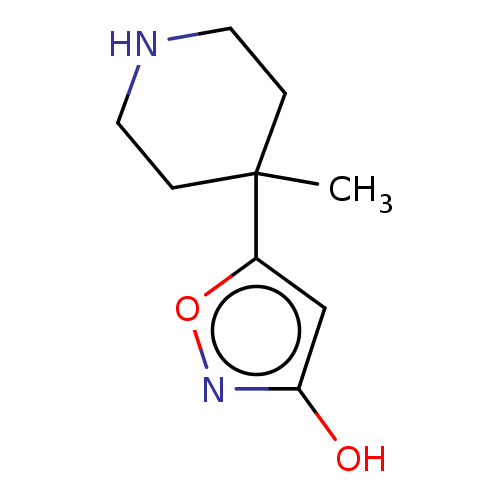
(CHEMBL1192583)Show InChI InChI=1S/C9H14N2O2/c1-9(2-4-10-5-3-9)7-6-8(12)11-13-7/h6,10H,2-5H2,1H3,(H,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Glu-plasminogen in buffer assessed as inhibition of interaction with fibrin preincubated for 15 mins followed b... |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020032
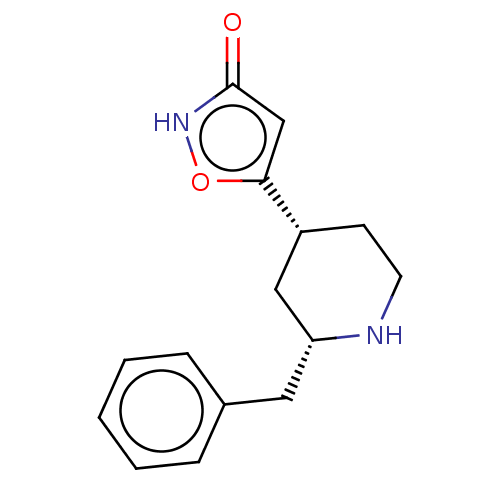
(CHEMBL3287846)Show SMILES O=c1cc(o[nH]1)[C@@H]1CCN[C@H](Cc2ccccc2)C1 |r| Show InChI InChI=1S/C15H18N2O2/c18-15-10-14(19-17-15)12-6-7-16-13(9-12)8-11-4-2-1-3-5-11/h1-5,10,12-13,16H,6-9H2,(H,17,18)/t12-,13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Glu-plasminogen in buffer assessed as inhibition of interaction with fibrin preincubated for 15 mins followed b... |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50020034
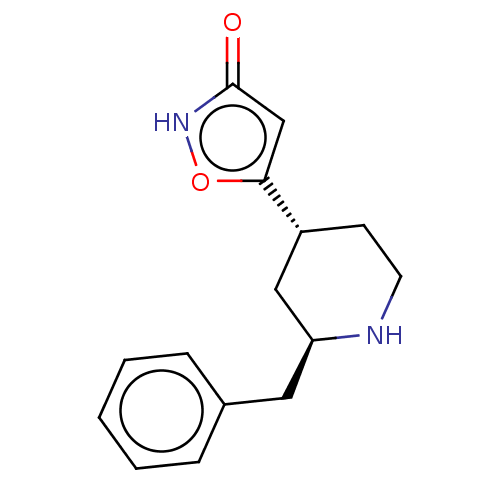
(CHEMBL3287848)Show SMILES O=c1cc(o[nH]1)[C@@H]1CCN[C@@H](Cc2ccccc2)C1 |r| Show InChI InChI=1S/C15H18N2O2/c18-15-10-14(19-17-15)12-6-7-16-13(9-12)8-11-4-2-1-3-5-11/h1-5,10,12-13,16H,6-9H2,(H,17,18)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.69E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Glu-plasminogen in buffer assessed as inhibition of interaction with fibrin preincubated for 15 mins followed b... |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020036

(CHEMBL3287851)Show SMILES CC(C)(C)C[C@H]1C[C@H](CCN1)c1cc(=O)[nH]o1 |r| Show InChI InChI=1S/C13H22N2O2/c1-13(2,3)8-10-6-9(4-5-14-10)11-7-12(16)15-17-11/h7,9-10,14H,4-6,8H2,1-3H3,(H,15,16)/t9-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020037

(CHEMBL3287854)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](CCc2ccccc2)C1 |r| Show InChI InChI=1S/C16H20N2O2/c19-16-11-15(20-18-16)13-8-9-17-14(10-13)7-6-12-4-2-1-3-5-12/h1-5,11,13-14,17H,6-10H2,(H,18,19)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020035

(CHEMBL3287849)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](Cc2ccccc2)C1 |r| Show InChI InChI=1S/C15H18N2O2/c18-15-10-14(19-17-15)12-6-7-16-13(9-12)8-11-4-2-1-3-5-11/h1-5,10,12-13,16H,6-9H2,(H,17,18)/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020036

(CHEMBL3287851)Show SMILES CC(C)(C)C[C@H]1C[C@H](CCN1)c1cc(=O)[nH]o1 |r| Show InChI InChI=1S/C13H22N2O2/c1-13(2,3)8-10-6-9(4-5-14-10)11-7-12(16)15-17-11/h7,9-10,14H,4-6,8H2,1-3H3,(H,15,16)/t9-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020037

(CHEMBL3287854)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](CCc2ccccc2)C1 |r| Show InChI InChI=1S/C16H20N2O2/c19-16-11-15(20-18-16)13-8-9-17-14(10-13)7-6-12-4-2-1-3-5-12/h1-5,11,13-14,17H,6-10H2,(H,18,19)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50020035

(CHEMBL3287849)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](Cc2ccccc2)C1 |r| Show InChI InChI=1S/C15H18N2O2/c18-15-10-14(19-17-15)12-6-7-16-13(9-12)8-11-4-2-1-3-5-11/h1-5,10,12-13,16H,6-9H2,(H,17,18)/t12-,13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50020036

(CHEMBL3287851)Show SMILES CC(C)(C)C[C@H]1C[C@H](CCN1)c1cc(=O)[nH]o1 |r| Show InChI InChI=1S/C13H22N2O2/c1-13(2,3)8-10-6-9(4-5-14-10)11-7-12(16)15-17-11/h7,9-10,14H,4-6,8H2,1-3H3,(H,15,16)/t9-,10+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50020037

(CHEMBL3287854)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](CCc2ccccc2)C1 |r| Show InChI InChI=1S/C16H20N2O2/c19-16-11-15(20-18-16)13-8-9-17-14(10-13)7-6-12-4-2-1-3-5-12/h1-5,11,13-14,17H,6-10H2,(H,18,19)/t13-,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020035

(CHEMBL3287849)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](Cc2ccccc2)C1 |r| Show InChI InChI=1S/C15H18N2O2/c18-15-10-14(19-17-15)12-6-7-16-13(9-12)8-11-4-2-1-3-5-11/h1-5,10,12-13,16H,6-9H2,(H,17,18)/t12-,13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020037

(CHEMBL3287854)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](CCc2ccccc2)C1 |r| Show InChI InChI=1S/C16H20N2O2/c19-16-11-15(20-18-16)13-8-9-17-14(10-13)7-6-12-4-2-1-3-5-12/h1-5,11,13-14,17H,6-10H2,(H,18,19)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020036

(CHEMBL3287851)Show SMILES CC(C)(C)C[C@H]1C[C@H](CCN1)c1cc(=O)[nH]o1 |r| Show InChI InChI=1S/C13H22N2O2/c1-13(2,3)8-10-6-9(4-5-14-10)11-7-12(16)15-17-11/h7,9-10,14H,4-6,8H2,1-3H3,(H,15,16)/t9-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020035

(CHEMBL3287849)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](Cc2ccccc2)C1 |r| Show InChI InChI=1S/C15H18N2O2/c18-15-10-14(19-17-15)12-6-7-16-13(9-12)8-11-4-2-1-3-5-11/h1-5,10,12-13,16H,6-9H2,(H,17,18)/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020037

(CHEMBL3287854)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](CCc2ccccc2)C1 |r| Show InChI InChI=1S/C16H20N2O2/c19-16-11-15(20-18-16)13-8-9-17-14(10-13)7-6-12-4-2-1-3-5-12/h1-5,11,13-14,17H,6-10H2,(H,18,19)/t13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020035

(CHEMBL3287849)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](Cc2ccccc2)C1 |r| Show InChI InChI=1S/C15H18N2O2/c18-15-10-14(19-17-15)12-6-7-16-13(9-12)8-11-4-2-1-3-5-11/h1-5,10,12-13,16H,6-9H2,(H,17,18)/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020037

(CHEMBL3287854)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](CCc2ccccc2)C1 |r| Show InChI InChI=1S/C16H20N2O2/c19-16-11-15(20-18-16)13-8-9-17-14(10-13)7-6-12-4-2-1-3-5-12/h1-5,11,13-14,17H,6-10H2,(H,18,19)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020036

(CHEMBL3287851)Show SMILES CC(C)(C)C[C@H]1C[C@H](CCN1)c1cc(=O)[nH]o1 |r| Show InChI InChI=1S/C13H22N2O2/c1-13(2,3)8-10-6-9(4-5-14-10)11-7-12(16)15-17-11/h7,9-10,14H,4-6,8H2,1-3H3,(H,15,16)/t9-,10+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020035

(CHEMBL3287849)Show SMILES O=c1cc(o[nH]1)[C@H]1CCN[C@@H](Cc2ccccc2)C1 |r| Show InChI InChI=1S/C15H18N2O2/c18-15-10-14(19-17-15)12-6-7-16-13(9-12)8-11-4-2-1-3-5-11/h1-5,10,12-13,16H,6-9H2,(H,17,18)/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020036

(CHEMBL3287851)Show SMILES CC(C)(C)C[C@H]1C[C@H](CCN1)c1cc(=O)[nH]o1 |r| Show InChI InChI=1S/C13H22N2O2/c1-13(2,3)8-10-6-9(4-5-14-10)11-7-12(16)15-17-11/h7,9-10,14H,4-6,8H2,1-3H3,(H,15,16)/t9-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020036

(CHEMBL3287851)Show SMILES CC(C)(C)C[C@H]1C[C@H](CCN1)c1cc(=O)[nH]o1 |r| Show InChI InChI=1S/C13H22N2O2/c1-13(2,3)8-10-6-9(4-5-14-10)11-7-12(16)15-17-11/h7,9-10,14H,4-6,8H2,1-3H3,(H,15,16)/t9-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 5: 538-43 (2014)
Article DOI: 10.1021/ml400526d
BindingDB Entry DOI: 10.7270/Q2QJ7JV4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data