

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM50273289 (2-Cyano-4-(1-methyl-piperidin-4-yloxy)-6-[(spiro[2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50252500 (6-(4-chlorobenzyl)-7-(3,3-dimethylbutyl)-7H-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263668 (2-Cyano-4-[2-(1-methyl-piperidin-4-yl)-ethoxy]-6-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50273288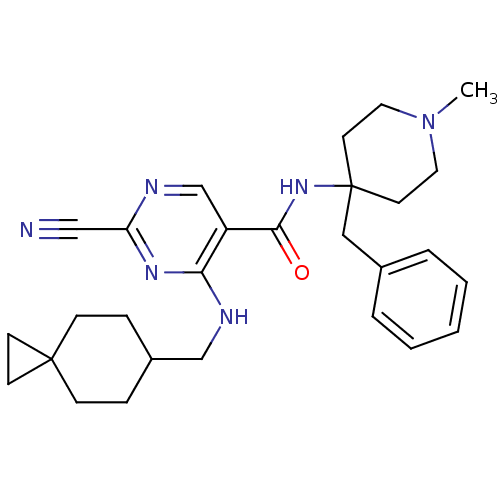 (2-Cyano-4-[(spiro[2.5]oct-6-ylmethyl)-amino]-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50272786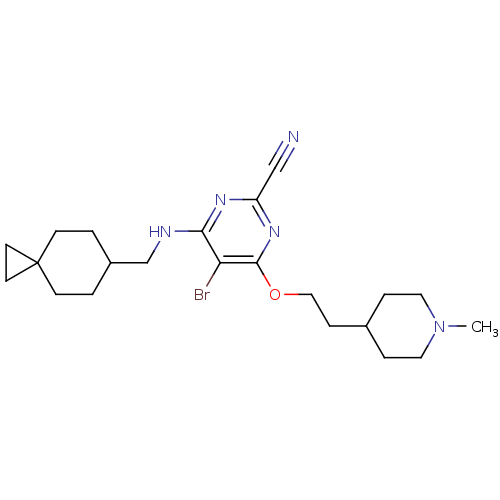 (5-Bromo-4-[2-(1-methyl-piperidin-4-yl)-ethoxy]-6-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50251889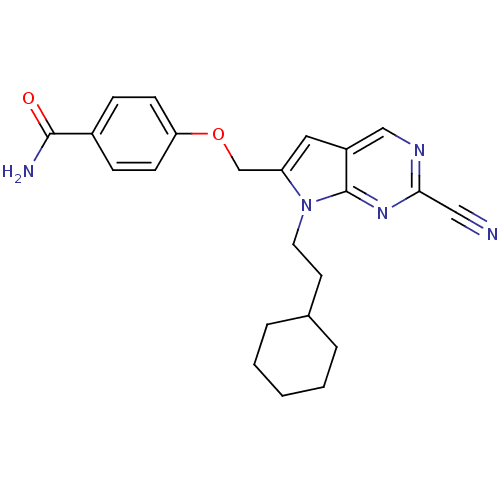 (4-((2-cyano-7-(2-cyclohexylethyl)-7H-pyrrolo[2,3-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263609 (2-cyano-4-((1-(2-hydroxyethyl)piperidin-4-yl)metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50273220 (2-Cyano-4-[(spiro[2.5]oct-6-ylmethyl)-amino]-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263557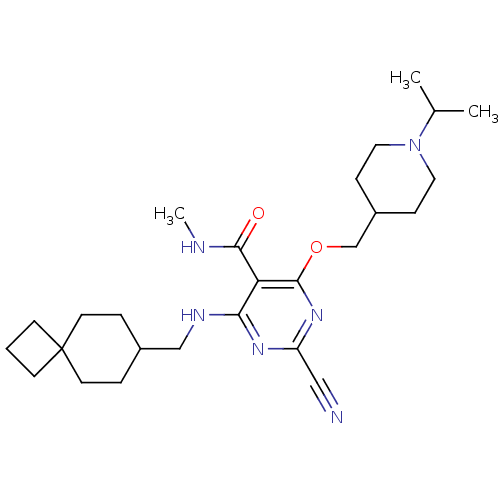 (2-cyano-4-((1-isopropylpiperidin-4-yl)methoxy)-N-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263555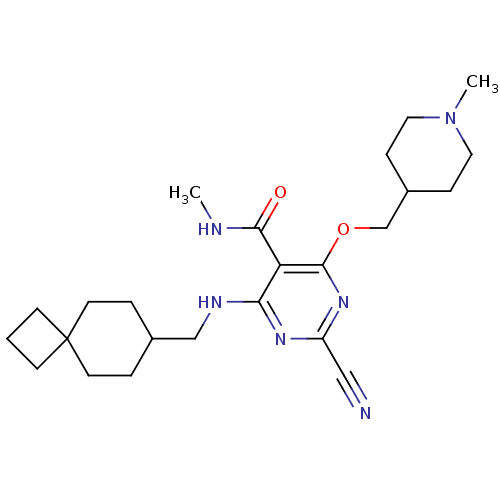 (2-cyano-N-methyl-4-((1-methylpiperidin-4-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263554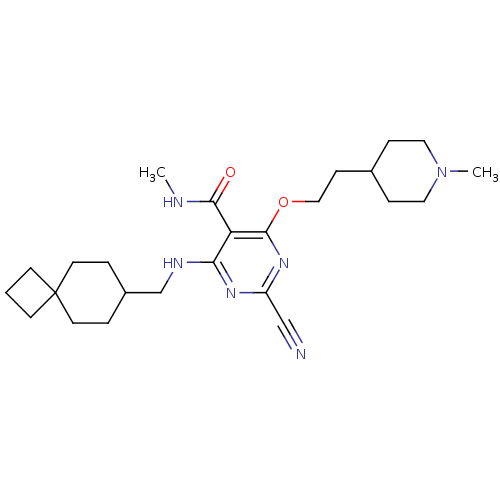 (2-cyano-N-methyl-4-(2-(1-methylpiperidin-4-yl)etho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50273256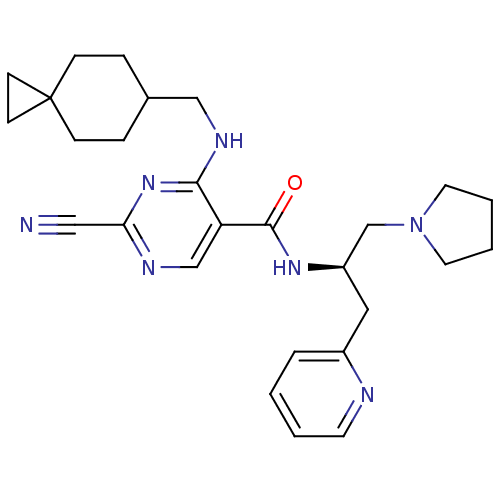 (2-Cyano-4-[(spiro[2.5]oct-6-ylmethyl)-amino]-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263611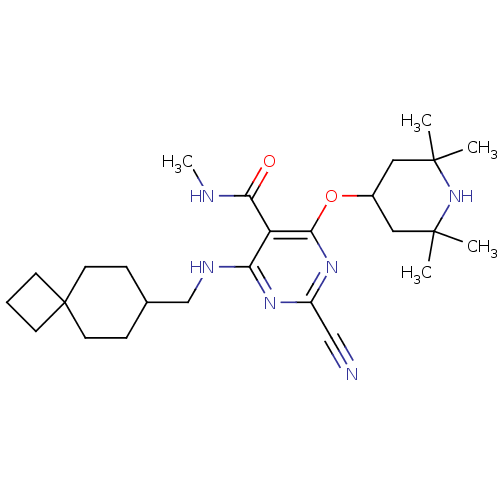 (2-cyano-N-methyl-4-(spiro[3.5]nonan-7-ylmethylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263610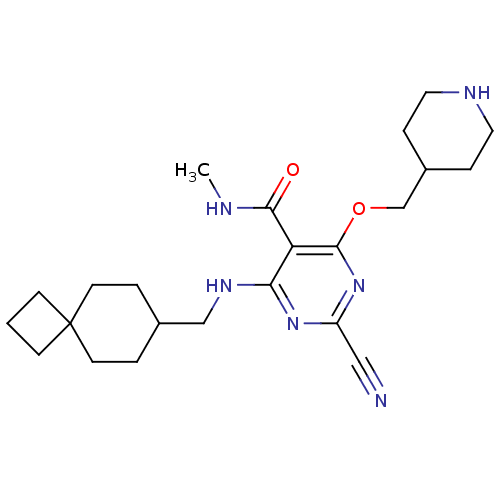 (2-cyano-N-methyl-4-(piperidin-4-ylmethoxy)-6-(spir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25138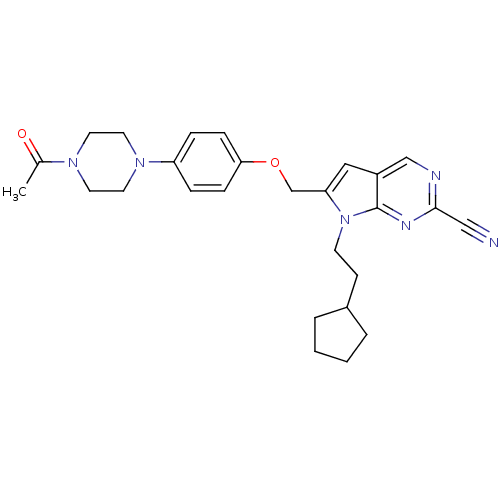 (2-cyano-pyrropyrimidine, 7d | 7-(2-cyclopentylethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50252656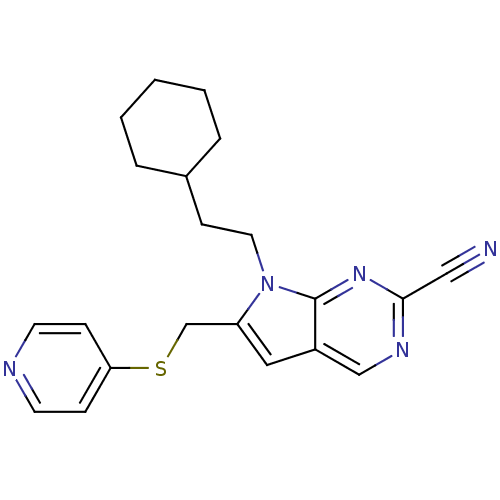 (7-(2-cyclohexylethyl)-6-((pyridin-4-yloxy)methyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50251887 (3-(3-((2-cyano-7-(2-cyclohexylethyl)-7H-pyrrolo[2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263667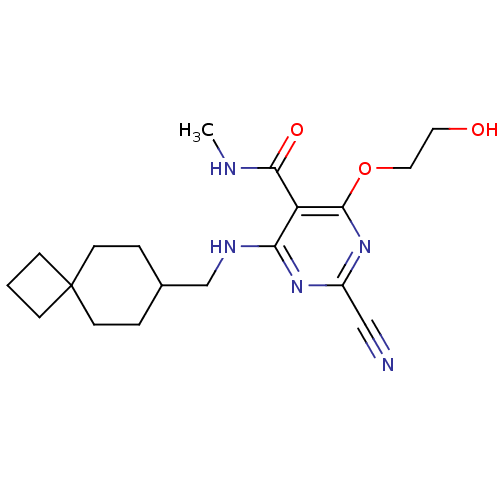 (2-cyano-4-(2-hydroxyethoxy)-N-methyl-6-(spiro[3.5]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50252502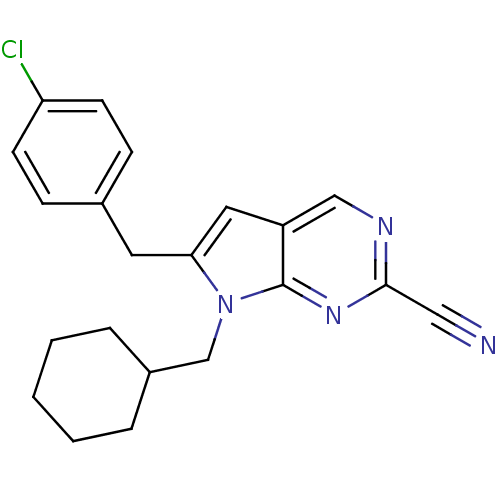 (6-(4-chlorobenzyl)-7-(cyclohexylmethyl)-7H-pyrrolo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50273286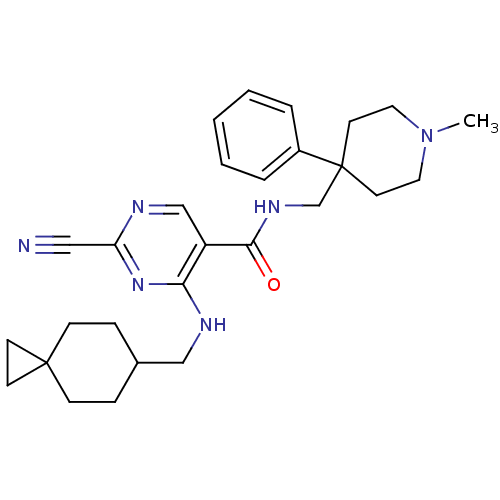 (2-Cyano-4-[(spiro[2.5]oct-6-ylmethyl)-amino]-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50273287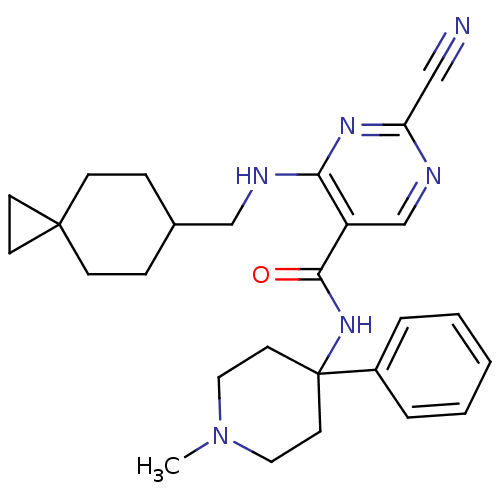 (2-Cyano-4-[(spiro[2.5]oct-6-ylmethyl)-amino]-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50251888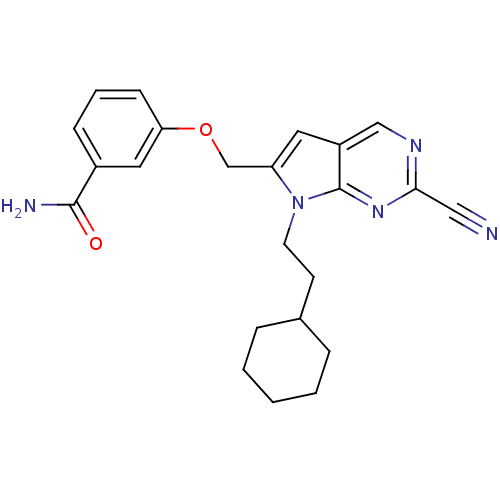 (3-((2-cyano-7-(2-cyclohexylethyl)-7H-pyrrolo[2,3-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50252543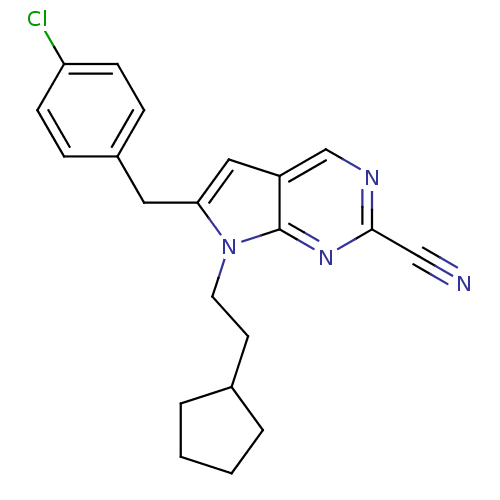 (6-(4-chlorobenzyl)-7-(2-cyclopentylethyl)-7H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50251942 (CHEMBL520427 | N-(4-((2-cyano-7-(2-cyclohexylethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25136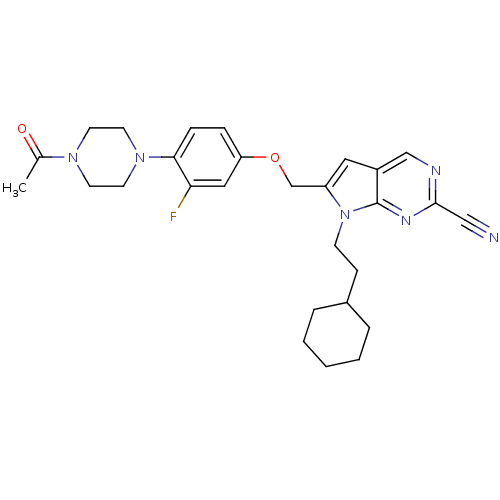 (2-cyano-pyrropyrimidine, 7b | 7-(2-cyclohexylethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25134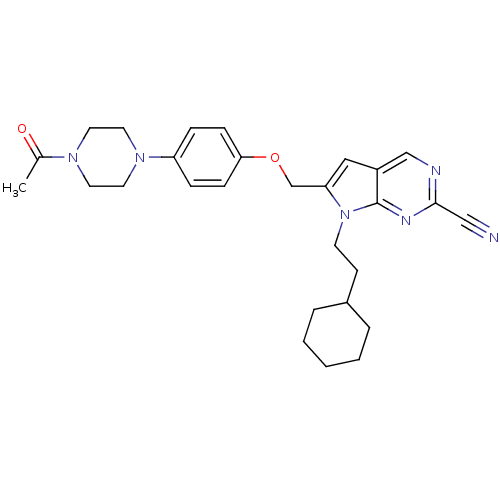 (2-cyano-pyrropyrimidine, 2 | 7-(2-cyclohexylethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50273178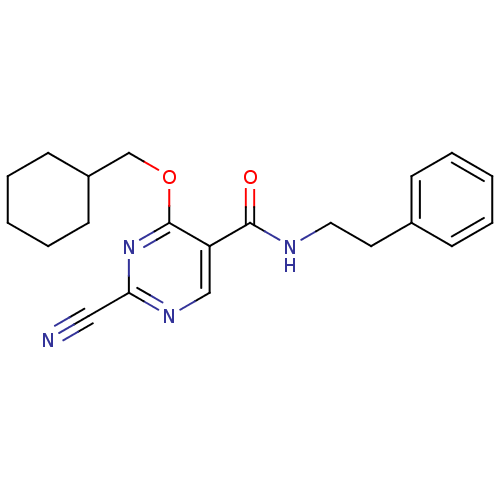 (2-cyano-4-(cyclohexylmethoxy)-N-phenethylpyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25134 (2-cyano-pyrropyrimidine, 2 | 7-(2-cyclohexylethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263612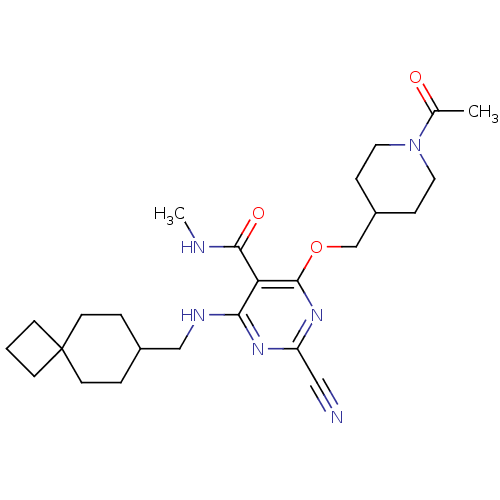 (4-((1-acetylpiperidin-4-yl)methoxy)-2-cyano-N-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25141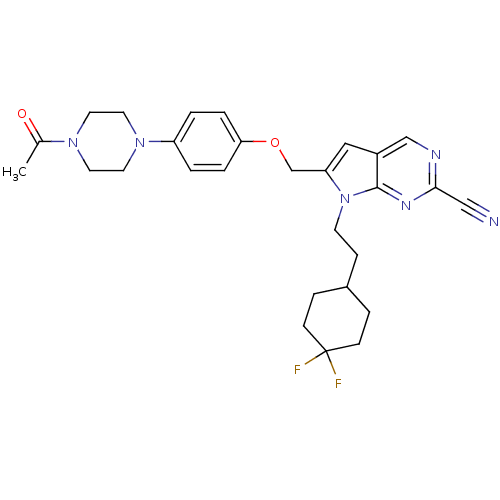 (2-cyano-pyrropyrimidine, 7g | 7-[2-(4,4-difluorocy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50272626 (2-Cyano-4-[2-(1-methyl-piperidin-4-yl)-ethoxy]-6-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM25134 (2-cyano-pyrropyrimidine, 2 | 7-(2-cyclohexylethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50252654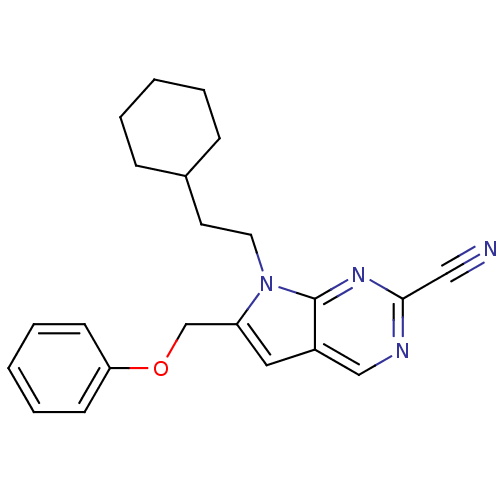 (7-(2-cyclohexylethyl)-6-(phenoxymethyl)-7H-pyrrolo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19731 (2-Cyano-pyrimidine, 16a | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25137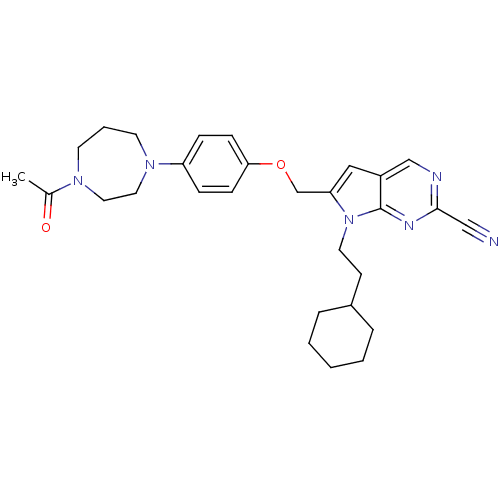 (2-cyano-pyrropyrimidine, 7c | 7-(2-cyclohexylethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50252655 (7-(2-cyclohexylethyl)-6-((pyridin-2-yloxy)methyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263511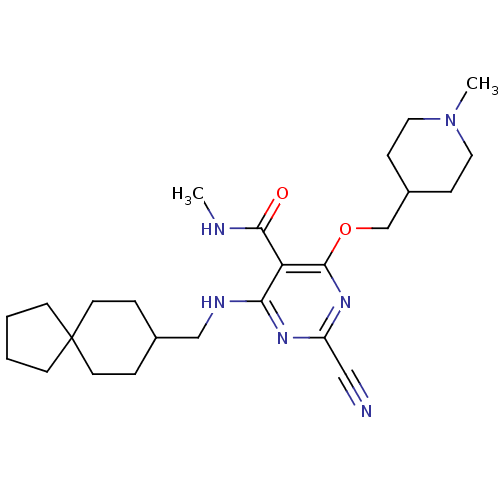 (2-cyano-N-methyl-4-((1-methylpiperidin-4-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50252036 (CHEMBL481169 | N-(4-((2-cyano-7-(2-cyclohexylethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50252038 (6-((4-(1-acetyl-1,2,3,6-tetrahydropyridin-4-yl)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50273178 (2-cyano-4-(cyclohexylmethoxy)-N-phenethylpyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM25135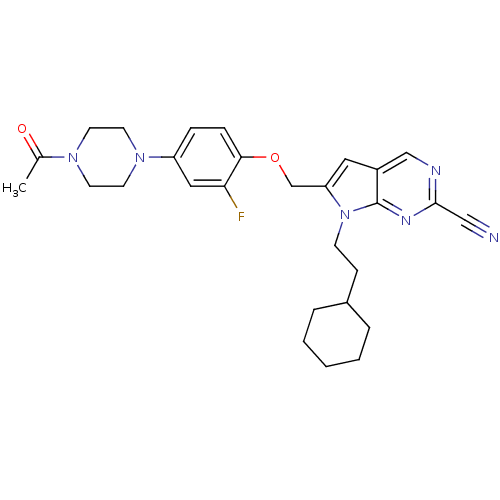 (2-cyano-pyrropyrimidine, 7a | 7-(2-cyclohexylethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50273218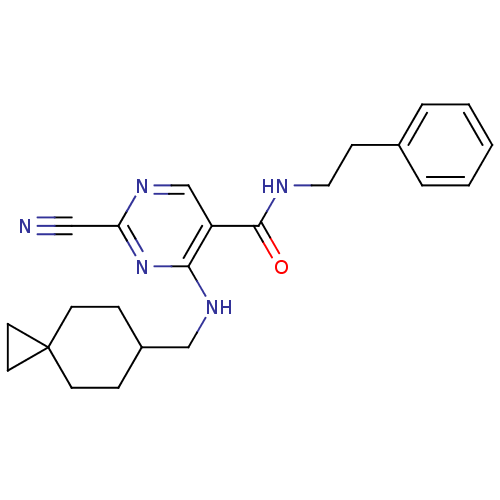 (2-Cyano-4-[(spiro[2.5]oct-6-ylmethyl)-amino]-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50273176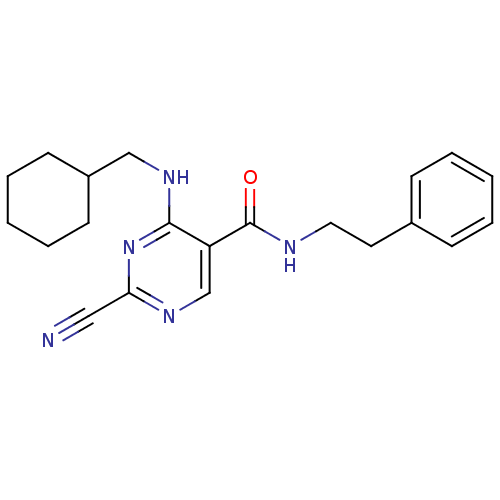 (2-cyano-4-(cyclohexylmethylamino)-N-phenethylpyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50252037 (6-((4-(1-acetylpiperidin-4-yl)phenoxy)methyl)-7-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50251886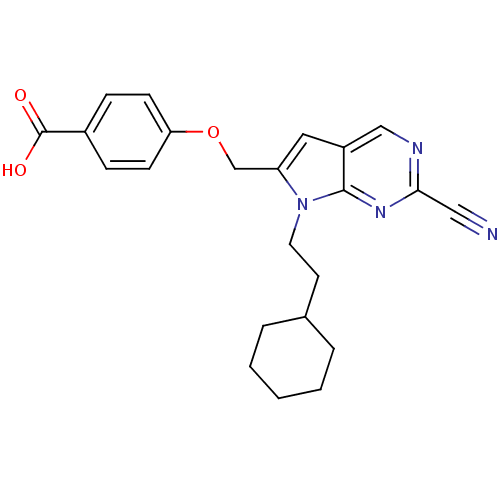 (4-((2-cyano-7-(2-cyclohexylethyl)-7H-pyrrolo[2,3-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50252543 (6-(4-chlorobenzyl)-7-(2-cyclopentylethyl)-7H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25139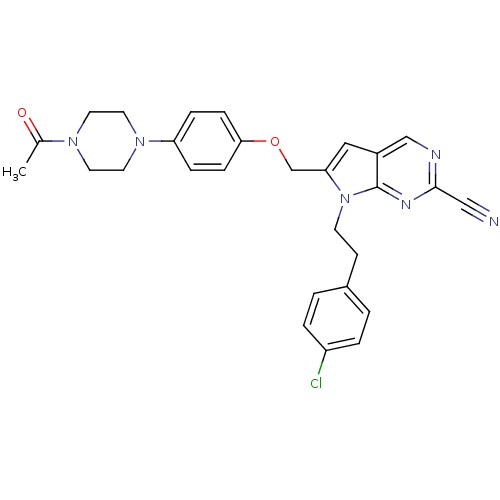 (2-cyano-pyrropyrimidine, 7e | 7-[2-(4-chlorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25135 (2-cyano-pyrropyrimidine, 7a | 7-(2-cyclohexylethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50252500 (6-(4-chlorobenzyl)-7-(3,3-dimethylbutyl)-7H-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50252547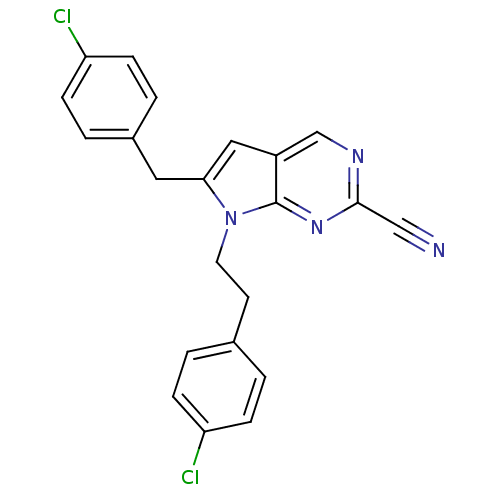 (6-(4-chlorobenzyl)-7-(4-chlorophenethyl)-7H-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 209 total ) | Next | Last >> |