

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50059510 (8-Methoxy-3-phenyl-1,2,3,4-tetrahydro-chromeno[3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50315177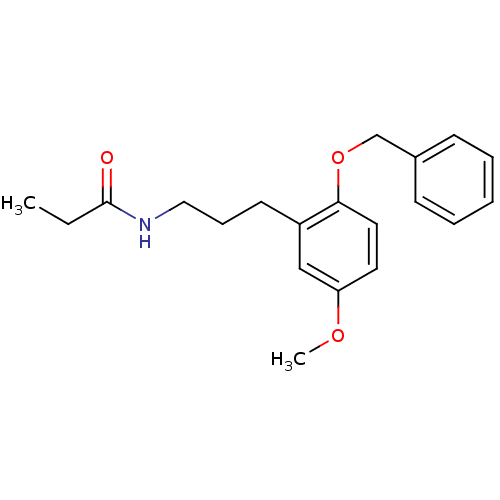 (CHEMBL1091161 | N-(3-(2-(benzyloxy)-5-methoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2582-5 (2010) Article DOI: 10.1016/j.bmcl.2010.02.084 BindingDB Entry DOI: 10.7270/Q2S75GG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14073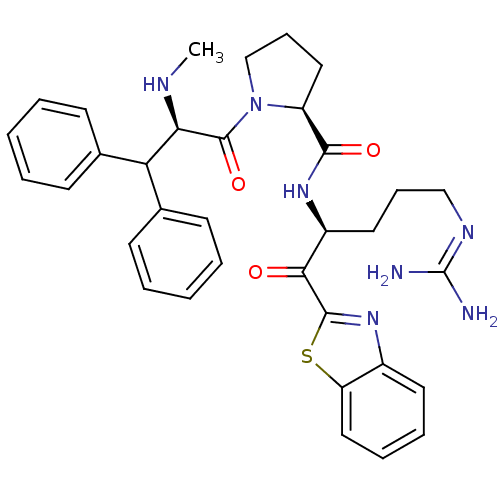 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000650 | -72.4 | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50315171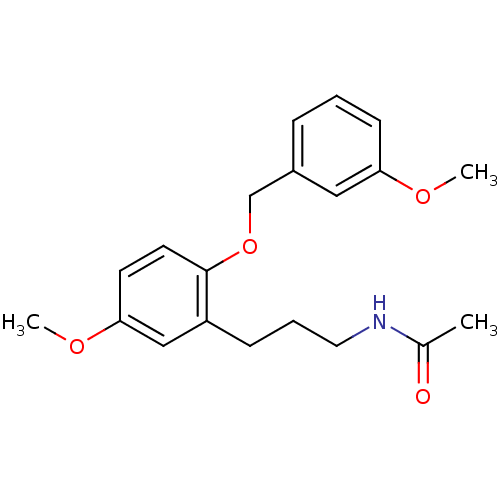 (CHEMBL1092646 | N-(3-(5-methoxy-2-(3-methoxybenzyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2582-5 (2010) Article DOI: 10.1016/j.bmcl.2010.02.084 BindingDB Entry DOI: 10.7270/Q2S75GG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50315178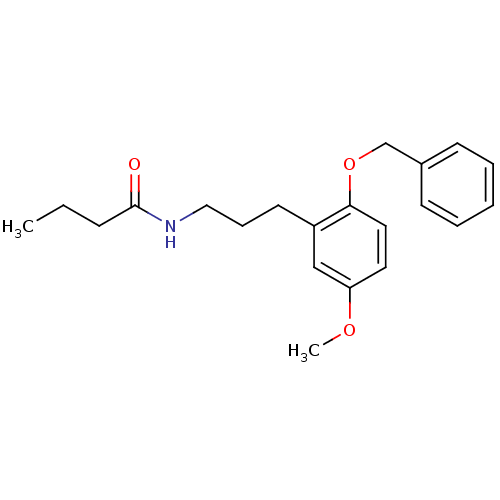 (CHEMBL1088825 | N-(3-(2-(benzyloxy)-5-methoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2582-5 (2010) Article DOI: 10.1016/j.bmcl.2010.02.084 BindingDB Entry DOI: 10.7270/Q2S75GG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50030745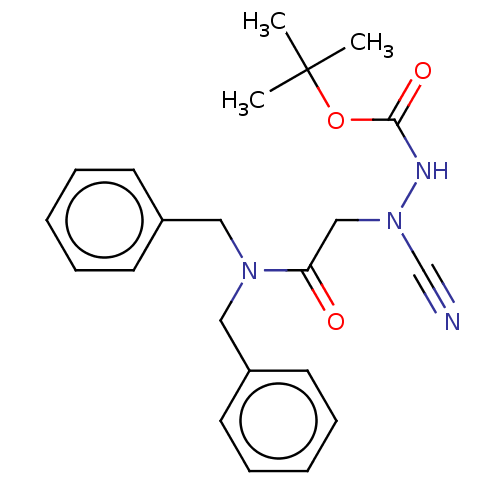 (CHEMBL3342185 | acs.jmedchem.1c00409_ST.412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin K using Z-Leu-Arg-AMC fluorogenic substrate incubated for 60 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50030746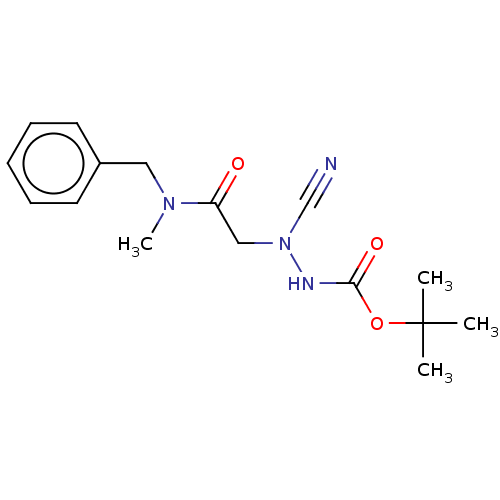 (CHEMBL3342184 | acs.jmedchem.1c00409_ST.413) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin K using Z-Leu-Arg-AMC fluorogenic substrate incubated for 60 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14065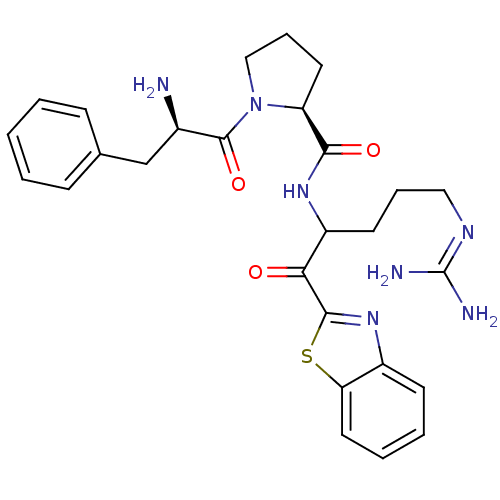 ((2S)-1-[(2R)-2-amino-3-phenylpropanoyl]-N-[1-(1,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00550 | -66.9 | 21 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14127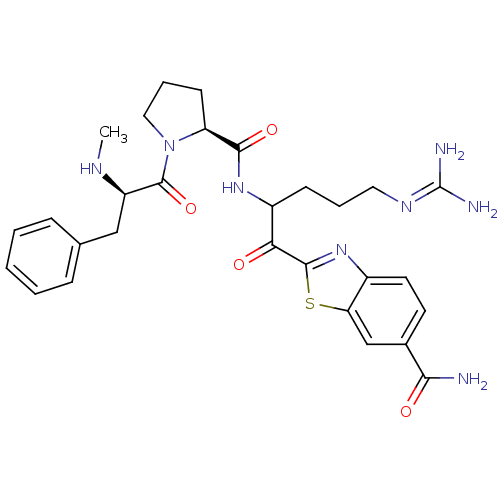 (2-(5-carbamimidamido-2-{[(2S)-1-[(2R)-2-(methylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1501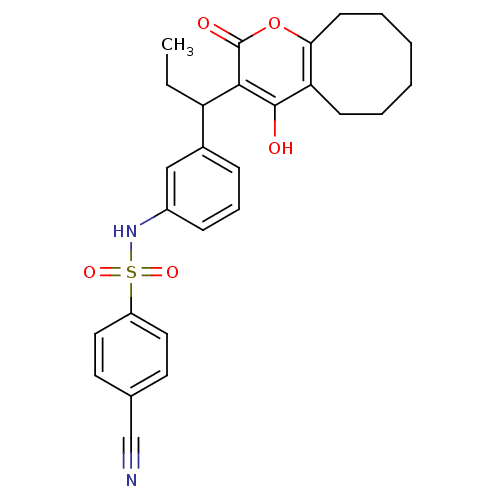 (4-Cyano-N-[3-[1-(5,6,7,8,9,10-hexahydro-4-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn Curated by ChEMBL | Assay Description Binding affinity of the compound towards HIV protease was determined | J Med Chem 39: 4125-30 (1996) Article DOI: 10.1021/jm960296c BindingDB Entry DOI: 10.7270/Q2KH0MFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] FabI (Escherichia coli) | BDBM8726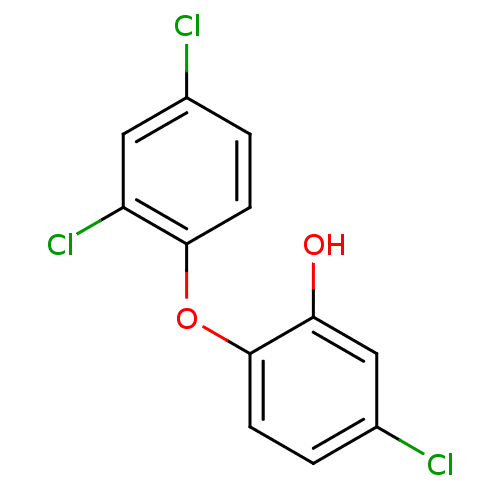 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM554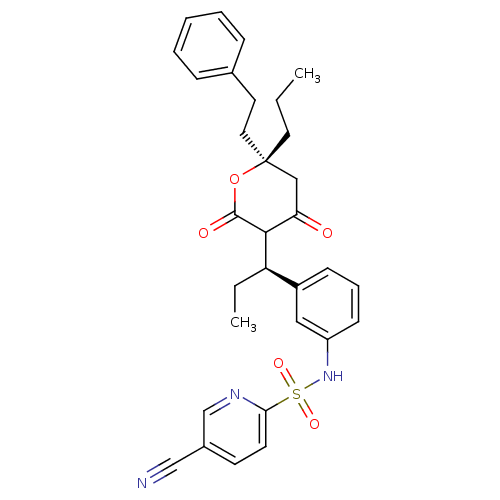 (5-cyano-N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | -63.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM554 (5-cyano-N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | -63.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM558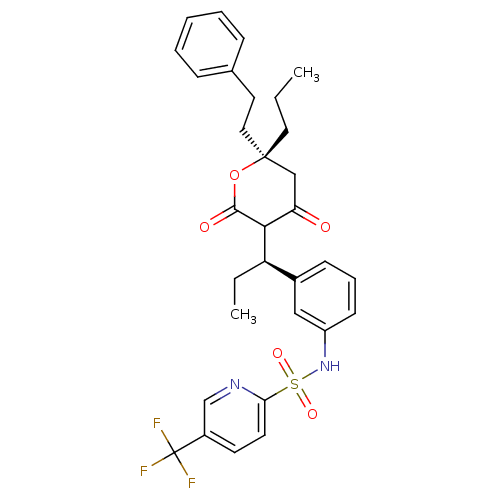 (N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.00800 | -62.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM558 (N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.00800 | -62.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036480 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM97445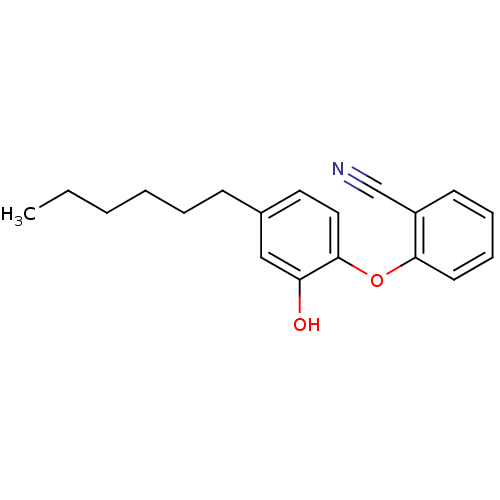 (PT119) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus enoyl ACP reductase | Eur J Med Chem 88: 66-73 (2014) Article DOI: 10.1016/j.ejmech.2014.09.008 BindingDB Entry DOI: 10.7270/Q25T3N3S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335280 (CHEMBL1651354 | N-(Benzylcarbamoyl)-leucyl-methyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50030747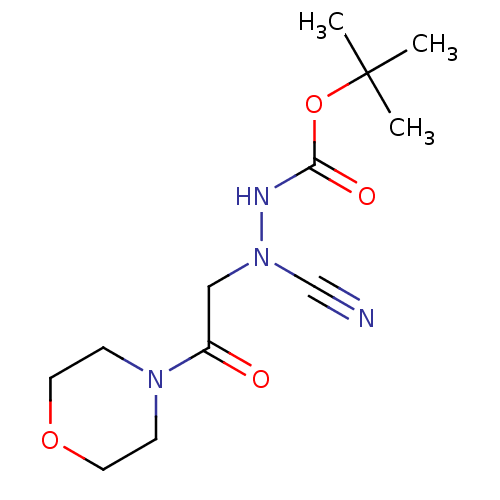 (CHEMBL3342183) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin K using Z-Leu-Arg-AMC fluorogenic substrate incubated for 60 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036476 (2,6-Dichloro-3-(4-methyl-piperazine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036478 (3-Carboxymethoxy-2,6-dichloro-benzoic acid 4-isopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036481 (2,6-Dichloro-3-[(2-dimethylamino-ethyl)-methyl-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036477 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50469862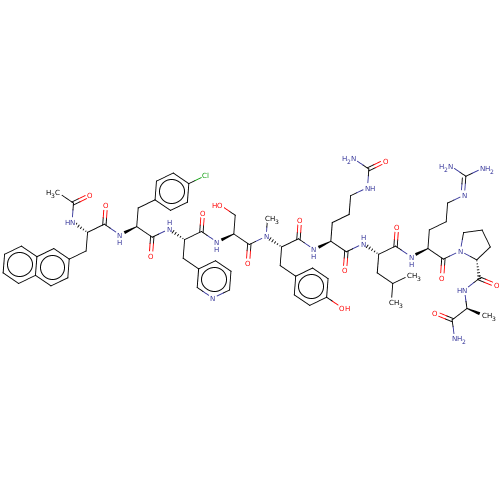 (CHEMBL409219) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description The negative logarithm of the concentration of antagonist that inhibits 50% of the binding of 125 I-labeled leuprolide to the rat pituitary LHRH rece... | J Med Chem 36: 928-33 (1993) Article DOI: 10.1021/jm00059a020 BindingDB Entry DOI: 10.7270/Q2Z03BW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036475 (2,6-Dichloro-3-(2-pyrrolidin-1-yl-ethoxy)-benzoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50450717 (CHEMBL317087) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Tested for inhibitor binding of wild-type HIV PR | J Med Chem 47: 2030-6 (2004) Article DOI: 10.1021/jm031105q BindingDB Entry DOI: 10.7270/Q2CN74PV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430590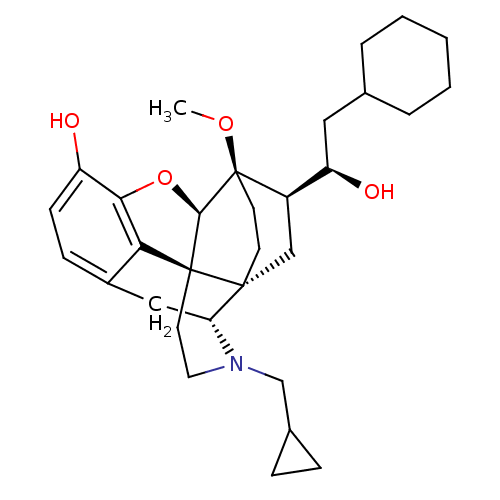 (CHEMBL2338742) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human KOR expressed in CHO cells | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335281 (CHEMBL1651355 | N-(Phenylcarbamoyl)-leucyl-methyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14076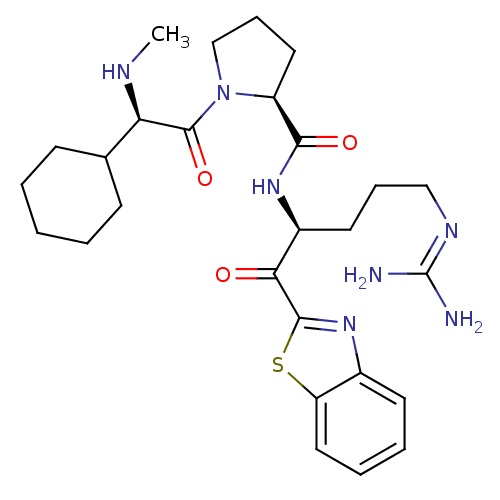 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | -63.8 | 5.30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM557 (N-{3-[(1R)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | -60.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM557 (N-{3-[(1R)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | -60.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50053910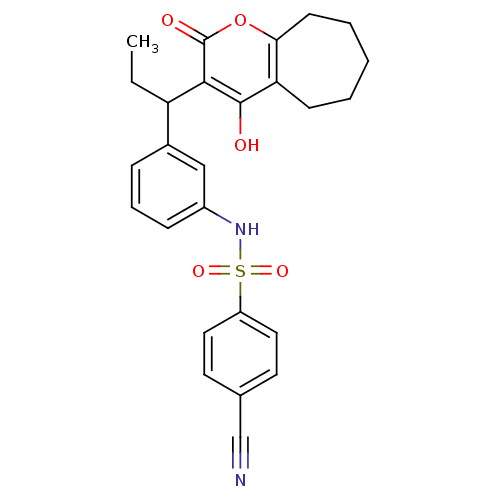 (4-Cyano-N-{3-[1-(4-hydroxy-2-oxo-2,5,6,7,8,9-hexah...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn Curated by ChEMBL | Assay Description Binding affinity of the compound towards HIV protease was determined | J Med Chem 39: 4125-30 (1996) Article DOI: 10.1021/jm960296c BindingDB Entry DOI: 10.7270/Q2KH0MFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430589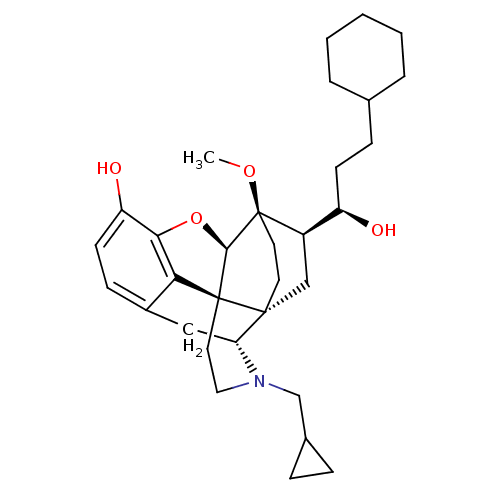 (CHEMBL2338716) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human KOR expressed in CHO cells | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50049553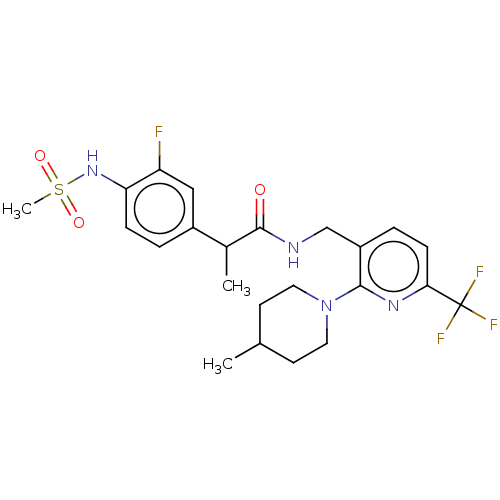 (CHEMBL2177428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activation by FLIPR assay | Bioorg Med Chem Lett 25: 2326-30 (2015) Article DOI: 10.1016/j.bmcl.2015.04.024 BindingDB Entry DOI: 10.7270/Q2Z60QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50049553 (CHEMBL2177428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay | Eur J Med Chem 93: 101-8 (2015) Article DOI: 10.1016/j.ejmech.2015.02.001 BindingDB Entry DOI: 10.7270/Q2N0188S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50073160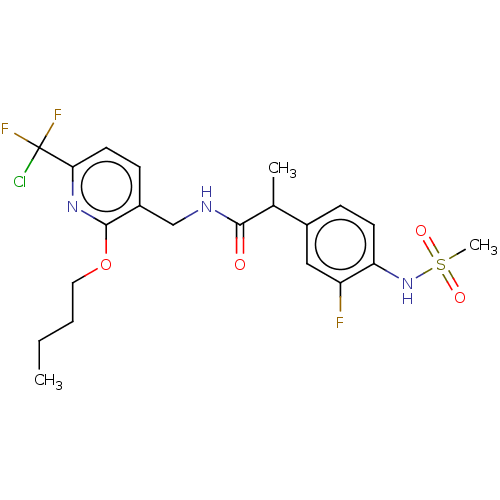 (CHEMBL3407762) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay | Eur J Med Chem 93: 101-8 (2015) Article DOI: 10.1016/j.ejmech.2015.02.001 BindingDB Entry DOI: 10.7270/Q2N0188S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Sus scrofa) | BDBM50155838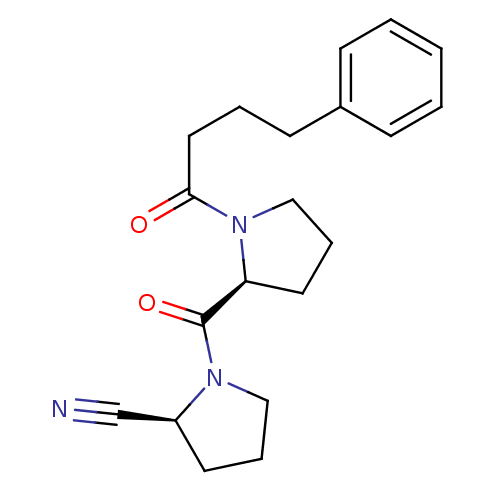 ((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kuopio Curated by ChEMBL | Assay Description Inhibitory activity against prolyl oligopeptidase of porcine brain homogenate | J Med Chem 47: 5605-7 (2004) Article DOI: 10.1021/jm049503w BindingDB Entry DOI: 10.7270/Q25D8R9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50029257 ((3-((4-(2-isopropoxyphenyl)piperazin-1-yl)methyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity to adrenergic alpha1A receptor (unknown origin) | Bioorg Med Chem 16: 4759-800 (2008) Article DOI: 10.1016/j.bmc.2008.02.091 BindingDB Entry DOI: 10.7270/Q2DV1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430588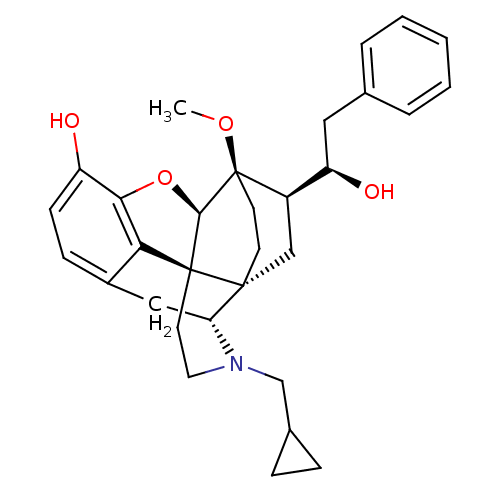 (CHEMBL2338717) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human KOR expressed in CHO cells | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50450726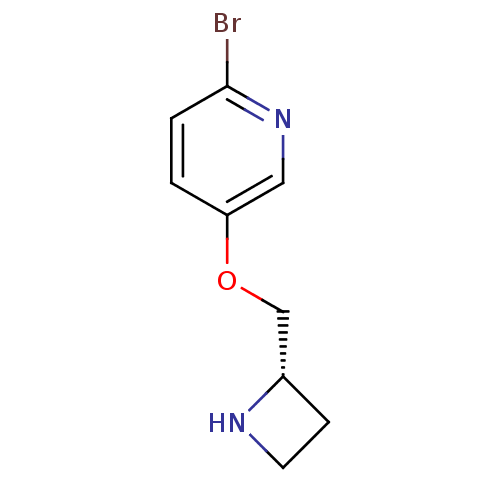 (CHEMBL407258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50351401 (CHEMBL1819091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335285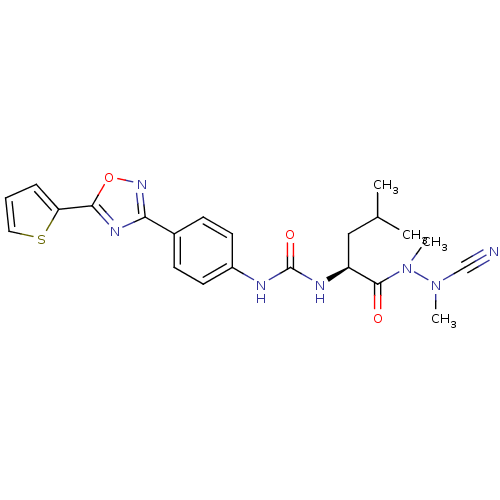 (CHEMBL1651350 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50450734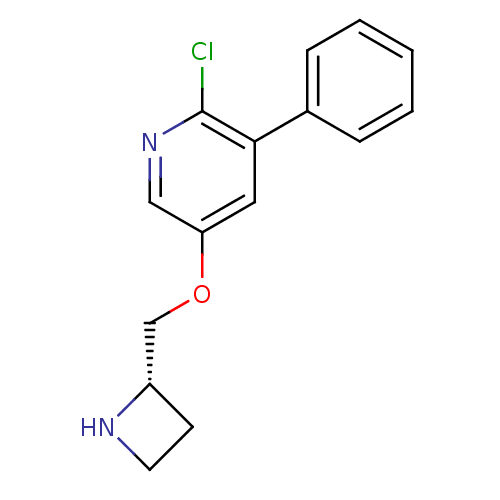 (CHEMBL318869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50450710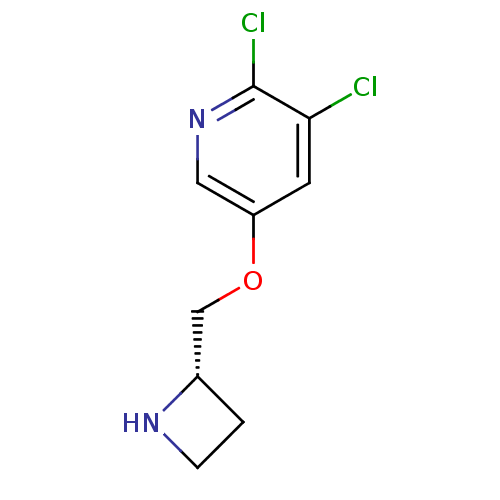 (CHEMBL97555) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50469858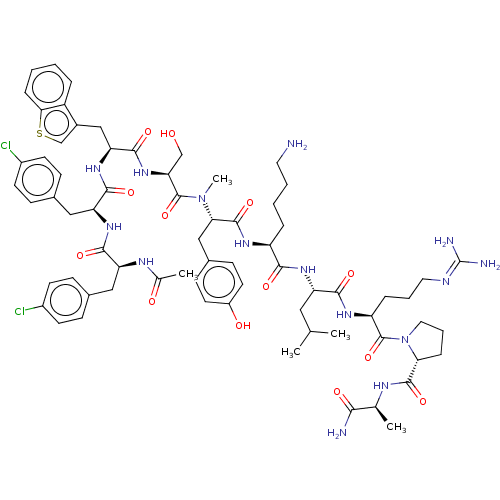 (CHEMBL268397) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description The negative logarithm of the concentration of antagonist that inhibits 50% of the binding of 125 I-labeled leuprolide to the rat pituitary LHRH rece... | J Med Chem 36: 928-33 (1993) Article DOI: 10.1021/jm00059a020 BindingDB Entry DOI: 10.7270/Q2Z03BW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50125967 (CHEMBL3627846) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) | Bioorg Med Chem Lett 25: 4893-8 (2015) Article DOI: 10.1016/j.bmcl.2015.05.080 BindingDB Entry DOI: 10.7270/Q2RJ4M9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50450720 (CHEMBL94683) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 in rat brain using [3H]-cytisine as radioligand | Bioorg Med Chem Lett 8: 2797-802 (1999) BindingDB Entry DOI: 10.7270/Q23J3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430615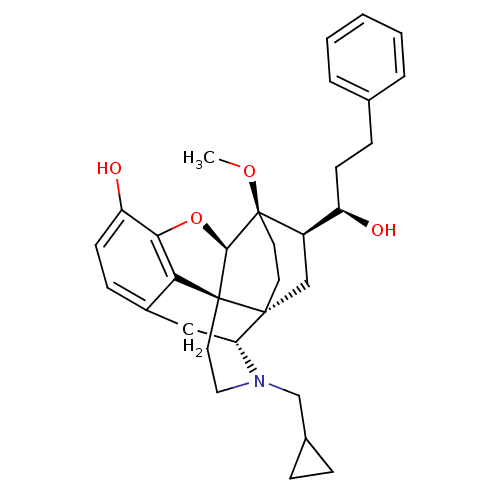 (CHEMBL2338718) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human KOR expressed in CHO cells | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50351399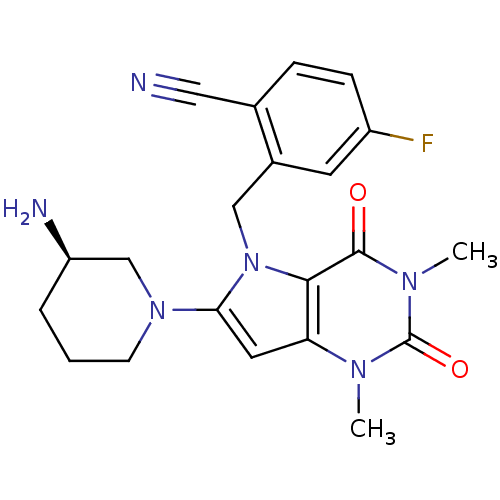 (CHEMBL1819089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 104881 total ) | Next | Last >> |