 Found 353 hits with Last Name = 'houston' and Initial = 'd'
Found 353 hits with Last Name = 'houston' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184767
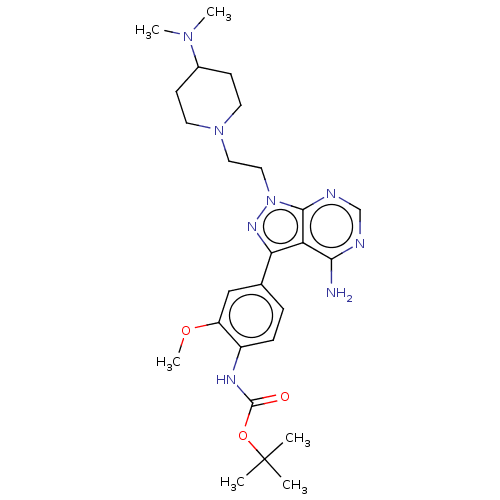
(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Competitive inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as su... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50367250

(3-DEAZAARISTEROMYCIN A | CHEMBL268272)Show SMILES Nc1nccc2n(cnc12)[C@@H]1C[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C12H16N4O3/c13-12-9-7(1-2-14-12)16(5-15-9)8-3-6(4-17)10(18)11(8)19/h1-2,5-6,8,10-11,17-19H,3-4H2,(H2,13,14)/t6-,8-,10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against S-adenosyl-homocysteine hydrolase |
J Med Chem 28: 471-7 (1985)
BindingDB Entry DOI: 10.7270/Q21C1XF3 |
More data for this
Ligand-Target Pair | |
Chitinase B
(Serratia marcescens) | BDBM10854
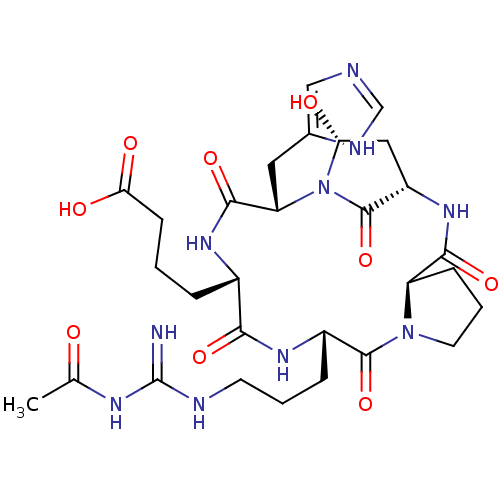
(4-[(1S,4R,10S,13S,16S,18R)-10-{3-[(acetamidomethan...)Show SMILES CC(=O)NC(=N)NCCC[C@@H]1NC(=O)[C@H](CCCC(O)=O)NC(=O)[C@H](Cc2cnc[nH]2)N2[C@H](O)C[C@H](NC(=O)[C@H]3CCCN3C1=O)C2=O |r| Show InChI InChI=1S/C29H42N10O9/c1-15(40)34-29(30)32-9-3-6-18-27(47)38-10-4-7-20(38)25(45)37-19-12-22(41)39(28(19)48)21(11-16-13-31-14-33-16)26(46)35-17(24(44)36-18)5-2-8-23(42)43/h13-14,17-22,41H,2-12H2,1H3,(H,31,33)(H,35,46)(H,36,44)(H,37,45)(H,42,43)(H3,30,32,34,40)/t17-,18-,19-,20+,21-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
University of Dundee
| Assay Description
The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... |
Chem Biol 12: 65-76 (2005)
Article DOI: 10.1016/j.chembiol.2004.10.013
BindingDB Entry DOI: 10.7270/Q23F4MV6 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215404
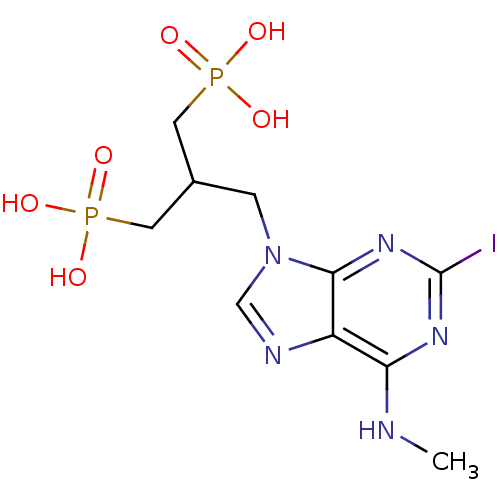
(CHEMBL227235 | [3-(2-iodo-6-methylaminopurin-9-yl)...)Show SMILES CNc1nc(I)nc2n(CC(CP(O)(O)=O)CP(O)(O)=O)cnc12 Show InChI InChI=1S/C10H16IN5O6P2/c1-12-8-7-9(15-10(11)14-8)16(5-13-7)2-6(3-23(17,18)19)4-24(20,21)22/h5-6H,2-4H2,1H3,(H,12,14,15)(H2,17,18,19)(H2,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50121987
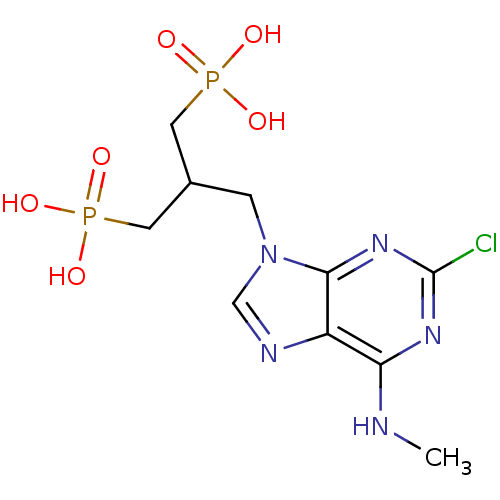
(CHEMBL153254 | [3-(2-Chloro-6-methylamino-purin-9-...)Show SMILES CNc1nc(Cl)nc2n(CC(CP(O)(O)=O)CP(O)(O)=O)cnc12 Show InChI InChI=1S/C10H16ClN5O6P2/c1-12-8-7-9(15-10(11)14-8)16(5-13-7)2-6(3-23(17,18)19)4-24(20,21)22/h5-6H,2-4H2,1H3,(H,12,14,15)(H2,17,18,19)(H2,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215406
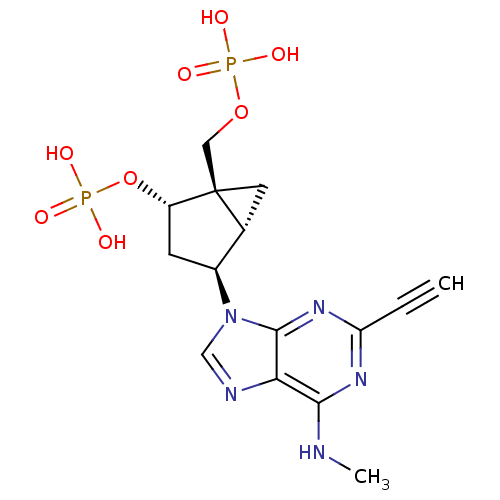
((1'R,2'S,4'S,5'S)-4-(2-ethynyl-6-methylaminopurin-...)Show SMILES CNc1nc(nc2n(cnc12)[C@H]1C[C@H](OP(O)(O)=O)[C@]2(COP(O)(O)=O)C[C@H]12)C#C Show InChI InChI=1S/C15H19N5O8P2/c1-3-11-18-13(16-2)12-14(19-11)20(7-17-12)9-4-10(28-30(24,25)26)15(5-8(9)15)6-27-29(21,22)23/h1,7-10H,4-6H2,2H3,(H,16,18,19)(H2,21,22,23)(H2,24,25,26)/t8-,9+,10+,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50088426

((1R,2S,3R,5R)-3-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C11H15N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h3-6,8-9,17-19H,1-2H2,(H2,12,13,14)/t5-,6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against S-adenosyl-homocysteine hydrolase |
J Med Chem 28: 471-7 (1985)
BindingDB Entry DOI: 10.7270/Q21C1XF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215400

((1'R,2'S,4'R,5'S)-phosphoric acid mono-[4-(6-methy...)Show SMILES CNc1nc(nc2n(cnc12)[C@H]1C[C@H](OP(O)(O)=O)[C@]2(COP(O)(O)=O)C[C@H]12)-c1ccccc1 Show InChI InChI=1S/C19H23N5O8P2/c1-20-17-15-18(23-16(22-17)11-5-3-2-4-6-11)24(10-21-15)13-7-14(32-34(28,29)30)19(8-12(13)19)9-31-33(25,26)27/h2-6,10,12-14H,7-9H2,1H3,(H,20,22,23)(H2,25,26,27)(H2,28,29,30)/t12-,13+,14+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215402
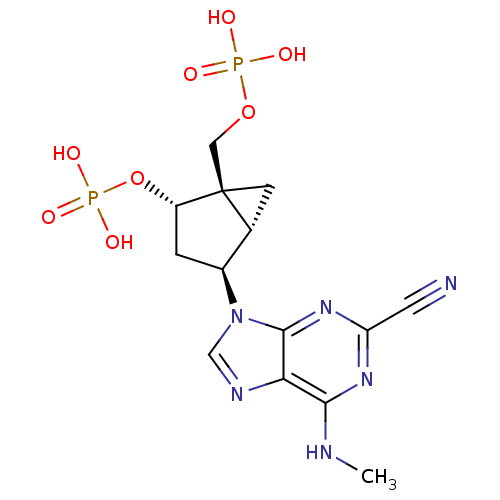
((1'R,2'S,4'R,5'S)-phosphoric acid mono-[4-(2-cyano...)Show SMILES CNc1nc(nc2n(cnc12)[C@H]1C[C@H](OP(O)(O)=O)[C@]2(COP(O)(O)=O)C[C@H]12)C#N Show InChI InChI=1S/C14H18N6O8P2/c1-16-12-11-13(19-10(4-15)18-12)20(6-17-11)8-2-9(28-30(24,25)26)14(3-7(8)14)5-27-29(21,22)23/h6-9H,2-3,5H2,1H3,(H,16,18,19)(H2,21,22,23)(H2,24,25,26)/t7-,8+,9+,14+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215403
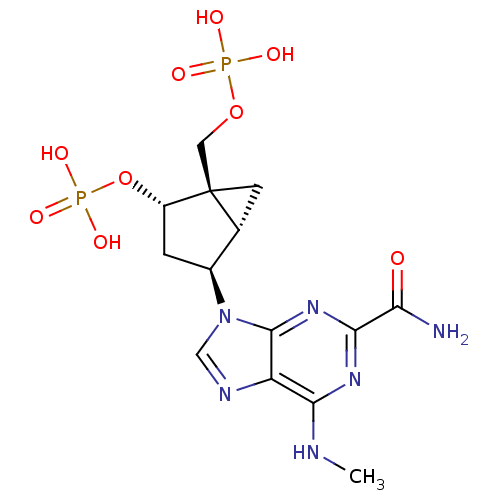
((1'R,2'S,4'R,5'S)-phosphoric acid mono-[4-(2-carba...)Show SMILES CNc1nc(nc2n(cnc12)[C@H]1C[C@H](OP(O)(O)=O)[C@]2(COP(O)(O)=O)C[C@H]12)C(N)=O Show InChI InChI=1S/C14H20N6O9P2/c1-16-11-9-13(19-12(18-11)10(15)21)20(5-17-9)7-2-8(29-31(25,26)27)14(3-6(7)14)4-28-30(22,23)24/h5-8H,2-4H2,1H3,(H2,15,21)(H,16,18,19)(H2,22,23,24)(H2,25,26,27)/t6-,7+,8+,14+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215401
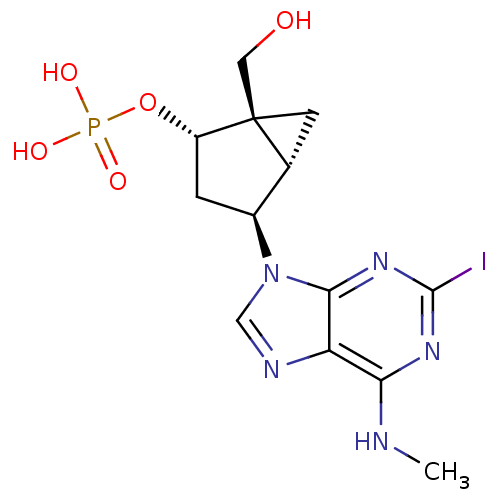
((1'R,2'S,4'S,5'S)-phosphoric acid mono-[1-hydroxym...)Show SMILES CNc1nc(I)nc2n(cnc12)[C@H]1C[C@H](OP(O)(O)=O)[C@]2(CO)C[C@H]12 Show InChI InChI=1S/C13H17IN5O5P/c1-15-10-9-11(18-12(14)17-10)19(5-16-9)7-2-8(24-25(21,22)23)13(4-20)3-6(7)13/h5-8,20H,2-4H2,1H3,(H,15,17,18)(H2,21,22,23)/t6-,7+,8+,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 706 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215405
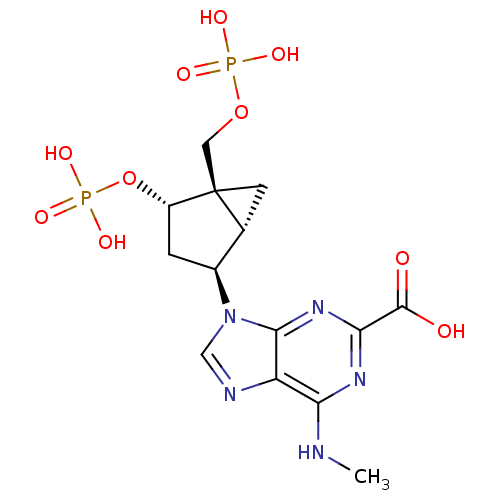
((1'R,2'S,4'R,5'S)-6-methylamino-9-(4-phosphonooxy-...)Show SMILES CNc1nc(nc2n(cnc12)[C@H]1C[C@H](OP(O)(O)=O)[C@]2(COP(O)(O)=O)C[C@H]12)C(O)=O Show InChI InChI=1S/C14H19N5O10P2/c1-15-10-9-12(18-11(17-10)13(20)21)19(5-16-9)7-2-8(29-31(25,26)27)14(3-6(7)14)4-28-30(22,23)24/h5-8H,2-4H2,1H3,(H,20,21)(H,15,17,18)(H2,22,23,24)(H2,25,26,27)/t6-,7+,8+,14+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Bos taurus (bovine)) | BDBM50026331
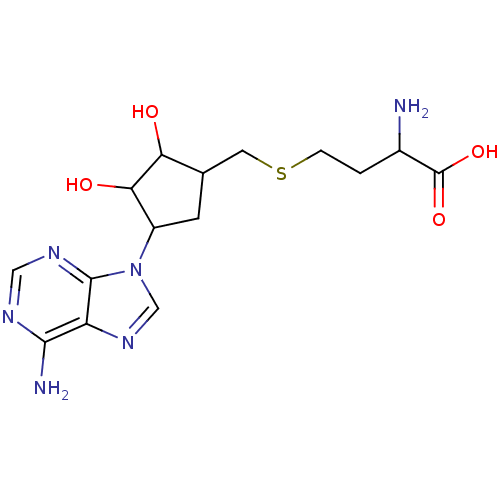
(2-Amino-4-[4-(6-amino-purin-9-yl)-2,3-dihydroxy-cy...)Show SMILES NC(CCSCC1CC(C(O)C1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C15H22N6O4S/c16-8(15(24)25)1-2-26-4-7-3-9(12(23)11(7)22)21-6-20-10-13(17)18-5-19-14(10)21/h5-9,11-12,22-23H,1-4,16H2,(H,24,25)(H2,17,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 7.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated against PNMT |
J Med Chem 28: 478-82 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1DZS |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Bos taurus (bovine)) | BDBM50026331

(2-Amino-4-[4-(6-amino-purin-9-yl)-2,3-dihydroxy-cy...)Show SMILES NC(CCSCC1CC(C(O)C1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C15H22N6O4S/c16-8(15(24)25)1-2-26-4-7-3-9(12(23)11(7)22)21-6-20-10-13(17)18-5-19-14(10)21/h5-9,11-12,22-23H,1-4,16H2,(H,24,25)(H2,17,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated against PNMT |
J Med Chem 28: 478-82 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1DZS |
More data for this
Ligand-Target Pair | |
Histamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50026331

(2-Amino-4-[4-(6-amino-purin-9-yl)-2,3-dihydroxy-cy...)Show SMILES NC(CCSCC1CC(C(O)C1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C15H22N6O4S/c16-8(15(24)25)1-2-26-4-7-3-9(12(23)11(7)22)21-6-20-10-13(17)18-5-19-14(10)21/h5-9,11-12,22-23H,1-4,16H2,(H,24,25)(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated against Histamine N-methyl-transferase |
J Med Chem 28: 478-82 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1DZS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Histamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50026331

(2-Amino-4-[4-(6-amino-purin-9-yl)-2,3-dihydroxy-cy...)Show SMILES NC(CCSCC1CC(C(O)C1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C15H22N6O4S/c16-8(15(24)25)1-2-26-4-7-3-9(12(23)11(7)22)21-6-20-10-13(17)18-5-19-14(10)21/h5-9,11-12,22-23H,1-4,16H2,(H,24,25)(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated against Histamine N-methyl-transferase |
J Med Chem 28: 478-82 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1DZS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Histamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated against Histamine N-methyl-transferase |
J Med Chem 28: 478-82 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1DZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated against Histamine N-methyl-transferase |
J Med Chem 28: 478-82 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1DZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phenylethanolamine N-methyltransferase
(Bos taurus (bovine)) | BDBM50026333
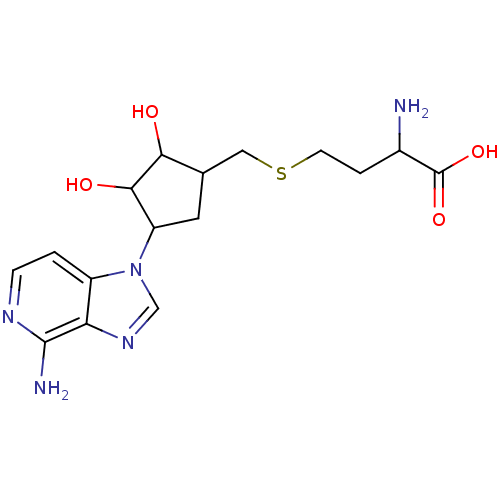
(2-Amino-4-[4-(4-amino-imidazo[4,5-c]pyridin-1-yl)-...)Show SMILES NC(CCSCC1CC(C(O)C1O)n1cnc2c(N)nccc12)C(O)=O Show InChI InChI=1S/C16H23N5O4S/c17-9(16(24)25)2-4-26-6-8-5-11(14(23)13(8)22)21-7-20-12-10(21)1-3-19-15(12)18/h1,3,7-9,11,13-14,22-23H,2,4-6,17H2,(H2,18,19)(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated against PNMT |
J Med Chem 28: 478-82 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1DZS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50026329
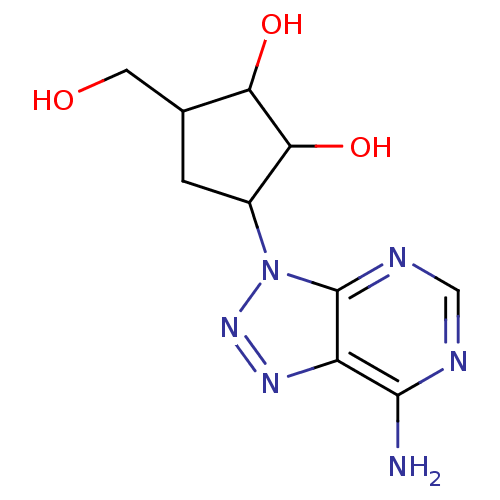
(3-(7-Amino-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5...)Show InChI InChI=1S/C10H14N6O3/c11-9-6-10(13-3-12-9)16(15-14-6)5-1-4(2-17)7(18)8(5)19/h3-5,7-8,17-19H,1-2H2,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against S-adenosyl-homocysteine hydrolase |
J Med Chem 28: 471-7 (1985)
BindingDB Entry DOI: 10.7270/Q21C1XF3 |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Bos taurus (bovine)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated against PNMT |
J Med Chem 28: 478-82 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1DZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phenylethanolamine N-methyltransferase
(Bos taurus (bovine)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated against PNMT |
J Med Chem 28: 478-82 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1DZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chitinase B
(Serratia marcescens) | BDBM10853
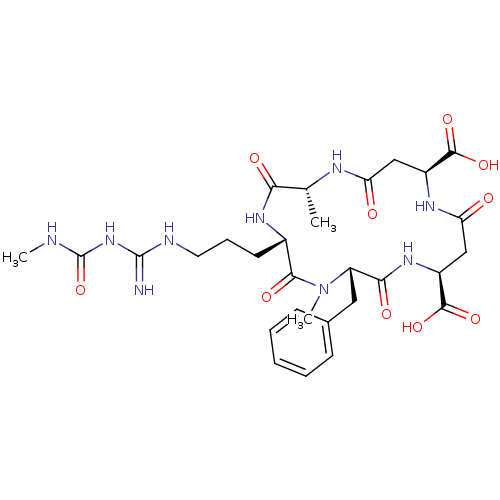
((2R,5S,8S,11S,15S)-8-benzyl-2,7-dimethyl-5-[3-({[(...)Show SMILES CNC(=O)NC(=N)NCCC[C@@H]1NC(=O)[C@@H](C)NC(=O)C[C@H](NC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)N(C)C1=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C29H41N9O10/c1-15-23(41)35-17(10-7-11-32-28(30)37-29(48)31-2)25(43)38(3)20(12-16-8-5-4-6-9-16)24(42)36-19(27(46)47)14-22(40)34-18(26(44)45)13-21(39)33-15/h4-6,8-9,15,17-20H,7,10-14H2,1-3H3,(H,33,39)(H,34,40)(H,35,41)(H,36,42)(H,44,45)(H,46,47)(H4,30,31,32,37,48)/t15-,17+,18+,19+,20+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.30E+4 | -26.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
University of Dundee
| Assay Description
The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... |
Chem Biol 12: 65-76 (2005)
Article DOI: 10.1016/j.chembiol.2004.10.013
BindingDB Entry DOI: 10.7270/Q23F4MV6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human ABL using EAIYAAPFAKKK as substrate in presence of [gamma-33P]ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50184767

(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human YES using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P] ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human FYN using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P]ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184776
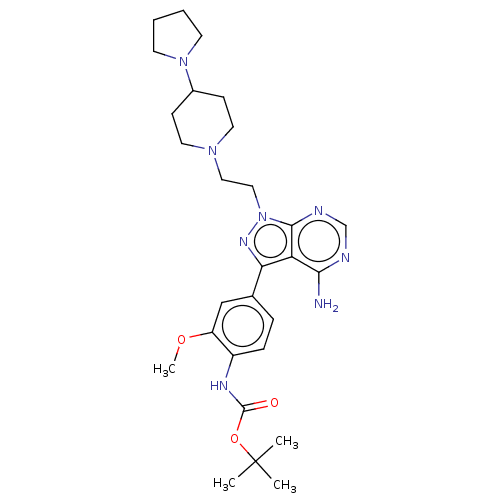
(CHEMBL3824233 | US10294227, Code 518)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N2CCCC2)c2ncnc(N)c12 Show InChI InChI=1S/C28H40N8O3/c1-28(2,3)39-27(37)32-21-8-7-19(17-22(21)38-4)24-23-25(29)30-18-31-26(23)36(33-24)16-15-34-13-9-20(10-14-34)35-11-5-6-12-35/h7-8,17-18,20H,5-6,9-16H2,1-4H3,(H,32,37)(H2,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human YES using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P] ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184767

(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184777
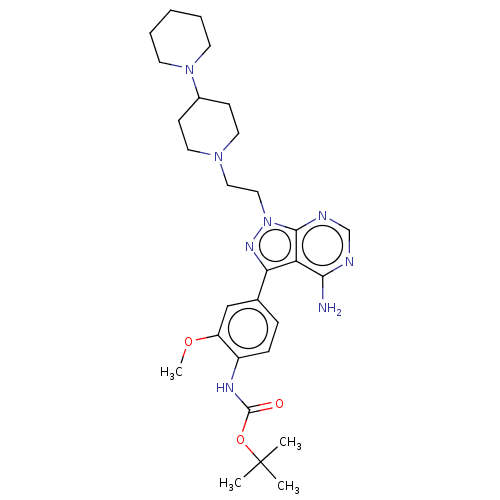
(CHEMBL3823104 | US10294227, Code 519)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N2CCCCC2)c2ncnc(N)c12 Show InChI InChI=1S/C29H42N8O3/c1-29(2,3)40-28(38)33-22-9-8-20(18-23(22)39-4)25-24-26(30)31-19-32-27(24)37(34-25)17-16-35-14-10-21(11-15-35)36-12-6-5-7-13-36/h8-9,18-19,21H,5-7,10-17H2,1-4H3,(H,33,38)(H2,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length AXL (R497 to Y821 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as substr... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length FLT3 (V592 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
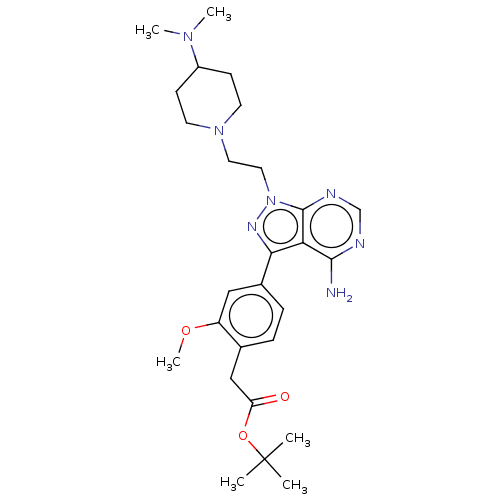
(Homo sapiens (Human)) | BDBM50184786
(CHEMBL3824116 | US10294227, Code 565)Show SMILES COc1cc(ccc1CC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C27H39N7O3/c1-27(2,3)37-22(35)16-18-7-8-19(15-21(18)36-6)24-23-25(28)29-17-30-26(23)34(31-24)14-13-33-11-9-20(10-12-33)32(4)5/h7-8,15,17,20H,9-14,16H2,1-6H3,(H2,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184785
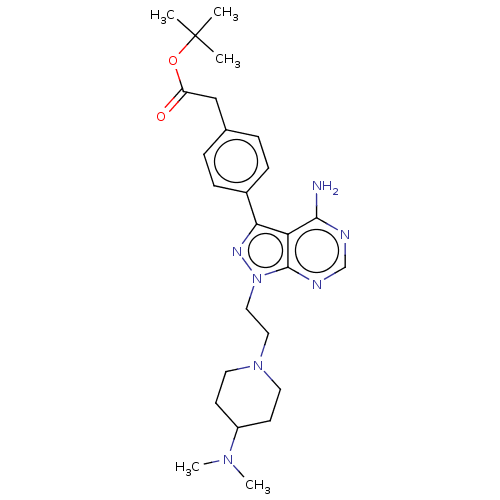
(CHEMBL3823620 | US10294227, Code 553)Show SMILES CN(C)C1CCN(CCn2nc(-c3ccc(CC(=O)OC(C)(C)C)cc3)c3c(N)ncnc23)CC1 Show InChI InChI=1S/C26H37N7O2/c1-26(2,3)35-21(34)16-18-6-8-19(9-7-18)23-22-24(27)28-17-29-25(22)33(30-23)15-14-32-12-10-20(11-13-32)31(4)5/h6-9,17,20H,10-16H2,1-5H3,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length FLT3 (V592 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 D835Y mutant (Q580 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as su... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50184767

(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human FYN using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P]ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384576

(CHEMBL2036808)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(F)cc1 |r,wU:13.12,wD:16.16,(54.06,-16.98,;54.06,-18.52,;55.39,-19.29,;55.39,-20.83,;56.72,-21.6,;58.06,-20.83,;58.06,-19.29,;59.39,-18.52,;60.72,-19.28,;62.2,-18.8,;63.11,-20.06,;62.2,-21.31,;62.67,-22.78,;61.64,-23.92,;62.12,-25.38,;61.08,-26.52,;59.58,-26.2,;58.54,-27.34,;59.11,-24.73,;60.14,-23.59,;60.72,-20.83,;59.39,-21.6,;62.67,-17.34,;64.18,-17.02,;64.66,-15.56,;63.63,-14.41,;64.1,-12.95,;62.11,-14.74,;61.64,-16.2,)| Show InChI InChI=1S/C22H29FN6/c1-2-3-12-25-22-26-13-19-20(16-6-8-17(23)9-7-16)28-29(21(19)27-22)14-15-4-10-18(24)11-5-15/h6-9,13,15,18H,2-5,10-12,14,24H2,1H3,(H,25,26,27)/t15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of MER (unknown origin) |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length RET (E713 to D1014 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184781
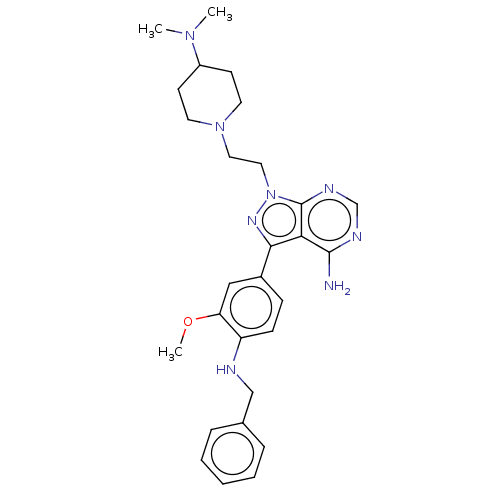
(CHEMBL3823543 | US10294227, Code 533)Show SMILES COc1cc(ccc1NCc1ccccc1)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C28H36N8O/c1-34(2)22-11-13-35(14-12-22)15-16-36-28-25(27(29)31-19-32-28)26(33-36)21-9-10-23(24(17-21)37-3)30-18-20-7-5-4-6-8-20/h4-10,17,19,22,30H,11-16,18H2,1-3H3,(H2,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 D835Y mutant (Q580 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as su... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Vitamin K epoxide reductase complex subunit 1
(Homo sapiens (Human)) | BDBM50535485
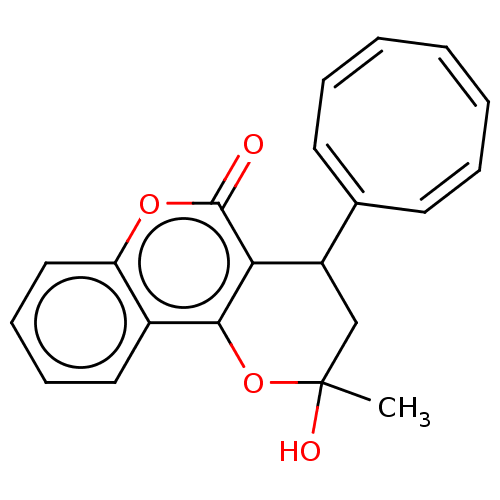
(CHEMBL4594145)Show SMILES CC1(O)CC(c2c(O1)c1ccccc1oc2=O)/C1=C/C=C\C=C/C=C\1 |c:22,24,26,t:20| Show InChI InChI=1S/C21H18O4/c1-21(23)13-16(14-9-5-3-2-4-6-10-14)18-19(25-21)15-11-7-8-12-17(15)24-20(18)22/h2-12,16,23H,13H2,1H3/b3-2-,4-2-,5-3-,6-4-,9-5-,10-6-,14-9+,14-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of VKOR (unknown origin) in HEK293 expressing FIXgla-PC incubated for 48 hrs by ELISA |
Bioorg Med Chem Lett 29: 1954-1956 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.039
BindingDB Entry DOI: 10.7270/Q2TQ652J |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length Aurora A (E122 to K401 residues) expressed in mammalian expression system using poly [Glu, Try] 4:1 as substrate i... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50455346

(CHEMBL4218175)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H31N7/c1-24-23-25-15-20-21(27-30(22(20)26-23)16-17-5-3-4-6-17)18-7-9-19(10-8-18)29-13-11-28(2)12-14-29/h7-10,15,17H,3-6,11-14,16H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length RET (E713 to D1014 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human partial length FLT3 ITD mutant expressed in bacterial expression system using poly [Glu, Try] 4:1 as substrate in presence of [ga... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50172079

(CHEMBL3809489)Show SMILES Nc1nc(Nc2ccc3CC[C@@H](CCc3c2)N2CCCC2)nn1-c1cc2CCCc3ccccc3-c2nn1 |r| Show InChI InChI=1S/C30H34N8/c31-29-33-30(32-24-13-10-20-11-14-25(15-12-22(20)18-24)37-16-3-4-17-37)36-38(29)27-19-23-8-5-7-21-6-1-2-9-26(21)28(23)35-34-27/h1-2,6,9-10,13,18-19,25H,3-5,7-8,11-12,14-17H2,(H3,31,32,33,36)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length VEGFR2 (R787 to P1253 residues) expressed in mammalian expression system using poly [Glu, Try] 4:1 as su... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50455354

(CHEMBL4208852)Show SMILES CNc1ncc2c(nn(CC3CCCC3)c2n1)-c1ccc(nc1)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N8/c1-23-22-25-14-18-20(27-30(21(18)26-22)15-16-5-3-4-6-16)17-7-8-19(24-13-17)29-11-9-28(2)10-12-29/h7-8,13-14,16H,3-6,9-12,15H2,1-2H3,(H,23,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length FLT3 (V592 to Y969 residues) expressed in bacterial expression system using poly [Glu, Try] 4:1 as subst... |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Vitamin K epoxide reductase complex subunit 1
(Homo sapiens (Human)) | BDBM50343352
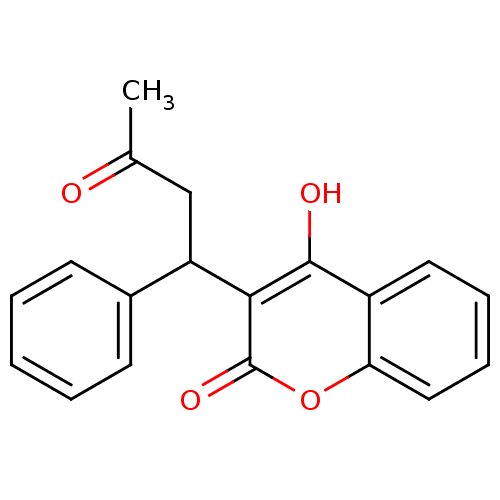
(2-hydroxy-3-(3-oxo-1-phenylbutyl)-4H-chromen-4-one...)Show InChI InChI=1S/C19H16O4/c1-12(20)11-15(13-7-3-2-4-8-13)17-18(21)14-9-5-6-10-16(14)23-19(17)22/h2-10,15,21H,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of VKOR (unknown origin) in HEK293 expressing FIXgla-PC incubated for 48 hrs by ELISA |
Bioorg Med Chem Lett 29: 1954-1956 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.039
BindingDB Entry DOI: 10.7270/Q2TQ652J |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human PDGFRalpha using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P]ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data