 Found 2927 hits with Last Name = 'hu' and Initial = 'yj'
Found 2927 hits with Last Name = 'hu' and Initial = 'yj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptide deformylase
(Escherichia coli (strain K12)) | BDBM50291695

(CHEMBL84822 | H-PHOSPHONATE DERIVATIVE)Show SMILES CCCC[C@H](OP(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C18H28N3O7P/c1-4-5-6-16(28-29(26)27)18(23)20-15(11-12(2)3)17(22)19-13-7-9-14(10-8-13)21(24)25/h7-10,12,15-16,29H,4-6,11H2,1-3H3,(H,19,22)(H,20,23)(H,26,27)/t15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Peptide deformylase |
Bioorg Med Chem Lett 8: 2479-82 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BTV |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Escherichia coli (strain K12)) | BDBM50071663
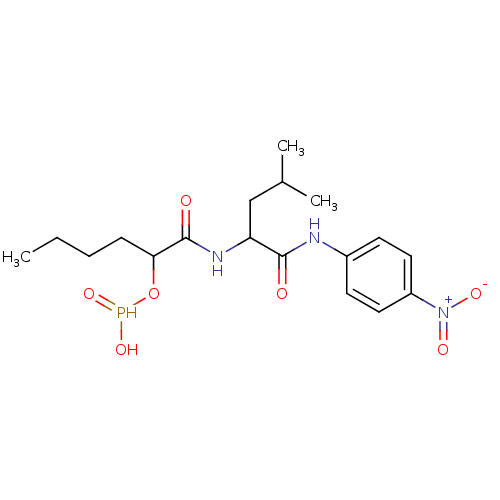
(CHEMBL309477 | H-PHOSPHONATE DERIVATIVE)Show SMILES CCCCC(O[PH+](O)[O-])C(=O)NC(CC(C)C)C(=O)Nc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C18H28N3O7P/c1-4-5-6-16(28-29(26)27)18(23)20-15(11-12(2)3)17(22)19-13-7-9-14(10-8-13)21(24)25/h7-10,12,15-16,26,29H,4-6,11H2,1-3H3,(H,19,22)(H,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Peptide deformylase |
Bioorg Med Chem Lett 8: 2479-82 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BTV |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
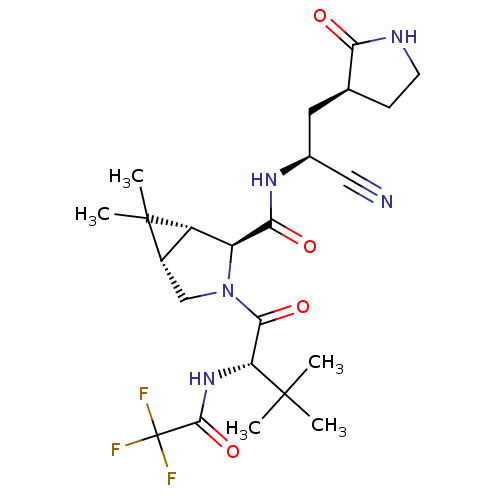
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50507479

(CHEMBL4447260)Show InChI InChI=1S/C18H18N2O2S/c1-21-16-11-15(4-5-18-3-2-10-23-18)12-17(13-16)22-9-8-20-7-6-19-14-20/h2-7,10-14H,8-9H2,1H3/b5-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP1A2 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso... |
ACS Med Chem Lett 9: 1247-1252 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00409
BindingDB Entry DOI: 10.7270/Q2V1284K |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50097721
(CHEMBL1879790 | EN300-11843)Show InChI InChI=1S/C29H38FN5O3S/c1-4-6-7-8-9-10-15-33(3)26(36)20-35-19-24(16-23-17-31-28(38)34(5-2)18-23)27(37)32-29(35)39-21-22-11-13-25(30)14-12-22/h11-14,17-19H,4-10,15-16,20-21H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50108048
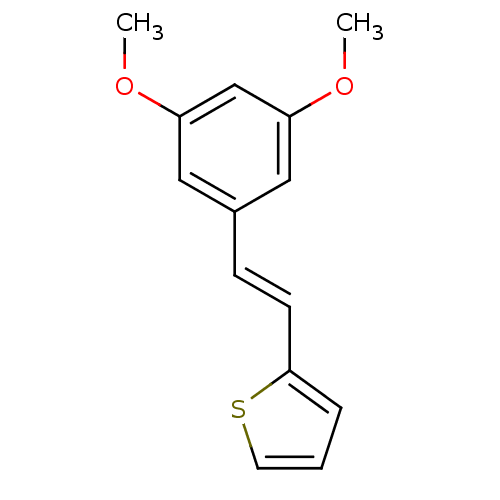
((E)-2-(3,5-dimethoxystyryl)thiophene | 2-[(E)-2-(3...)Show InChI InChI=1S/C14H14O2S/c1-15-12-8-11(9-13(10-12)16-2)5-6-14-4-3-7-17-14/h3-10H,1-2H3/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP1B1 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso... |
ACS Med Chem Lett 9: 1247-1252 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00409
BindingDB Entry DOI: 10.7270/Q2V1284K |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50108048

((E)-2-(3,5-dimethoxystyryl)thiophene | 2-[(E)-2-(3...)Show InChI InChI=1S/C14H14O2S/c1-15-12-8-11(9-13(10-12)16-2)5-6-14-4-3-7-17-14/h3-10H,1-2H3/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of ethoxyresorufin O-deethylation (EROD) in bicistronic bacterial membranes expressing human cytochrome P450 1B1 |
J Med Chem 45: 160-4 (2001)
BindingDB Entry DOI: 10.7270/Q2668CGD |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496902
(CVD-0018409 | PF-07321332 | US11351149, Example 13...)Show SMILES CC(C)(C)[C@H](NC(=O)C(F)(F)F)C(=O)N1C[C@H]2[C@@H]([C@H]1C(=O)N[C@@H](C[C@@H]1CCNC1=O)C#N)C2(C)C Show InChI InChI=1S/C23H32F3N5O4/c1-21(2,3)16(30-20(35)23(24,25)26)19(34)31-10-13-14(22(13,4)5)15(31)18(33)29-12(9-27)8-11-6-7-28-17(11)32/h11-16H,6-8,10H2,1-5H3,(H,28,32)(H,29,33)(H,30,35)/t11-,12-,13-,14-,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27707
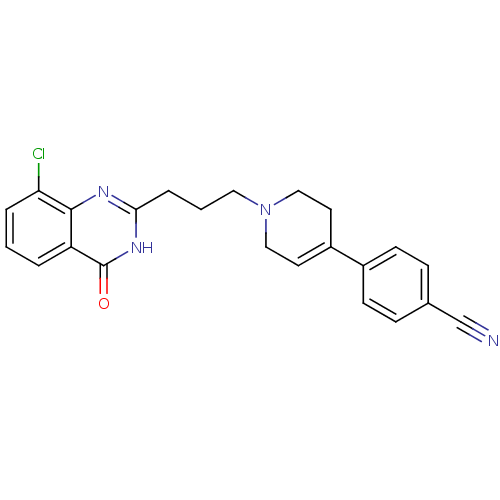
(4-{1-[3-(8-chloro-4-oxo-3,4-dihydroquinazolin-2-yl...)Show SMILES Clc1cccc2c1nc(CCCN1CCC(=CC1)c1ccc(cc1)C#N)[nH]c2=O |c:16| Show InChI InChI=1S/C23H21ClN4O/c24-20-4-1-3-19-22(20)26-21(27-23(19)29)5-2-12-28-13-10-18(11-14-28)17-8-6-16(15-25)7-9-17/h1,3-4,6-10H,2,5,11-14H2,(H,26,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165496
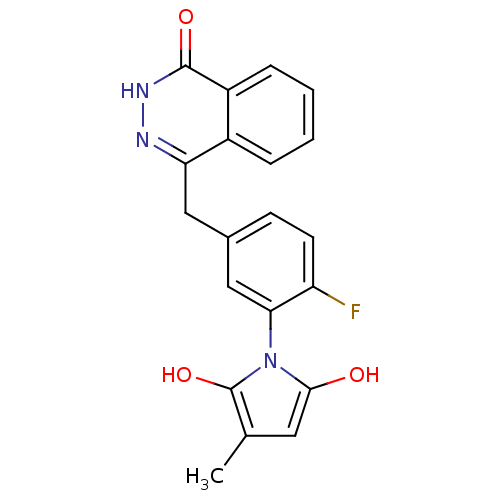
(1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...)Show SMILES Cc1cc(O)n(c1O)-c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F |(2.63,-45.05,;1.1,-44.9,;.32,-43.57,;-1.18,-43.9,;-2.34,-42.88,;-1.33,-45.44,;.08,-46.05,;.41,-47.56,;-2.66,-46.22,;-3.99,-45.46,;-5.31,-46.24,;-6.65,-45.47,;-6.65,-43.93,;-5.32,-43.16,;-5.32,-41.61,;-6.67,-40.84,;-6.68,-39.3,;-8,-41.62,;-9.34,-40.86,;-10.66,-41.63,;-10.67,-43.18,;-9.33,-43.95,;-7.99,-43.17,;-5.31,-47.78,;-3.98,-48.54,;-2.65,-47.77,;-1.31,-48.53,)| Show InChI InChI=1S/C20H16FN3O3/c1-11-8-18(25)24(20(11)27)17-10-12(6-7-15(17)21)9-16-13-4-2-3-5-14(13)19(26)23-22-16/h2-8,10,25,27H,9H2,1H3,(H,23,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50507479

(CHEMBL4447260)Show InChI InChI=1S/C18H18N2O2S/c1-21-16-11-15(4-5-18-3-2-10-23-18)12-17(13-16)22-9-8-20-7-6-19-14-20/h2-7,10-14H,8-9H2,1H3/b5-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP1B1 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso... |
ACS Med Chem Lett 9: 1247-1252 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00409
BindingDB Entry DOI: 10.7270/Q2V1284K |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165488
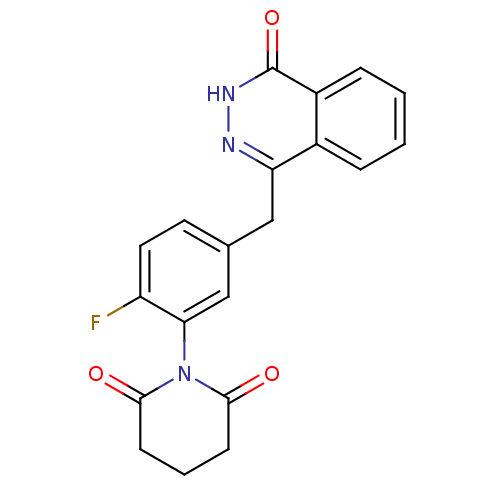
(1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1N1C(=O)CCCC1=O Show InChI InChI=1S/C20H16FN3O3/c21-15-9-8-12(11-17(15)24-18(25)6-3-7-19(24)26)10-16-13-4-1-2-5-14(13)20(27)23-22-16/h1-2,4-5,8-9,11H,3,6-7,10H2,(H,23,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165486
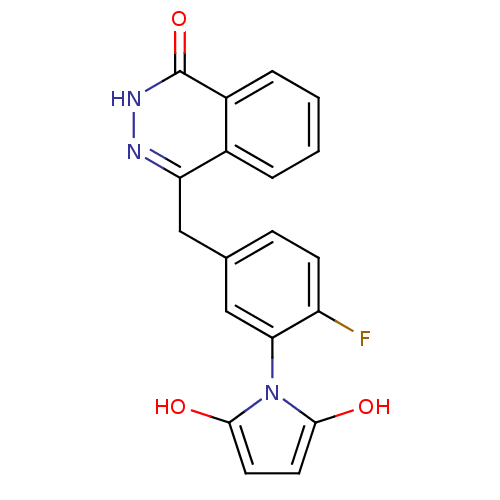
(1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...)Show SMILES Oc1ccc(O)n1-c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F |(35.28,-33.78,;34.95,-32.28,;35.97,-31.13,;35.19,-29.8,;33.69,-30.13,;32.54,-29.11,;33.55,-31.67,;32.22,-32.45,;30.88,-31.69,;29.56,-32.47,;28.22,-31.7,;28.22,-30.16,;29.56,-29.39,;29.55,-27.84,;28.2,-27.07,;28.19,-25.53,;26.87,-27.85,;25.53,-27.09,;24.21,-27.86,;24.21,-29.4,;25.54,-30.17,;26.88,-29.4,;29.56,-34,;30.89,-34.77,;32.23,-33.99,;33.56,-34.76,)| Show InChI InChI=1S/C19H14FN3O3/c20-14-6-5-11(10-16(14)23-17(24)7-8-18(23)25)9-15-12-3-1-2-4-13(12)19(26)22-21-15/h1-8,10,24-25H,9H2,(H,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50108052
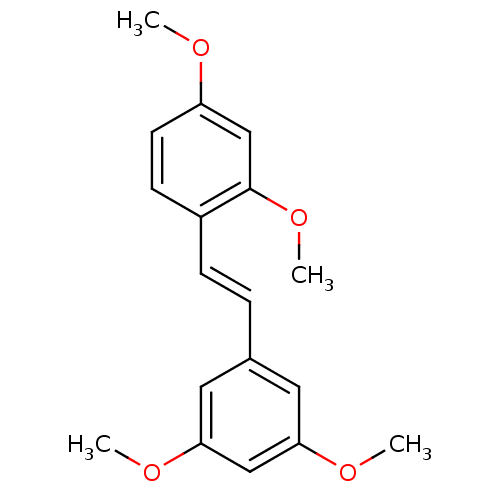
(1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...)Show InChI InChI=1S/C18H20O4/c1-19-15-8-7-14(18(12-15)22-4)6-5-13-9-16(20-2)11-17(10-13)21-3/h5-12H,1-4H3/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of ethoxyresorufin O-deethylation (EROD) in bicistronic bacterial membranes expressing human cytochrome P450 1B1 |
J Med Chem 45: 160-4 (2001)
BindingDB Entry DOI: 10.7270/Q2668CGD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50108052

(1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...)Show InChI InChI=1S/C18H20O4/c1-19-15-8-7-14(18(12-15)22-4)6-5-13-9-16(20-2)11-17(10-13)21-3/h5-12H,1-4H3/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP1B1 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso... |
ACS Med Chem Lett 9: 1247-1252 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00409
BindingDB Entry DOI: 10.7270/Q2V1284K |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165498
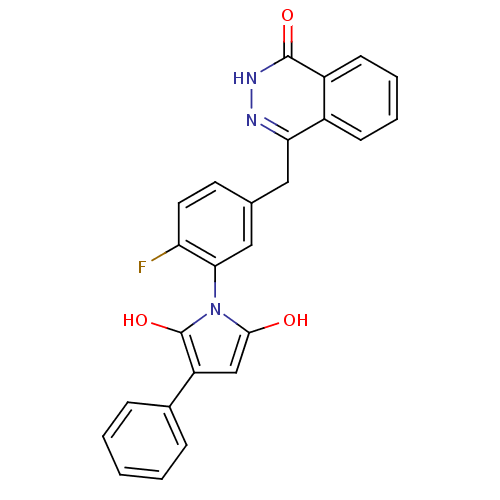
(1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...)Show SMILES Oc1cc(c(O)n1-c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)-c1ccccc1 |(14.66,-43.53,;15.81,-44.55,;17.31,-44.22,;18.09,-45.55,;17.07,-46.7,;17.4,-48.2,;15.67,-46.09,;14.34,-46.87,;13,-46.11,;11.68,-46.89,;10.34,-46.12,;10.34,-44.58,;11.68,-43.8,;11.67,-42.26,;10.32,-41.48,;10.31,-39.94,;8.99,-42.27,;7.65,-41.51,;6.33,-42.28,;6.33,-43.82,;7.66,-44.59,;9,-43.82,;11.68,-48.42,;13.01,-49.19,;14.35,-48.41,;15.68,-49.18,;19.63,-45.56,;20.38,-46.9,;21.92,-46.92,;22.71,-45.59,;21.94,-44.24,;20.41,-44.23,)| Show InChI InChI=1S/C25H18FN3O3/c26-20-11-10-15(12-21-17-8-4-5-9-18(17)24(31)28-27-21)13-22(20)29-23(30)14-19(25(29)32)16-6-2-1-3-7-16/h1-11,13-14,30,32H,12H2,(H,28,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50108048

((E)-2-(3,5-dimethoxystyryl)thiophene | 2-[(E)-2-(3...)Show InChI InChI=1S/C14H14O2S/c1-15-12-8-11(9-13(10-12)16-2)5-6-14-4-3-7-17-14/h3-10H,1-2H3/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of ethoxyresorufin O-deethylation (EROD) in bicistronic bacterial membranes expressing human cytochrome P450 1A2 |
J Med Chem 45: 160-4 (2001)
BindingDB Entry DOI: 10.7270/Q2668CGD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50108048

((E)-2-(3,5-dimethoxystyryl)thiophene | 2-[(E)-2-(3...)Show InChI InChI=1S/C14H14O2S/c1-15-12-8-11(9-13(10-12)16-2)5-6-14-4-3-7-17-14/h3-10H,1-2H3/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP1A2 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso... |
ACS Med Chem Lett 9: 1247-1252 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00409
BindingDB Entry DOI: 10.7270/Q2V1284K |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165478
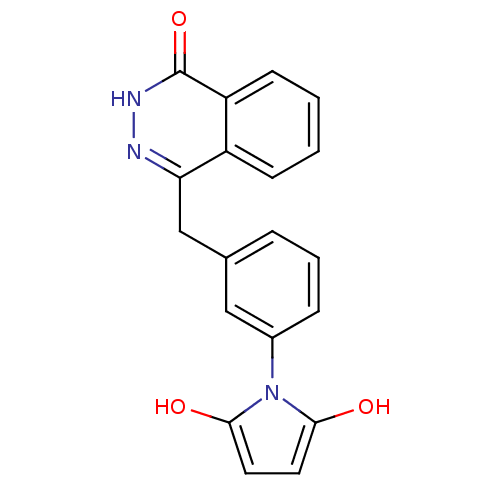
(1-(3-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)phe...)Show SMILES Oc1ccc(O)n1-c1cccc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C19H15N3O3/c23-17-8-9-18(24)22(17)13-5-3-4-12(10-13)11-16-14-6-1-2-7-15(14)19(25)21-20-16/h1-10,23-24H,11H2,(H,21,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50014323
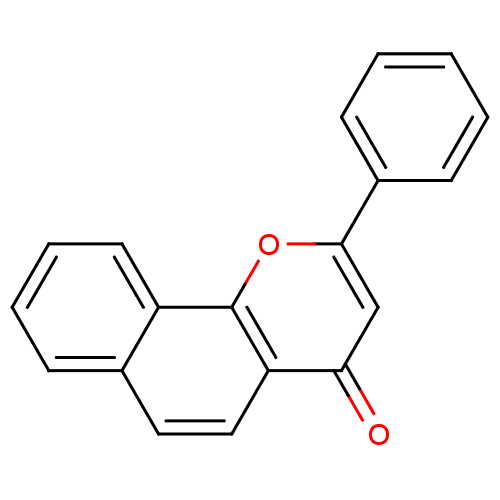
(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP1B1 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso... |
ACS Med Chem Lett 9: 1247-1252 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00409
BindingDB Entry DOI: 10.7270/Q2V1284K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27708
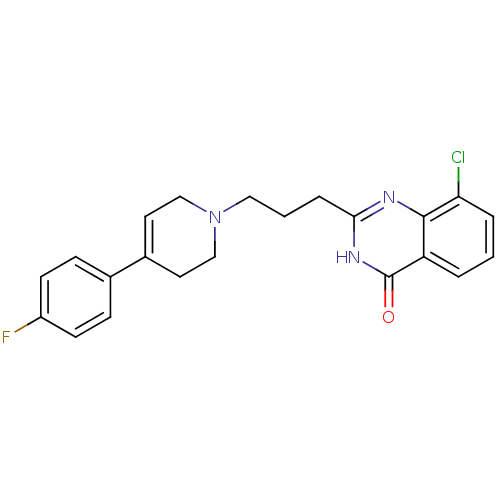
(8-chloro-2-{3-[4-(4-fluorophenyl)-1,2,3,6-tetrahyd...)Show SMILES Fc1ccc(cc1)C1=CCN(CCCc2nc3c(Cl)cccc3c(=O)[nH]2)CC1 |t:8| Show InChI InChI=1S/C22H21ClFN3O/c23-19-4-1-3-18-21(19)25-20(26-22(18)28)5-2-12-27-13-10-16(11-14-27)15-6-8-17(24)9-7-15/h1,3-4,6-10H,2,5,11-14H2,(H,25,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Mas-related G-protein coupled receptor member X1
(Homo sapiens (Human)) | BDBM50340753
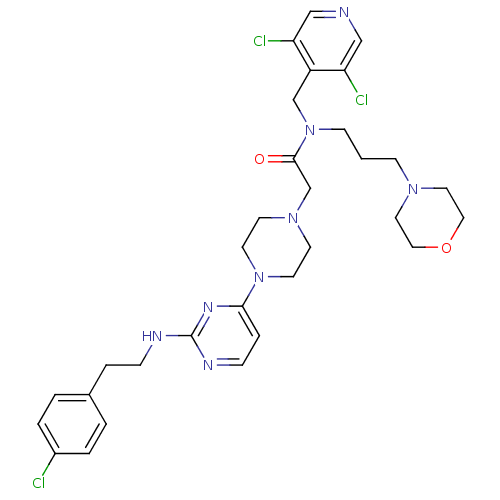
(2-(4-(2-(4-chlorophenethylamino)pyrimidin-4-yl)pip...)Show SMILES Clc1ccc(CCNc2nccc(n2)N2CCN(CC(=O)N(CCCN3CCOCC3)Cc3c(Cl)cncc3Cl)CC2)cc1 Show InChI InChI=1S/C31H39Cl3N8O2/c32-25-4-2-24(3-5-25)6-8-36-31-37-9-7-29(38-31)41-14-12-40(13-15-41)23-30(43)42(11-1-10-39-16-18-44-19-17-39)22-26-27(33)20-35-21-28(26)34/h2-5,7,9,20-21H,1,6,8,10-19,22-23H2,(H,36,37,38) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Antagonist activity at SNSR4 in human HEK293 cells by FLIPR assay |
Bioorg Med Chem Lett 21: 2102-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.138
BindingDB Entry DOI: 10.7270/Q2D21XW7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27709
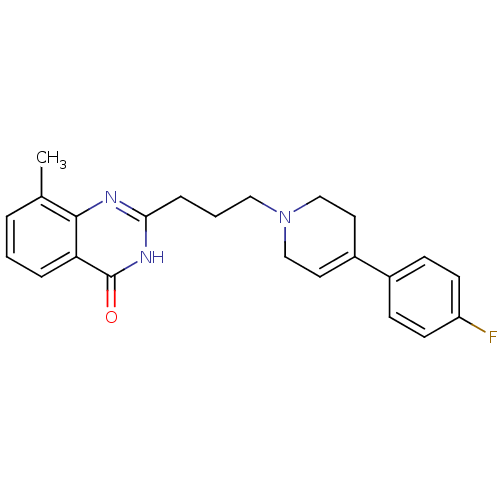
(2-{3-[4-(4-fluorophenyl)-1,2,3,6-tetrahydropyridin...)Show SMILES Cc1cccc2c1nc(CCCN1CCC(=CC1)c1ccc(F)cc1)[nH]c2=O |c:16| Show InChI InChI=1S/C23H24FN3O/c1-16-4-2-5-20-22(16)25-21(26-23(20)28)6-3-13-27-14-11-18(12-15-27)17-7-9-19(24)10-8-17/h2,4-5,7-11H,3,6,12-15H2,1H3,(H,25,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165472
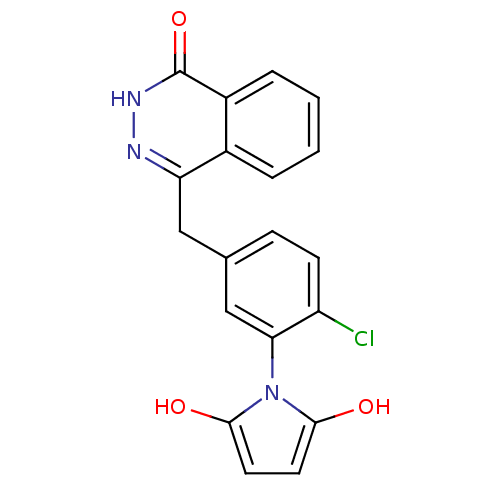
(1-(2-chloro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...)Show SMILES Oc1ccc(O)n1-c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1Cl |(18.5,-33.78,;18.16,-32.28,;19.19,-31.13,;18.41,-29.8,;16.9,-30.13,;15.75,-29.11,;16.76,-31.67,;15.43,-32.45,;14.1,-31.69,;12.77,-32.47,;11.44,-31.7,;11.43,-30.16,;12.77,-29.39,;12.76,-27.84,;11.42,-27.07,;11.41,-25.53,;10.09,-27.85,;8.75,-27.09,;7.42,-27.86,;7.42,-29.4,;8.75,-30.17,;10.1,-29.4,;12.77,-34,;14.11,-34.77,;15.44,-33.99,;16.78,-34.76,)| Show InChI InChI=1S/C19H14ClN3O3/c20-14-6-5-11(10-16(14)23-17(24)7-8-18(23)25)9-15-12-3-1-2-4-13(12)19(26)22-21-15/h1-8,10,24-25H,9H2,(H,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Mas-related G-protein coupled receptor member X1
(Homo sapiens (Human)) | BDBM50340743

(2-(4-(2-(4-chlorophenethylamino)pyrimidin-4-yl)pip...)Show SMILES Clc1ccc(CCNc2nccc(n2)N2CCN(CC(=O)N(Cc3ccco3)Cc3ccncc3)CC2)cc1 Show InChI InChI=1S/C29H32ClN7O2/c30-25-5-3-23(4-6-25)9-13-32-29-33-14-10-27(34-29)36-17-15-35(16-18-36)22-28(38)37(21-26-2-1-19-39-26)20-24-7-11-31-12-8-24/h1-8,10-12,14,19H,9,13,15-18,20-22H2,(H,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Antagonist activity at SNSR4 in human HEK293 cells by FLIPR assay |
Bioorg Med Chem Lett 21: 2102-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.138
BindingDB Entry DOI: 10.7270/Q2D21XW7 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM627791
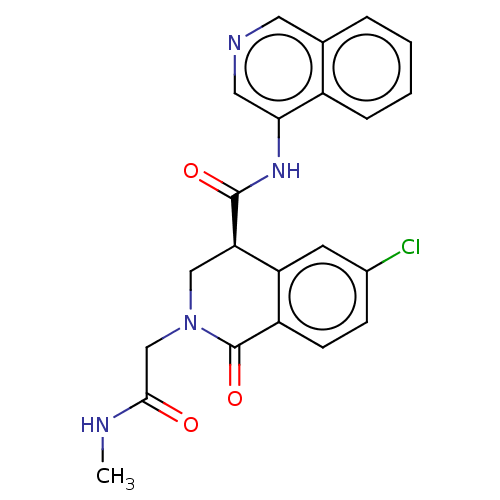
(CVD-0019230)Show SMILES CNC(=O)CN1C[C@@H](C(=O)Nc2cncc3ccccc23)c2cc(Cl)ccc2C1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165477
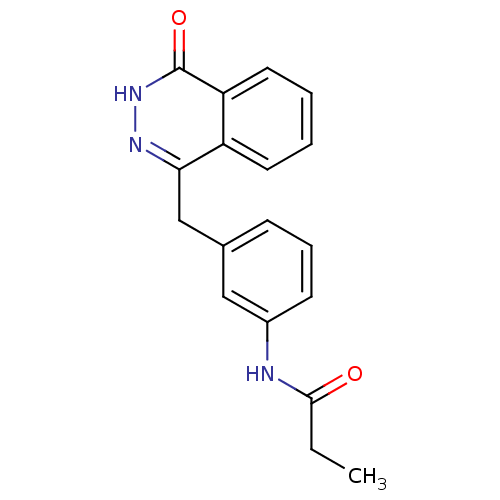
(CHEMBL371425 | N-(3-((4-oxo-3,4-dihydrophthalazin-...)Show InChI InChI=1S/C18H17N3O2/c1-2-17(22)19-13-7-5-6-12(10-13)11-16-14-8-3-4-9-15(14)18(23)21-20-16/h3-10H,2,11H2,1H3,(H,19,22)(H,21,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against angiotensin converting enzyme (ACE) |
Bioorg Med Chem Lett 4: 2799-2804 (1994)
Article DOI: 10.1016/S0960-894X(01)80597-7
BindingDB Entry DOI: 10.7270/Q2NP24XX |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM495599

(CVD-0016335 | STE-KUL-d79e3d6a-3)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](NC(=O)CCCC#C)C(C)(C)C)C(=O)NN(CCC(N)=O)C(=O)CCl Show InChI InChI=1S/C23H38ClN5O5/c1-7-8-9-10-18(31)27-20(23(4,5)6)22(34)26-16(13-15(2)3)21(33)28-29(19(32)14-24)12-11-17(25)30/h1,15-16,20H,8-14H2,2-6H3,(H2,25,30)(H,26,34)(H,27,31)(H,28,33)/t16-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27705
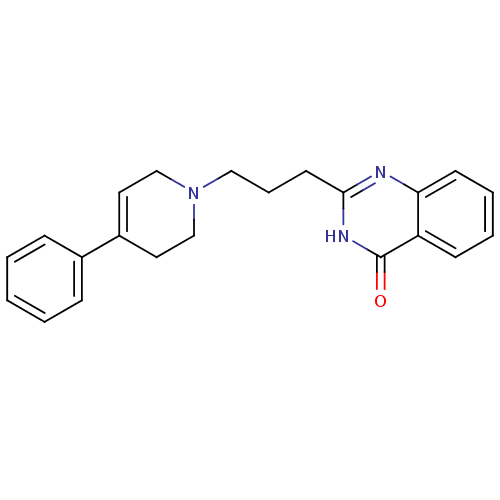
(2-[3-(4-phenyl-1,2,3,6-tetrahydropyridin-1-yl)prop...)Show SMILES O=c1[nH]c(CCCN2CCC(=CC2)c2ccccc2)nc2ccccc12 |c:10| Show InChI InChI=1S/C22H23N3O/c26-22-19-9-4-5-10-20(19)23-21(24-22)11-6-14-25-15-12-18(13-16-25)17-7-2-1-3-8-17/h1-5,7-10,12H,6,11,13-16H2,(H,23,24,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50379718

(CHEMBL2011127)Show SMILES CC(C)N1Cc2c(nc(nc2N(C)Cc2cc3ccccc3cn2)N2CCN(CC2)C(C)=O)C1=O Show InChI InChI=1S/C26H31N7O2/c1-17(2)33-16-22-23(25(33)35)28-26(32-11-9-31(10-12-32)18(3)34)29-24(22)30(4)15-21-13-19-7-5-6-8-20(19)14-27-21/h5-8,13-14,17H,9-12,15-16H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X3 receptor expressed in RLE cells assessed as inhibition of (alpha,beta)me-ATP-induced intracellular calcium level by... |
Bioorg Med Chem Lett 22: 2565-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.124
BindingDB Entry DOI: 10.7270/Q29W0GGF |
More data for this
Ligand-Target Pair | |
Mas-related G-protein coupled receptor member X1
(Homo sapiens (Human)) | BDBM50340744
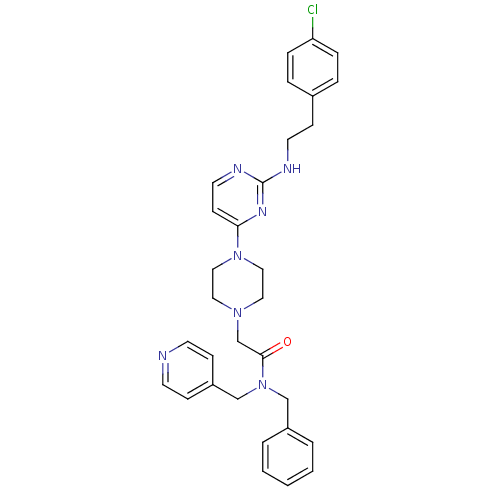
(CHEMBL1762699 | N-benzyl-2-(4-(2-(4-chlorophenethy...)Show SMILES Clc1ccc(CCNc2nccc(n2)N2CCN(CC(=O)N(Cc3ccccc3)Cc3ccncc3)CC2)cc1 Show InChI InChI=1S/C31H34ClN7O/c32-28-8-6-25(7-9-28)12-16-34-31-35-17-13-29(36-31)38-20-18-37(19-21-38)24-30(40)39(22-26-4-2-1-3-5-26)23-27-10-14-33-15-11-27/h1-11,13-15,17H,12,16,18-24H2,(H,34,35,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Antagonist activity at SNSR4 in human HEK293 cells by FLIPR assay |
Bioorg Med Chem Lett 21: 2102-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.138
BindingDB Entry DOI: 10.7270/Q2D21XW7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50220868
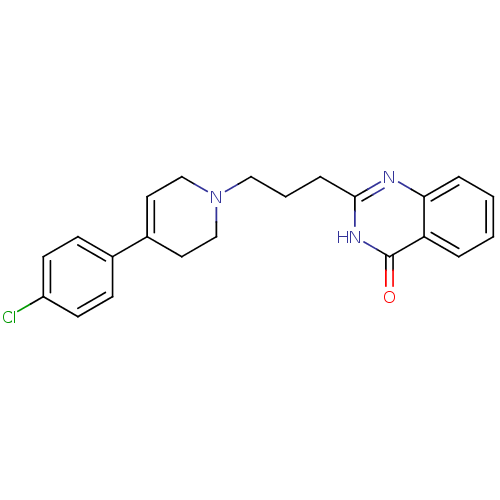
(2-(3-(4-(4-chlorophenyl)-5,6-dihydropyridin-1(2H)-...)Show SMILES Clc1ccc(cc1)C1=CCN(CCCc2nc3ccccc3c(=O)[nH]2)CC1 |t:8| Show InChI InChI=1S/C22H22ClN3O/c23-18-9-7-16(8-10-18)17-11-14-26(15-12-17)13-3-6-21-24-20-5-2-1-4-19(20)22(27)25-21/h1-2,4-5,7-11H,3,6,12-15H2,(H,24,25,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Mas-related G-protein coupled receptor member X1
(Homo sapiens (Human)) | BDBM50340745
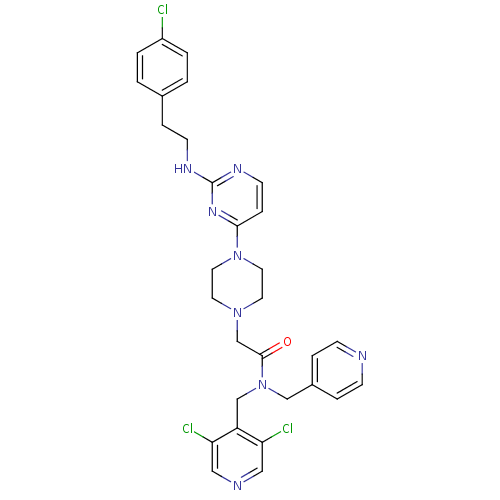
(2-(4-(2-(4-chlorophenethylamino)pyrimidin-4-yl)pip...)Show SMILES Clc1ccc(CCNc2nccc(n2)N2CCN(CC(=O)N(Cc3ccncc3)Cc3c(Cl)cncc3Cl)CC2)cc1 Show InChI InChI=1S/C30H31Cl3N8O/c31-24-3-1-22(2-4-24)7-11-36-30-37-12-8-28(38-30)40-15-13-39(14-16-40)21-29(42)41(19-23-5-9-34-10-6-23)20-25-26(32)17-35-18-27(25)33/h1-6,8-10,12,17-18H,7,11,13-16,19-21H2,(H,36,37,38) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Antagonist activity at SNSR4 in human HEK293 cells by FLIPR assay |
Bioorg Med Chem Lett 21: 2102-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.138
BindingDB Entry DOI: 10.7270/Q2D21XW7 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50379717
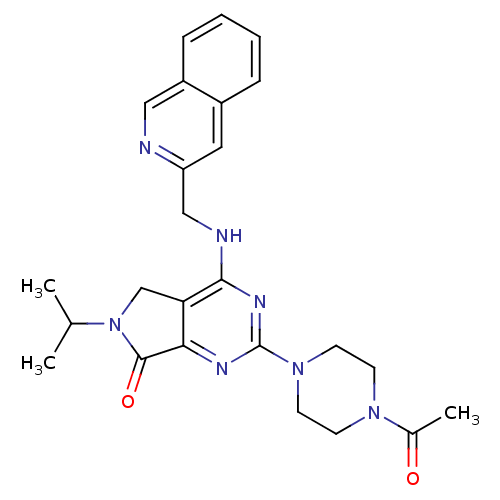
(CHEMBL2011126)Show SMILES CC(C)N1Cc2c(nc(nc2NCc2cc3ccccc3cn2)N2CCN(CC2)C(C)=O)C1=O Show InChI InChI=1S/C25H29N7O2/c1-16(2)32-15-21-22(24(32)34)28-25(31-10-8-30(9-11-31)17(3)33)29-23(21)27-14-20-12-18-6-4-5-7-19(18)13-26-20/h4-7,12-13,16H,8-11,14-15H2,1-3H3,(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X3 receptor expressed in RLE cells assessed as inhibition of (alpha,beta)me-ATP-induced intracellular calcium level by... |
Bioorg Med Chem Lett 22: 2565-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.124
BindingDB Entry DOI: 10.7270/Q29W0GGF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50379716
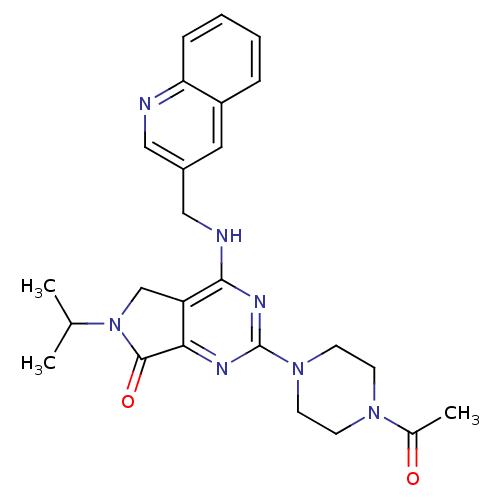
(CHEMBL2011125)Show SMILES CC(C)N1Cc2c(nc(nc2NCc2cnc3ccccc3c2)N2CCN(CC2)C(C)=O)C1=O Show InChI InChI=1S/C25H29N7O2/c1-16(2)32-15-20-22(24(32)34)28-25(31-10-8-30(9-11-31)17(3)33)29-23(20)27-14-18-12-19-6-4-5-7-21(19)26-13-18/h4-7,12-13,16H,8-11,14-15H2,1-3H3,(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X3 receptor expressed in RLE cells assessed as inhibition of (alpha,beta)me-ATP-induced intracellular calcium level by... |
Bioorg Med Chem Lett 22: 2565-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.124
BindingDB Entry DOI: 10.7270/Q29W0GGF |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50120265
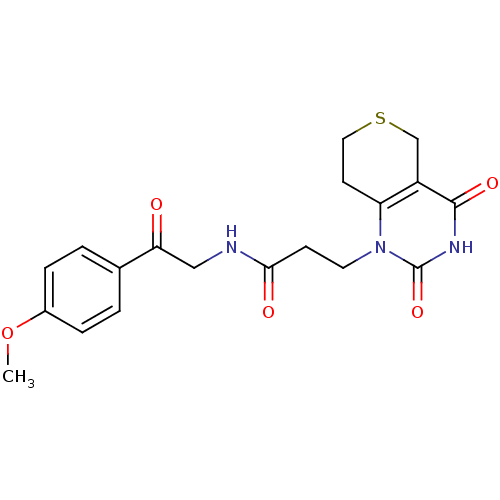
(3-(2,4-Dioxo-3,4,7,8-tetrahydro-2H,5H-thiopyrano[4...)Show SMILES COc1ccc(cc1)C(=O)CNC(=O)CCn1c2CCSCc2c(=O)[nH]c1=O Show InChI InChI=1S/C19H21N3O5S/c1-27-13-4-2-12(3-5-13)16(23)10-20-17(24)6-8-22-15-7-9-28-11-14(15)18(25)21-19(22)26/h2-5H,6-11H2,1H3,(H,20,24)(H,21,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50255501

(3-(2,4-dioxo-2,3,4,5,7,8-hexahydro-1H-thiopyrano[4...)Show SMILES O=C(CCn1c2CCSCc2c(=O)[nH]c1=O)NCC(=O)c1ccc(cc1)-c1ccnnc1 Show InChI InChI=1S/C22H21N5O4S/c28-19(15-3-1-14(2-4-15)16-5-8-24-25-11-16)12-23-20(29)6-9-27-18-7-10-32-13-17(18)21(30)26-22(27)31/h1-5,8,11H,6-7,9-10,12-13H2,(H,23,29)(H,26,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50255360
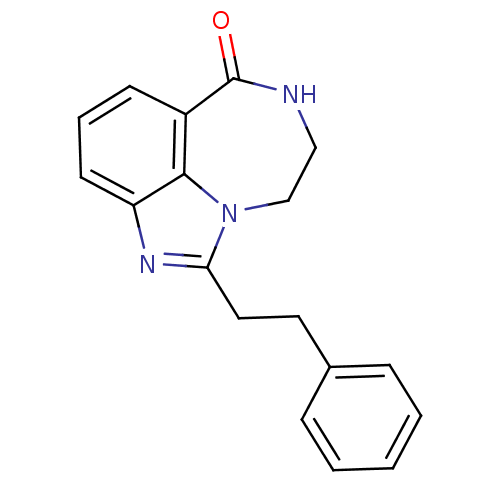
(1-Phenethyl-8,9-dihydro-7H-2,7,9a-triaza-benzo[cd]...)Show InChI InChI=1S/C18H17N3O/c22-18-14-7-4-8-15-17(14)21(12-11-19-18)16(20-15)10-9-13-5-2-1-3-6-13/h1-8H,9-12H2,(H,19,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM627915
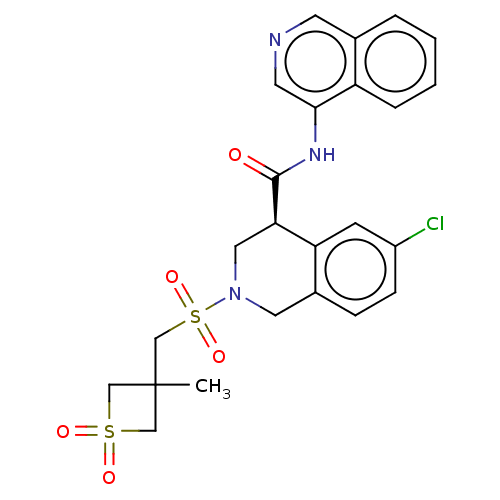
(CVD-0019273)Show SMILES CC1(CS(=O)(=O)N2C[C@@H](C(=O)Nc3cncc4ccccc34)c3cc(Cl)ccc3C2)CS(=O)(=O)C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50255630
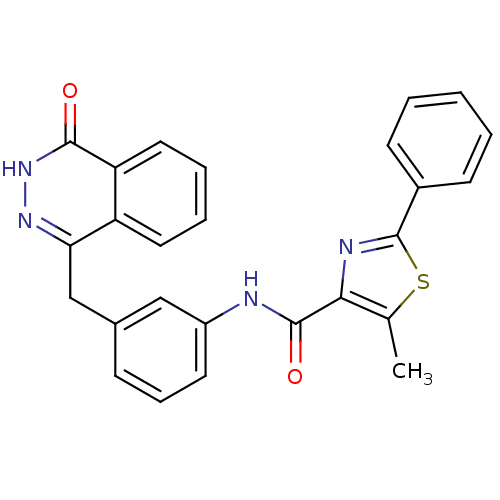
(5-methyl-N-(3-((4-oxo-3,4-dihydrophthalazin-1-yl)m...)Show SMILES Cc1sc(nc1C(=O)Nc1cccc(Cc2n[nH]c(=O)c3ccccc23)c1)-c1ccccc1 Show InChI InChI=1S/C26H20N4O2S/c1-16-23(28-26(33-16)18-9-3-2-4-10-18)25(32)27-19-11-7-8-17(14-19)15-22-20-12-5-6-13-21(20)24(31)30-29-22/h2-14H,15H2,1H3,(H,27,32)(H,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM625991
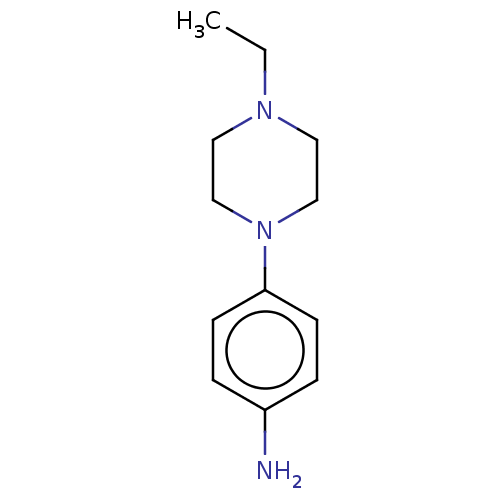
(EN300-11760) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165484
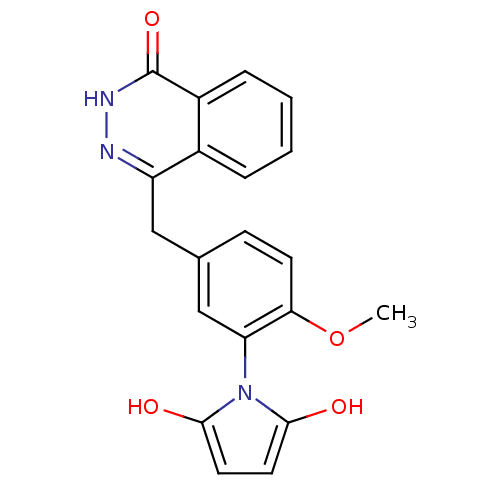
(1-(2-methoxy-5-((4-oxo-3,4-dihydrophthalazin-1-yl)...)Show SMILES COc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1-n1c(O)ccc1O |(-3.66,1.78,;-4.47,.48,;-3.75,-.88,;-2.22,-.93,;-1.48,-2.29,;-2.3,-3.61,;-1.58,-4.97,;-.04,-5.02,;.68,-6.38,;2.21,-6.44,;3.03,-5.13,;4.57,-5.19,;2.31,-3.77,;3.13,-2.47,;2.41,-1.1,;.86,-1.05,;.05,-2.35,;.77,-3.71,;-3.85,-3.55,;-4.57,-2.19,;-6.11,-2.13,;-7.06,-3.34,;-6.64,-4.82,;-8.51,-2.81,;-8.45,-1.27,;-6.97,-.85,;-6.44,.6,)| Show InChI InChI=1S/C20H17N3O4/c1-27-17-7-6-12(11-16(17)23-18(24)8-9-19(23)25)10-15-13-4-2-3-5-14(13)20(26)22-21-15/h2-9,11,24-25H,10H2,1H3,(H,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM626053
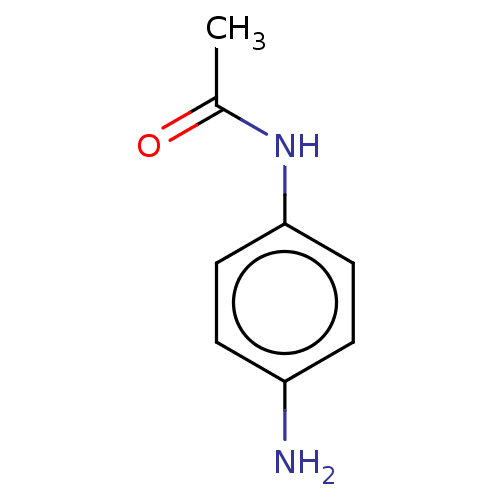
(EN300-17406) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165474
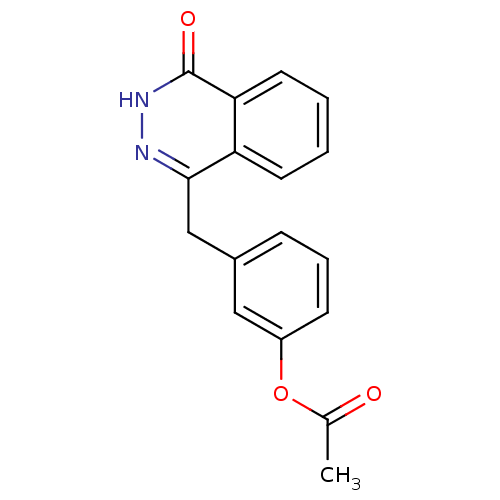
(3-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)phenyl...)Show InChI InChI=1S/C17H14N2O3/c1-11(20)22-13-6-4-5-12(9-13)10-16-14-7-2-3-8-15(14)17(21)19-18-16/h2-9H,10H2,1H3,(H,19,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM495290
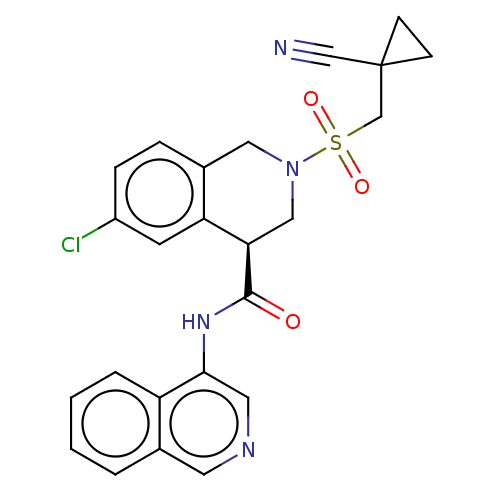
(CVD-0018692 | MAT-POS-e194df51-1)Show SMILES Clc1ccc2CN(C[C@@H](C(=O)Nc3cncc4ccccc34)c2c1)S(=O)(=O)CC1(CC1)C#N Show InChI InChI=1S/C24H21ClN4O3S/c25-18-6-5-17-12-29(33(31,32)15-24(14-26)7-8-24)13-21(20(17)9-18)23(30)28-22-11-27-10-16-3-1-2-4-19(16)22/h1-6,9-11,21H,7-8,12-13,15H2,(H,28,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50379714
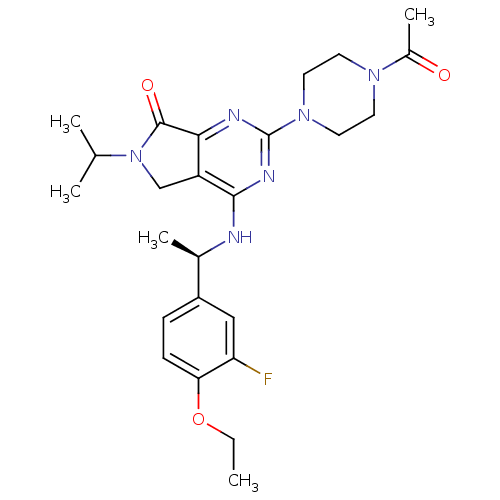
(CHEMBL2011123)Show SMILES CCOc1ccc(cc1F)[C@@H](C)Nc1nc(nc2C(=O)N(Cc12)C(C)C)N1CCN(CC1)C(C)=O |r| Show InChI InChI=1S/C25H33FN6O3/c1-6-35-21-8-7-18(13-20(21)26)16(4)27-23-19-14-32(15(2)3)24(34)22(19)28-25(29-23)31-11-9-30(10-12-31)17(5)33/h7-8,13,15-16H,6,9-12,14H2,1-5H3,(H,27,28,29)/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X3 receptor expressed in RLE cells assessed as inhibition of (alpha,beta)me-ATP-induced intracellular calcium level by... |
Bioorg Med Chem Lett 22: 2565-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.124
BindingDB Entry DOI: 10.7270/Q29W0GGF |
More data for this
Ligand-Target Pair | |
Mas-related G-protein coupled receptor member X1
(Homo sapiens (Human)) | BDBM50340746
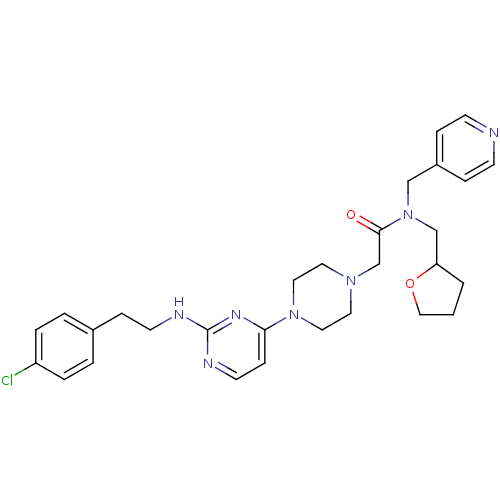
(2-(4-(2-(4-chlorophenethylamino)pyrimidin-4-yl)pip...)Show SMILES Clc1ccc(CCNc2nccc(n2)N2CCN(CC(=O)N(CC3CCCO3)Cc3ccncc3)CC2)cc1 Show InChI InChI=1S/C29H36ClN7O2/c30-25-5-3-23(4-6-25)9-13-32-29-33-14-10-27(34-29)36-17-15-35(16-18-36)22-28(38)37(21-26-2-1-19-39-26)20-24-7-11-31-12-8-24/h3-8,10-12,14,26H,1-2,9,13,15-22H2,(H,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Antagonist activity at SNSR4 in human HEK293 cells by FLIPR assay |
Bioorg Med Chem Lett 21: 2102-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.138
BindingDB Entry DOI: 10.7270/Q2D21XW7 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM627860
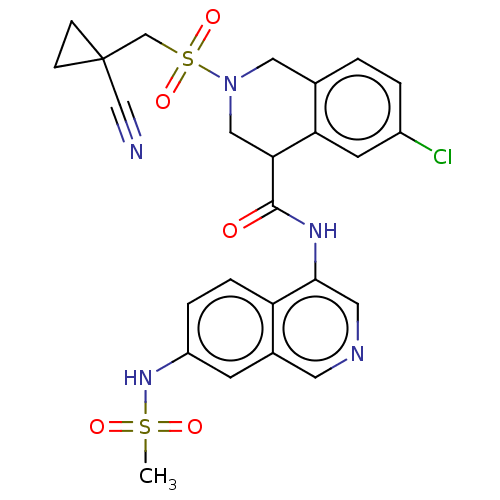
(CVD-0018385 | EDJ-MED-be9e6f63-3)Show SMILES CS(=O)(=O)Nc1ccc2c(NC(=O)C3CN(Cc4ccc(Cl)cc34)S(=O)(=O)CC3(CC3)C#N)cncc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM627860

(CVD-0018385 | EDJ-MED-be9e6f63-3)Show SMILES CS(=O)(=O)Nc1ccc2c(NC(=O)C3CN(Cc4ccc(Cl)cc34)S(=O)(=O)CC3(CC3)C#N)cncc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data