

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128532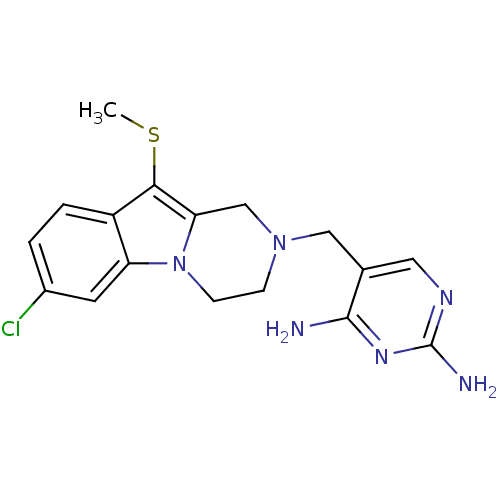 (5-(7-Chloro-10-methylsulfanyl-3,4-dihydro-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128535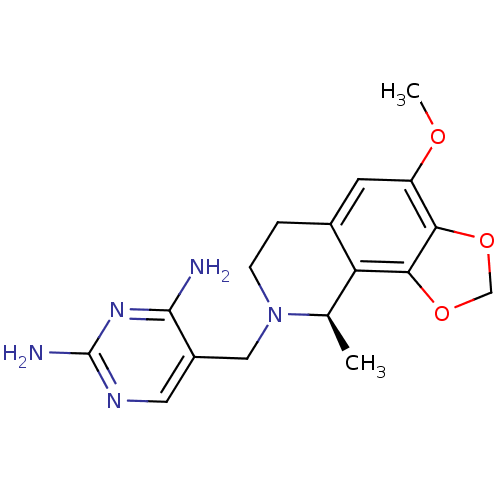 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266345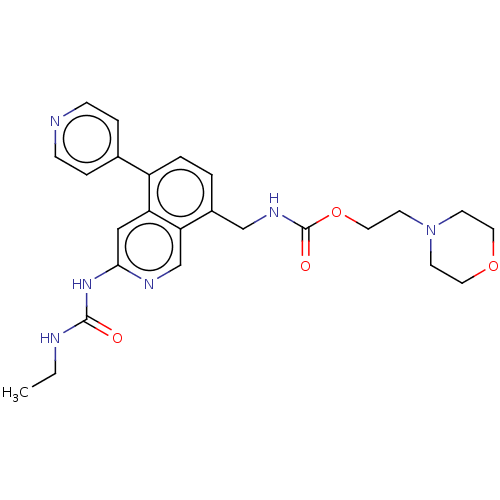 (CHEMBL4094690) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266347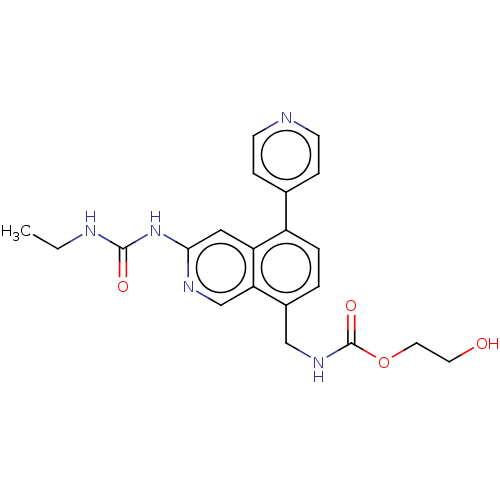 (CHEMBL4070524) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266348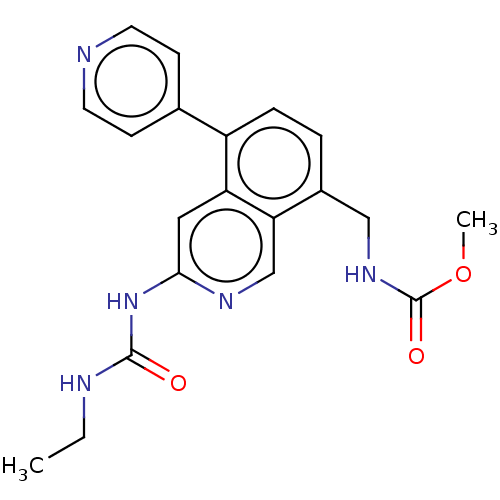 (CHEMBL4064539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128540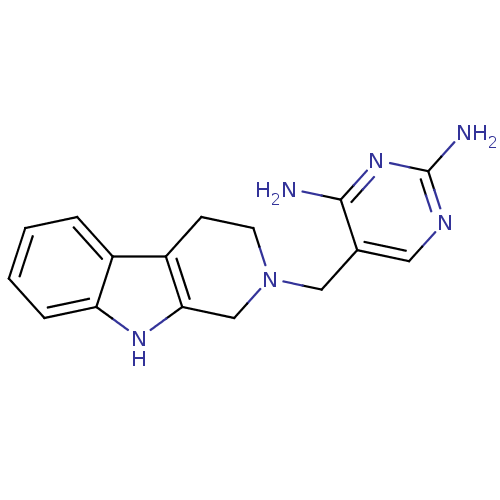 (5-(1,3,4,9-Tetrahydro-beta-carbolin-2-ylmethyl)-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266393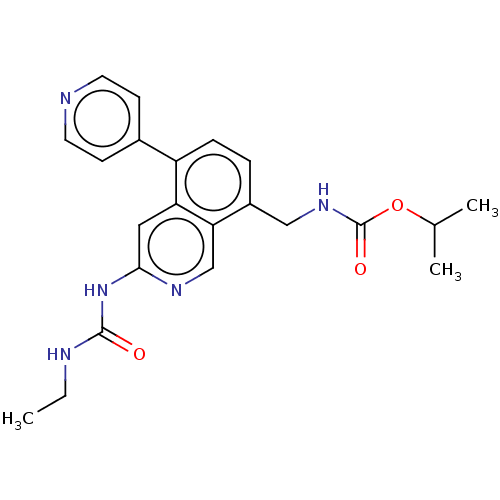 (CHEMBL4099988) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128534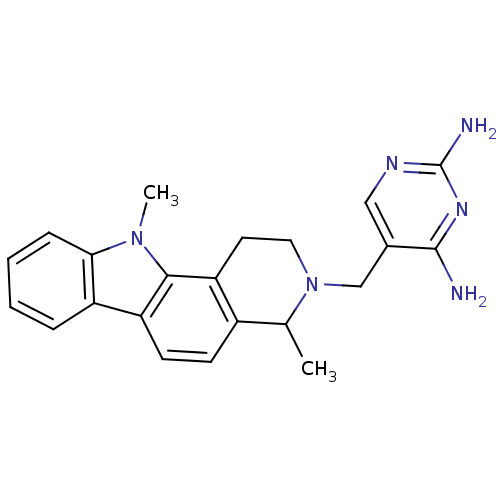 (5-(4,11-Dimethyl-1,2,4,11-tetrahydro-pyrido[4,3-a]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266374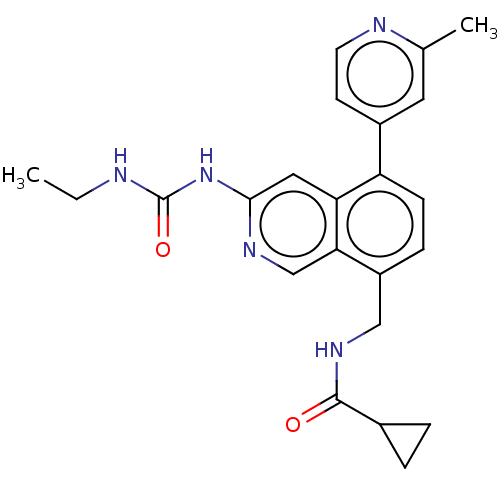 (CHEMBL4099469) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50089194 ((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-2-hydroxymethyl-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against isolated Escherichia coli peptidyl deformylase (PDF) enzyme containing iron. | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128538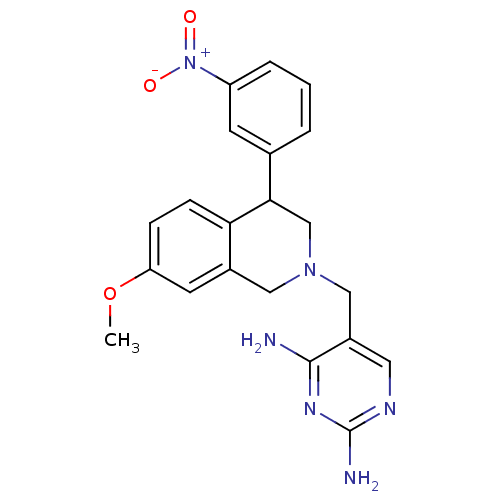 (5-[7-Methoxy-4-(3-nitro-phenyl)-3,4-dihydro-1H-iso...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128533 (5-[4-(2,4-Dichloro-phenyl)-6,7-dimethoxy-3,4-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266349 (CHEMBL4092234) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266343 (CHEMBL4078126) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266344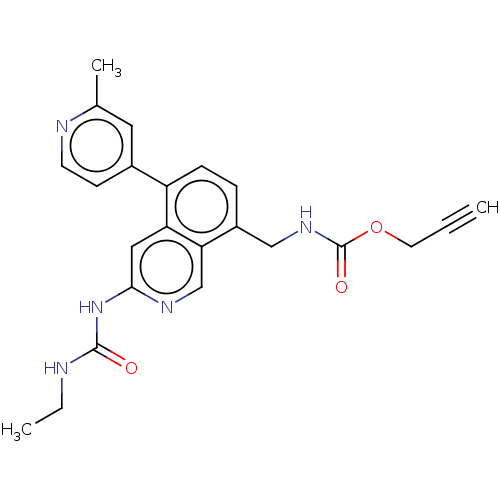 (CHEMBL4061762) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266351 (CHEMBL4096181) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266352 (CHEMBL4086015) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266355 (CHEMBL4066198) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266342 (CHEMBL4101490) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266356 (CHEMBL4074808) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266366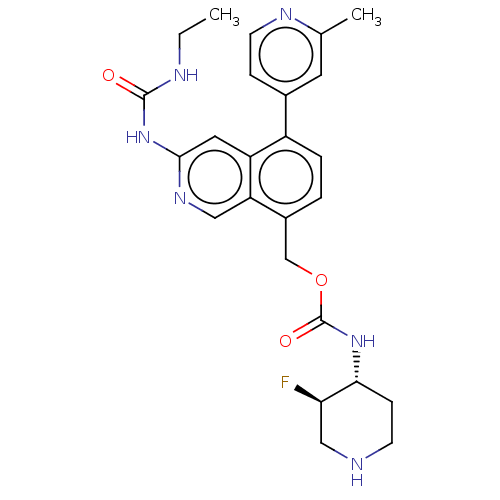 (CHEMBL4091200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266367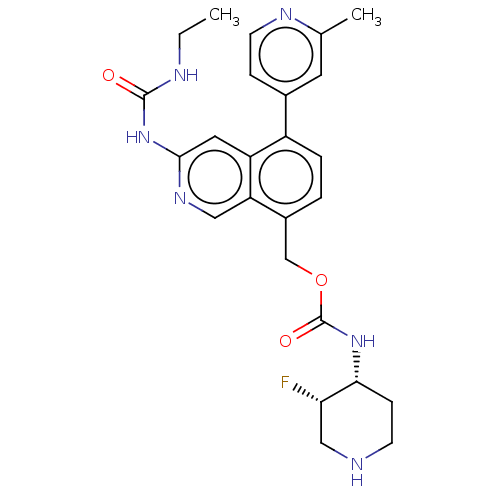 (CHEMBL4071960) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266368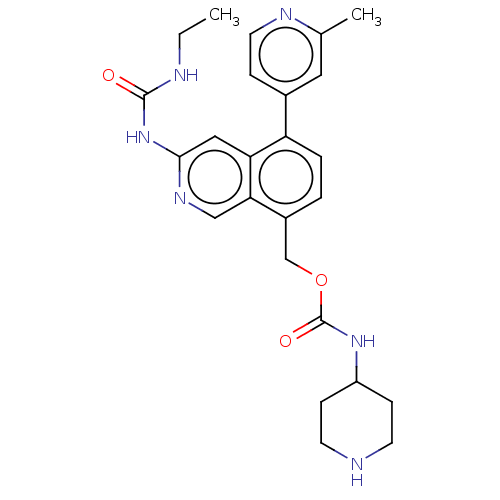 (CHEMBL4098937) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266369 (CHEMBL4087439) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266370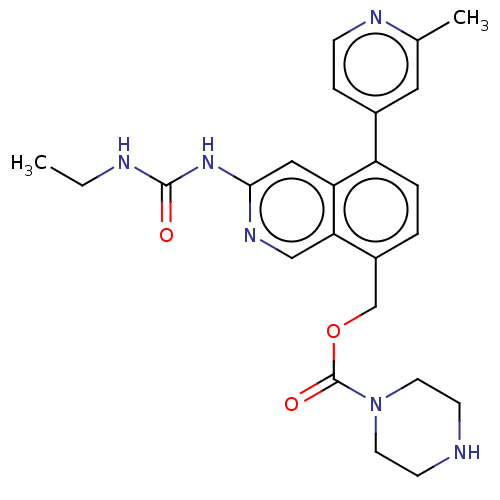 (CHEMBL4081451) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266375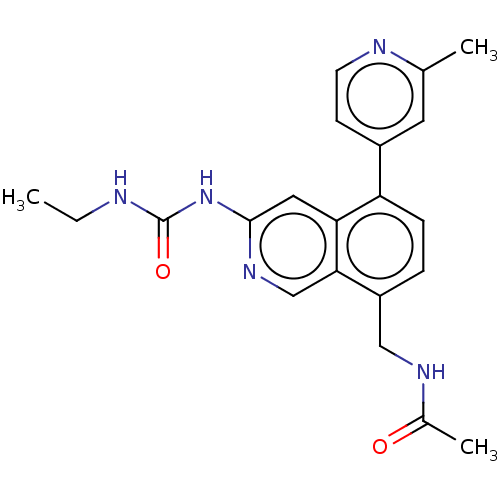 (CHEMBL4100423) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266378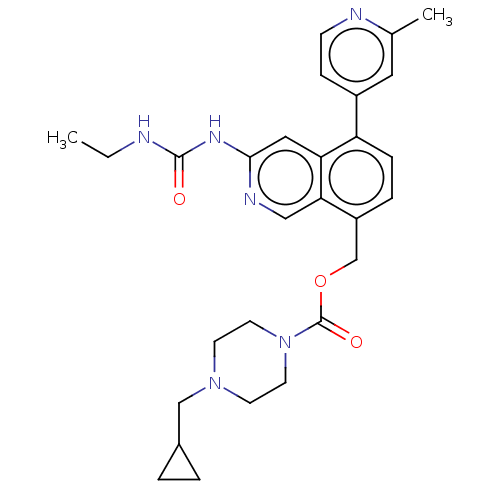 (CHEMBL4066059) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266379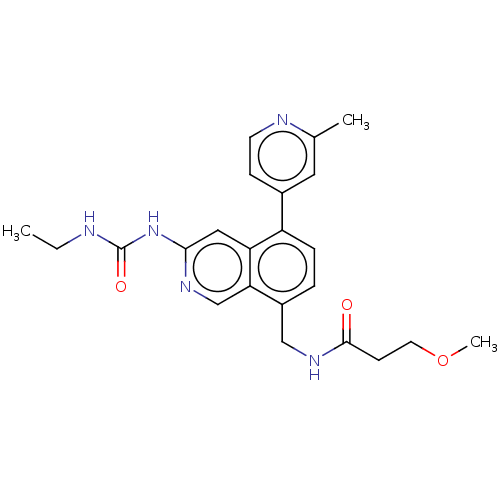 (CHEMBL4083966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266394 (CHEMBL4070393) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50266350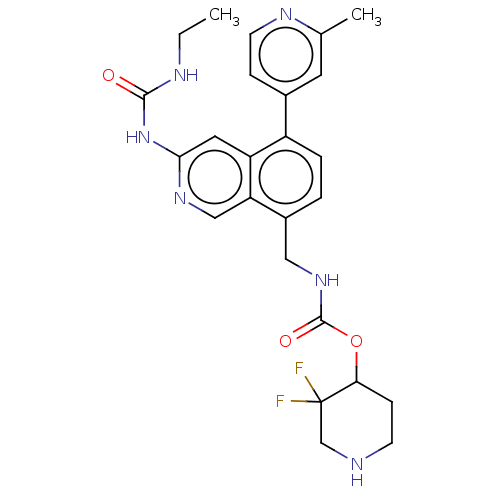 (CHEMBL4091332) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus N-terminal His6-tagged DNA gyrase subunit GyrA/GyrB supercoiling activity expressed in Escherichia coli BL21 (DE3... | J Med Chem 60: 3755-3775 (2017) Article DOI: 10.1021/acs.jmedchem.6b01834 BindingDB Entry DOI: 10.7270/Q2SQ92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50408913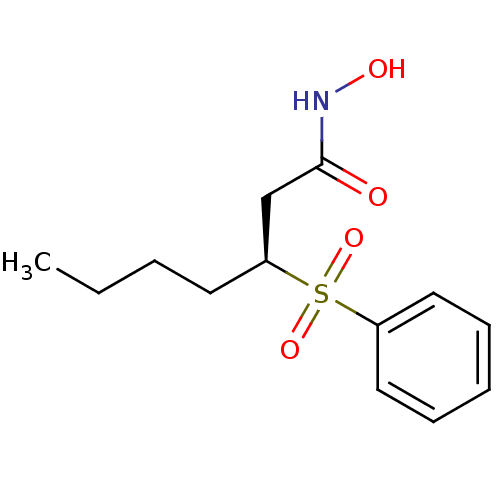 (CHEMBL2092878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of human Matrix metalloprotease-12 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50089199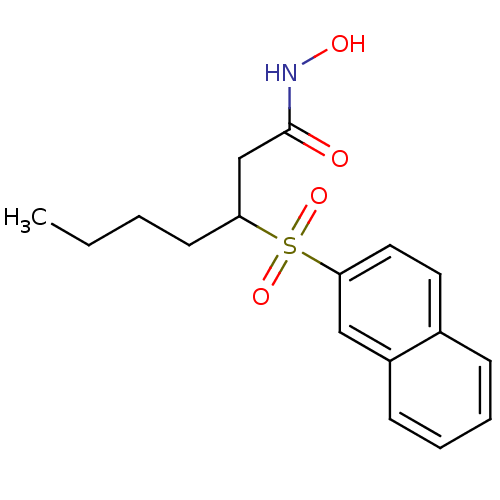 (3-(Naphthalene-2-sulfonyl)-heptanoic acid hydroxya...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-13 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128535 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128534 (5-(4,11-Dimethyl-1,2,4,11-tetrahydro-pyrido[4,3-a]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128536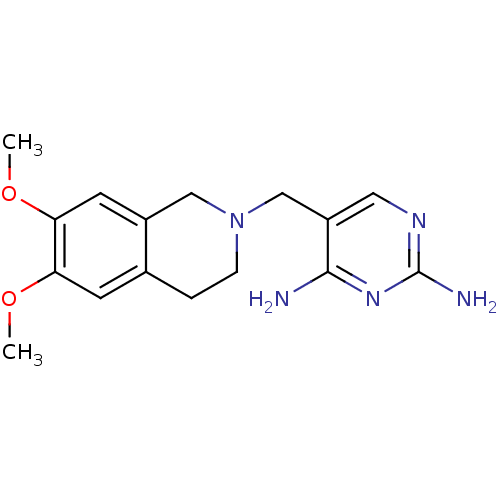 (5-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-ylme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50089201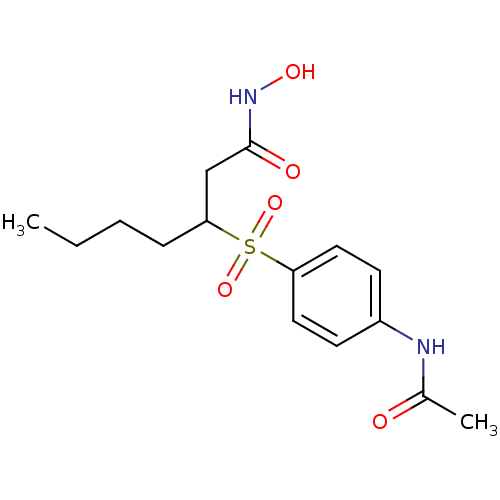 (3-(4-Acetylamino-benzenesulfonyl)-heptanoic acid h...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against isolated Escherichia coli peptidyl deformylase (PDF) enzyme containing iron. | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50067069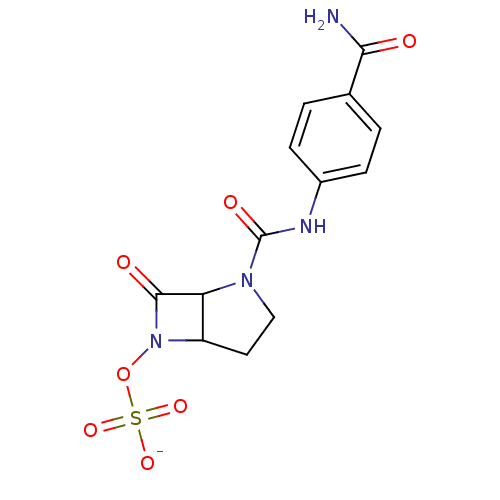 (CHEMBL128220 | sodium salt-Sulfuric acid mono-[2-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of beta-lactamase of Citrobacter freundii 1982 (class C) | J Med Chem 41: 3972-5 (1998) Article DOI: 10.1021/jm9800245 BindingDB Entry DOI: 10.7270/Q2571CPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50089196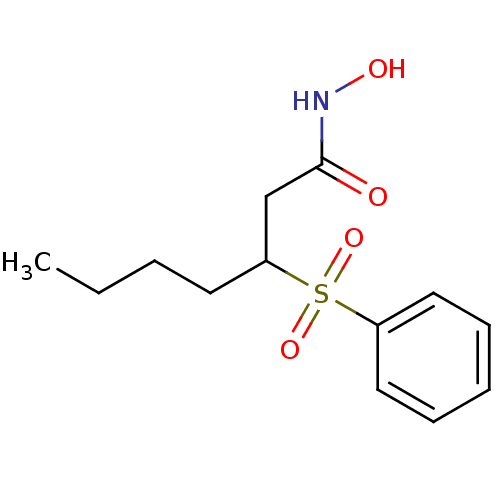 (3-Benzenesulfonyl-heptanoic acid hydroxyamide | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of human Matrix metalloprotease-12 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50067069 (CHEMBL128220 | sodium salt-Sulfuric acid mono-[2-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of beta-lactamase of Citrobacter freundii 1982 (class C) | J Med Chem 41: 3972-5 (1998) Article DOI: 10.1021/jm9800245 BindingDB Entry DOI: 10.7270/Q2571CPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128537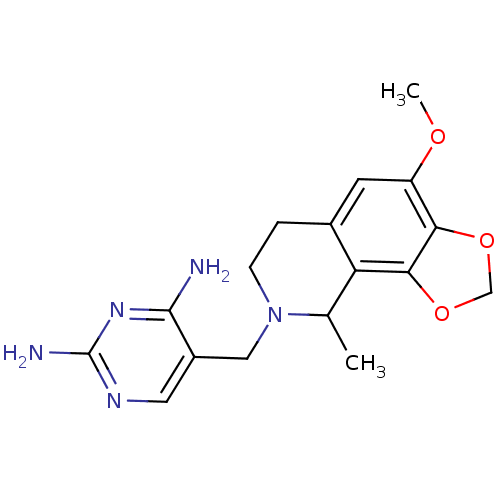 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50089199 (3-(Naphthalene-2-sulfonyl)-heptanoic acid hydroxya...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of human Matrix metalloprotease-12 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50089198 (3-Cyclohexanesulfonyl-heptanoic acid hydroxyamide ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of human Matrix metalloprotease-12 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067060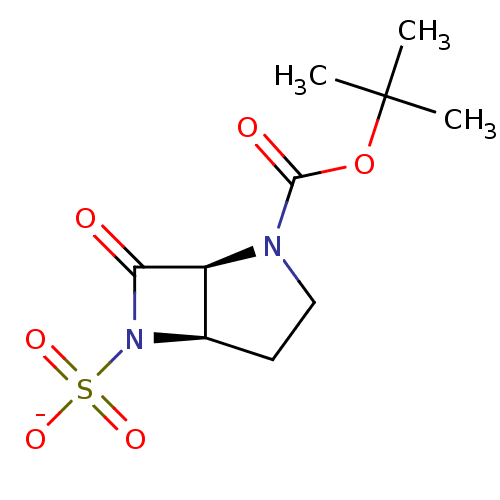 (CHEMBL128523 | Sodium; (1S,5R)-2-tert-butoxycarbon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli TEM-3 Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50369525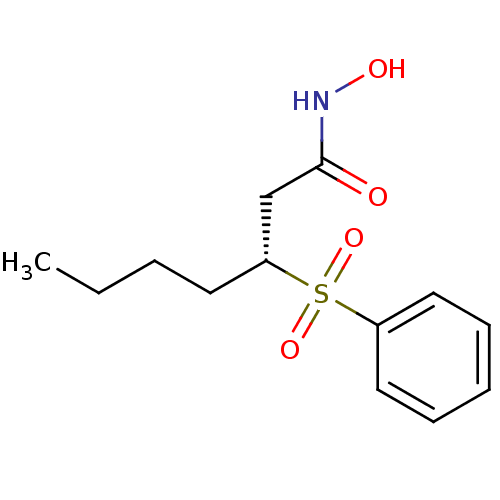 (CHEMBL1788203) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against isolated Escherichia coli peptidyl deformylase (PDF) enzyme containing iron. | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50067070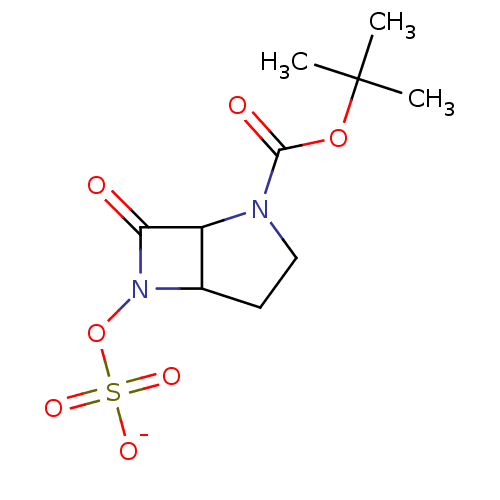 (CHEMBL355579 | sodium salt -tert-butyl 7-oxo-6-(su...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of beta-lactamase of Citrobacter freundii 1982 (class C) | J Med Chem 41: 3972-5 (1998) Article DOI: 10.1021/jm9800245 BindingDB Entry DOI: 10.7270/Q2571CPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128529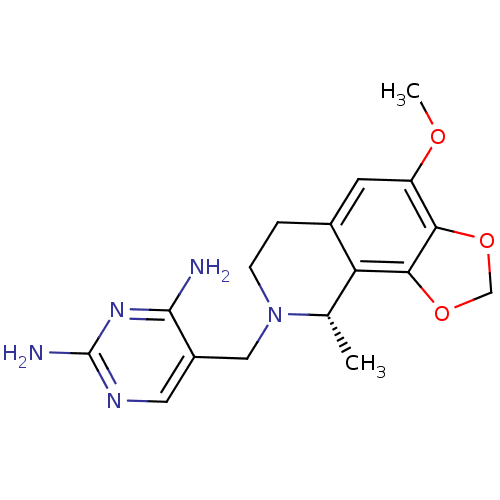 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50089196 (3-Benzenesulfonyl-heptanoic acid hydroxyamide | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-13 | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50089199 (3-(Naphthalene-2-sulfonyl)-heptanoic acid hydroxya...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against isolated Escherichia coli peptidyl deformylase (PDF) enzyme containing iron. | J Med Chem 43: 2324-31 (2000) BindingDB Entry DOI: 10.7270/Q2QF8TJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128537 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 479 total ) | Next | Last >> |