

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Mus musculus (mouse)) | BDBM50277545 (4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM159843 (BDBM159925 | US9040528, 2 | US9040528, 2(b)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103538 (CHEMBL3357561) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM50277545 (4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Aurora A kinase expressed in insect Sf9 cells by radioactive flashplate assay | Proc Natl Acad Sci USA 104: 4106-11 (2007) Article DOI: 10.1073/pnas.0608798104 BindingDB Entry DOI: 10.7270/Q25B03CS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50277545 (4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM50097413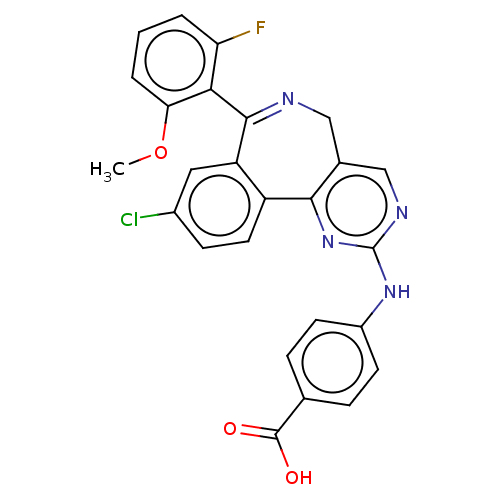 (CHEMBL3586473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103539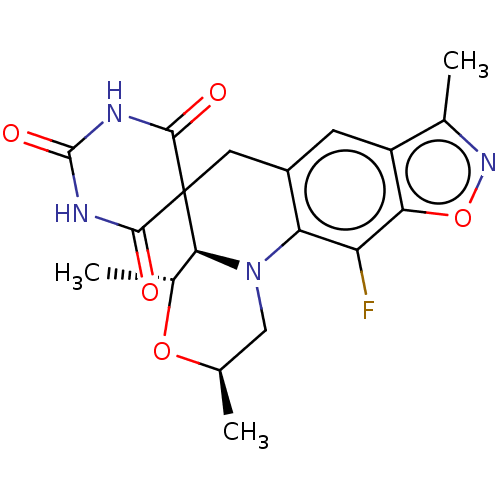 (CHEMBL3357982) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50097413 (CHEMBL3586473) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103540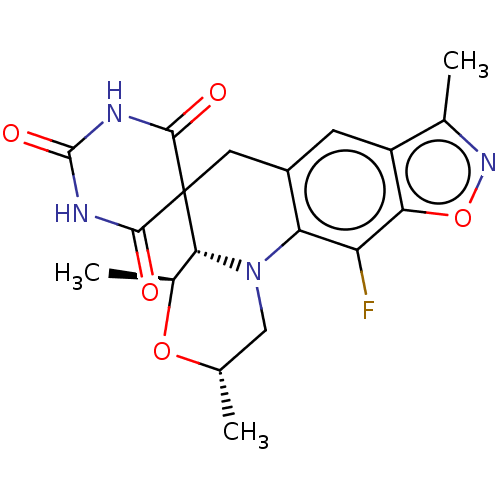 (CHEMBL3357983) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM50097414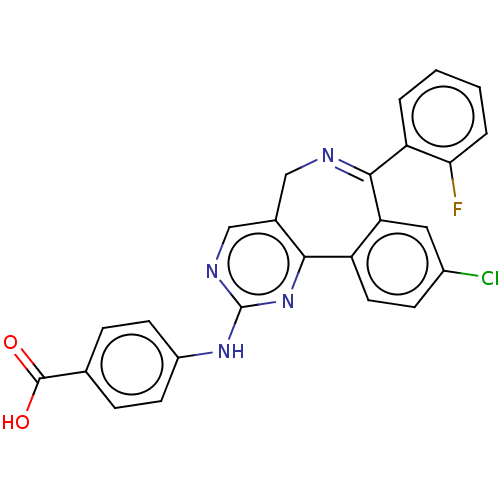 (CHEMBL3586468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Aurora A Thr288 autophosphorylation in human HeLa cells after 1 hr | Proc Natl Acad Sci USA 104: 4106-11 (2007) Article DOI: 10.1073/pnas.0608798104 BindingDB Entry DOI: 10.7270/Q25B03CS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM159845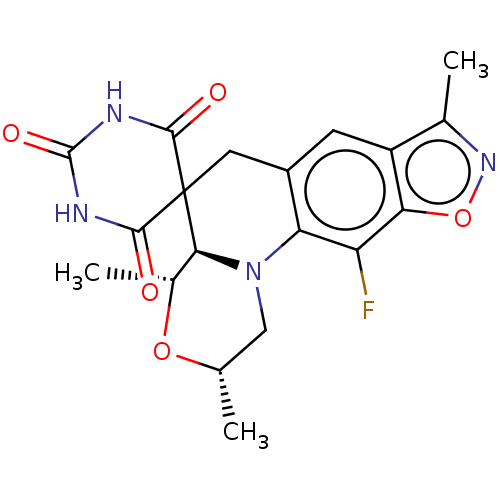 (US9040528, 2(a)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >83 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1 (Homo sapiens (Human)) | BDBM50097413 (CHEMBL3586473) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Binding affinity to GABAA alpha-1 benzodiazepine binding site (unknown origin) | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50097414 (CHEMBL3586468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Mus musculus) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Aurora B kinase expressed in insect Sf9 cells by radioactive flashplate assay | Proc Natl Acad Sci USA 104: 4106-11 (2007) Article DOI: 10.1073/pnas.0608798104 BindingDB Entry DOI: 10.7270/Q25B03CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM160047 (US9040528, 180) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM159842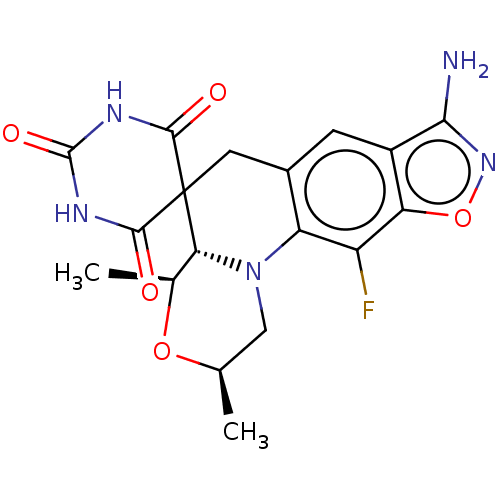 (US9040528, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM21690 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1 (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Binding affinity to GABAA alpha-1 benzodiazepine binding site (unknown origin) | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103535 (CHEMBL3357988) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103541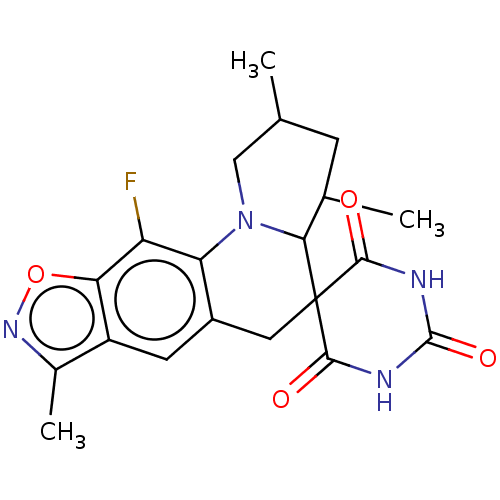 (CHEMBL3357990) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103537 (CHEMBL3357984) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM159943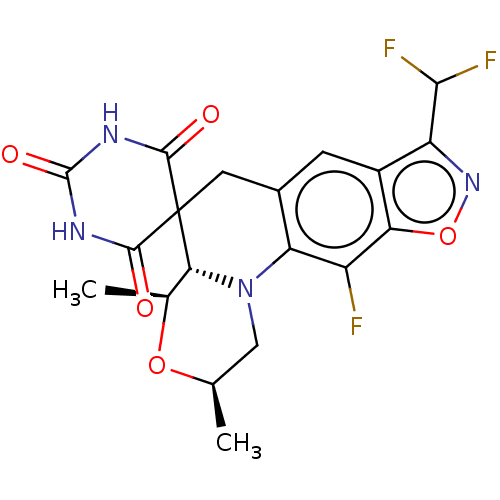 (US9040528, 93 | US9040528, 93(b)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1 (Homo sapiens (Human)) | BDBM50277545 (4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Binding affinity to GABAA alpha-1 benzodiazepine binding site (unknown origin) | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM159940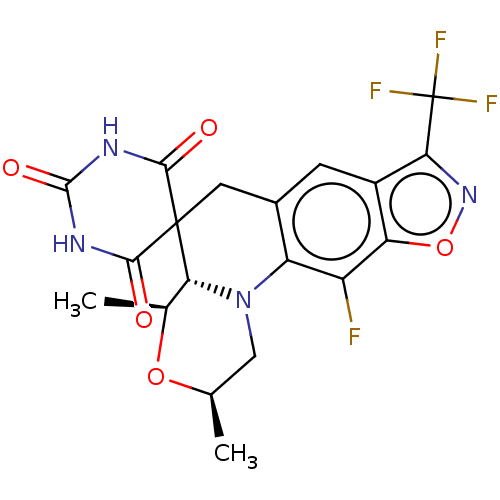 (US9040528, 92 | US9040528, 92(b)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM159895 (US9040528, 51) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50277545 (4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase B in human HCT116 cells assessed as inhibition of histone H3 phosphorylation by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103536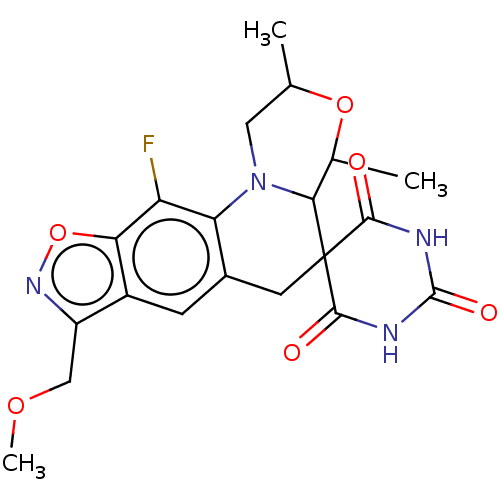 (CHEMBL3357987) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM50097418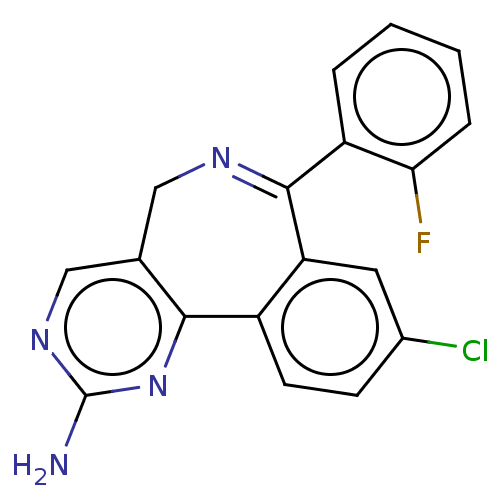 (CHEMBL41816) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50097413 (CHEMBL3586473) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase B in human HCT116 cells assessed as inhibition of histone H3 phosphorylation by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of LCK by radioactive flashplate assay | Proc Natl Acad Sci USA 104: 4106-11 (2007) Article DOI: 10.1073/pnas.0608798104 BindingDB Entry DOI: 10.7270/Q25B03CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103542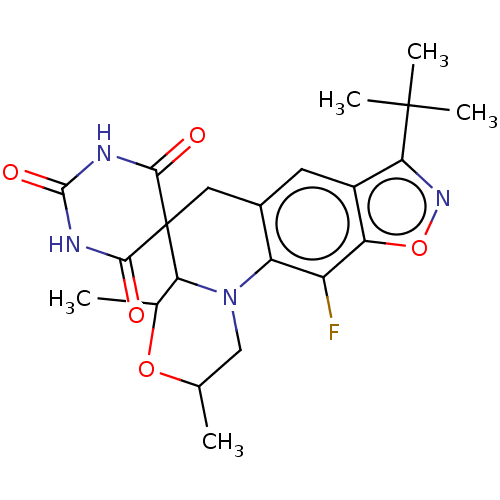 (CHEMBL3357989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase B in human HCT116 cells assessed as inhibition of histone H3 phosphorylation by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Aurora B Ser10 phosphorylation in human HeLa cells after 1 hr | Proc Natl Acad Sci USA 104: 4106-11 (2007) Article DOI: 10.1073/pnas.0608798104 BindingDB Entry DOI: 10.7270/Q25B03CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50097418 (CHEMBL41816) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50097418 (CHEMBL41816) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase B in human HCT116 cells assessed as inhibition of histone H3 phosphorylation by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50097414 (CHEMBL3586468) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase B in human HCT116 cells assessed as inhibition of histone H3 phosphorylation by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of CK2 by radioactive flashplate assay | Proc Natl Acad Sci USA 104: 4106-11 (2007) Article DOI: 10.1073/pnas.0608798104 BindingDB Entry DOI: 10.7270/Q25B03CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of CHK2 by radioactive flashplate assay | Proc Natl Acad Sci USA 104: 4106-11 (2007) Article DOI: 10.1073/pnas.0608798104 BindingDB Entry DOI: 10.7270/Q25B03CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50116067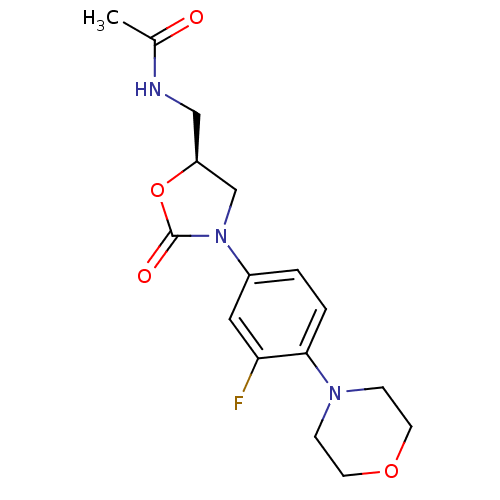 ((Linezolid)N-[3-(3-Fluoro-4-morpholin-4-yl-phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM159843 (BDBM159925 | US9040528, 2 | US9040528, 2(b)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of PLK1 by radioactive flashplate assay | Proc Natl Acad Sci USA 104: 4106-11 (2007) Article DOI: 10.1073/pnas.0608798104 BindingDB Entry DOI: 10.7270/Q25B03CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of CHK1 by radioactive flashplate assay | Proc Natl Acad Sci USA 104: 4106-11 (2007) Article DOI: 10.1073/pnas.0608798104 BindingDB Entry DOI: 10.7270/Q25B03CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of CDK1 by radioactive flashplate assay | Proc Natl Acad Sci USA 104: 4106-11 (2007) Article DOI: 10.1073/pnas.0608798104 BindingDB Entry DOI: 10.7270/Q25B03CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||