 Found 141 hits with Last Name = 'hunter' and Initial = 'am'
Found 141 hits with Last Name = 'hunter' and Initial = 'am' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50130561
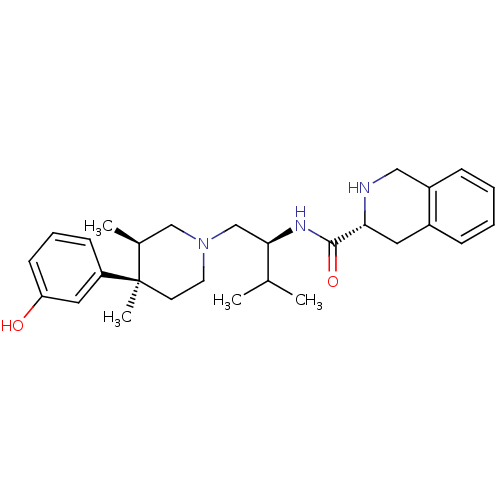
((R)-N-((S)-1-((3R,4R)-4-(3-hydroxyphenyl)-3,4-dime...)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C28H39N3O2/c1-19(2)26(30-27(33)25-14-21-8-5-6-9-22(21)16-29-25)18-31-13-12-28(4,20(3)17-31)23-10-7-11-24(32)15-23/h5-11,15,19-20,25-26,29,32H,12-14,16-18H2,1-4H3,(H,30,33)/t20-,25+,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50327257
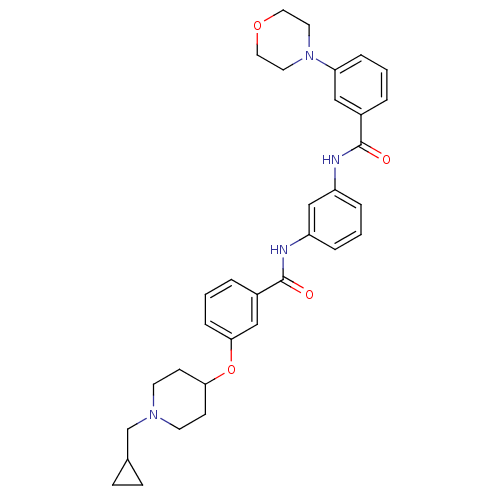
(3-(1-(cyclopropylmethyl)piperidin-4-yloxy)-N-(3-(3...)Show SMILES O=C(Nc1cccc(NC(=O)c2cccc(c2)N2CCOCC2)c1)c1cccc(OC2CCN(CC3CC3)CC2)c1 Show InChI InChI=1S/C33H38N4O4/c38-32(25-4-1-8-29(20-25)37-16-18-40-19-17-37)34-27-6-3-7-28(22-27)35-33(39)26-5-2-9-31(21-26)41-30-12-14-36(15-13-30)23-24-10-11-24/h1-9,20-22,24,30H,10-19,23H2,(H,34,38)(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50325855
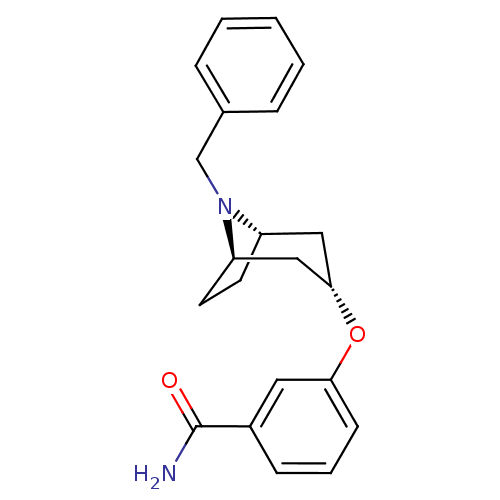
(CHEMBL1223951 | exo-3-((1R,3s,5S)-8-benzyl-8-azabi...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3Cc2ccccc2)c1 |r,TLB:17:16:15.9.10:13.12| Show InChI InChI=1S/C21H24N2O2/c22-21(24)16-7-4-8-19(11-16)25-20-12-17-9-10-18(13-20)23(17)14-15-5-2-1-3-6-15/h1-8,11,17-18,20H,9-10,12-14H2,(H2,22,24)/t17-,18+,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50327259
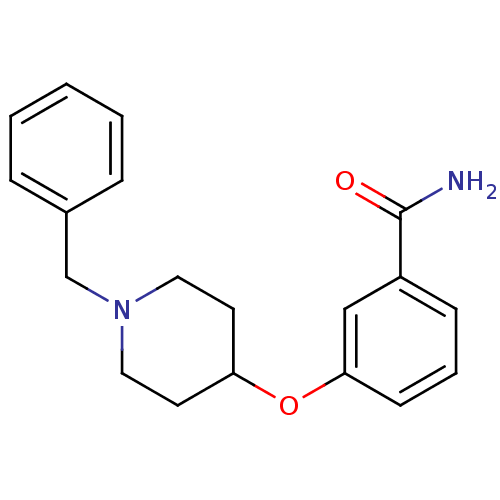
(3-(1-benzylpiperidin-4-yloxy)benzamide | CHEMBL125...)Show InChI InChI=1S/C19H22N2O2/c20-19(22)16-7-4-8-18(13-16)23-17-9-11-21(12-10-17)14-15-5-2-1-3-6-15/h1-8,13,17H,9-12,14H2,(H2,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50327258
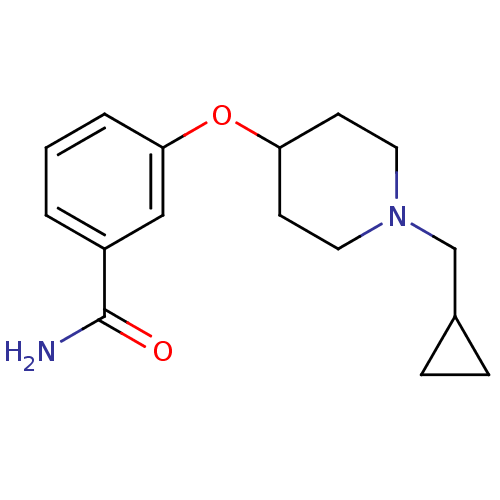
(3-(1-(cyclopropylmethyl)piperidin-4-yloxy)benzamid...)Show InChI InChI=1S/C16H22N2O2/c17-16(19)13-2-1-3-15(10-13)20-14-6-8-18(9-7-14)11-12-4-5-12/h1-3,10,12,14H,4-9,11H2,(H2,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327248
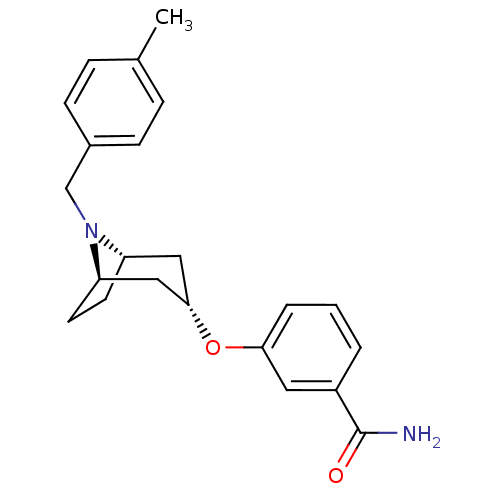
(CHEMBL1257821 | endo-3-(8-(4-methylbenzyl)-8-azabi...)Show SMILES Cc1ccc(CN2[C@H]3CC[C@@H]2C[C@H](C3)Oc2cccc(c2)C(N)=O)cc1 |r,TLB:5:6:12.11.13:9.8| Show InChI InChI=1S/C22H26N2O2/c1-15-5-7-16(8-6-15)14-24-18-9-10-19(24)13-21(12-18)26-20-4-2-3-17(11-20)22(23)25/h2-8,11,18-19,21H,9-10,12-14H2,1H3,(H2,23,25)/t18-,19+,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327247
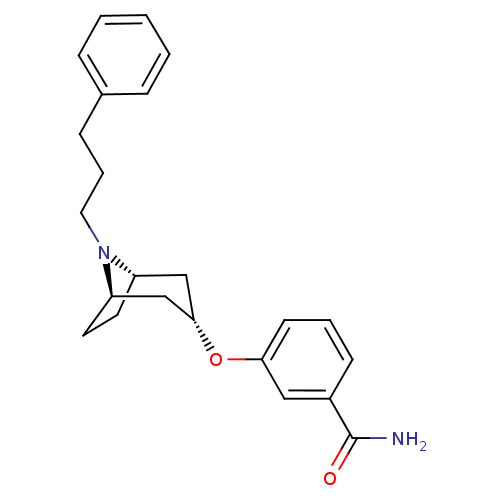
(CHEMBL1257820 | endo-3-(8-(3-phenylpropyl)-8-azabi...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3CCCc2ccccc2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C23H28N2O2/c24-23(26)18-9-4-10-21(14-18)27-22-15-19-11-12-20(16-22)25(19)13-5-8-17-6-2-1-3-7-17/h1-4,6-7,9-10,14,19-20,22H,5,8,11-13,15-16H2,(H2,24,26)/t19-,20+,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327244

(3-Endo-(8-((5-methylthiophen-2-yl)methyl)-8-azabic...)Show SMILES Cc1ccc(CN2[C@H]3CC[C@@H]2C[C@H](C3)Oc2cccc(c2)C(N)=O)s1 |r,TLB:5:6:12.11.13:9.8| Show InChI InChI=1S/C20H24N2O2S/c1-13-5-8-19(25-13)12-22-15-6-7-16(22)11-18(10-15)24-17-4-2-3-14(9-17)20(21)23/h2-5,8-9,15-16,18H,6-7,10-12H2,1H3,(H2,21,23)/t15-,16+,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327246
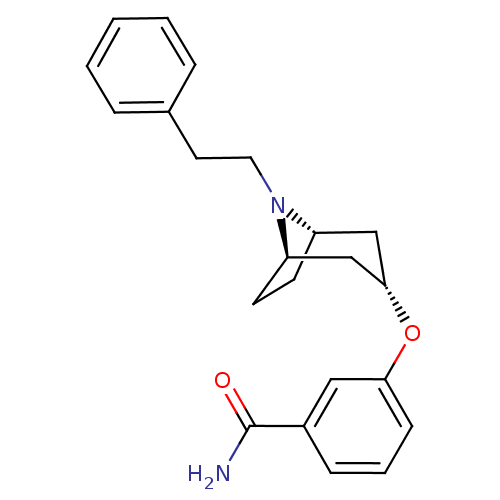
(CHEMBL1257698 | endo-3-(8-phenethyl-8-azabicyclo[3...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3CCc2ccccc2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C22H26N2O2/c23-22(25)17-7-4-8-20(13-17)26-21-14-18-9-10-19(15-21)24(18)12-11-16-5-2-1-3-6-16/h1-8,13,18-19,21H,9-12,14-15H2,(H2,23,25)/t18-,19+,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327243
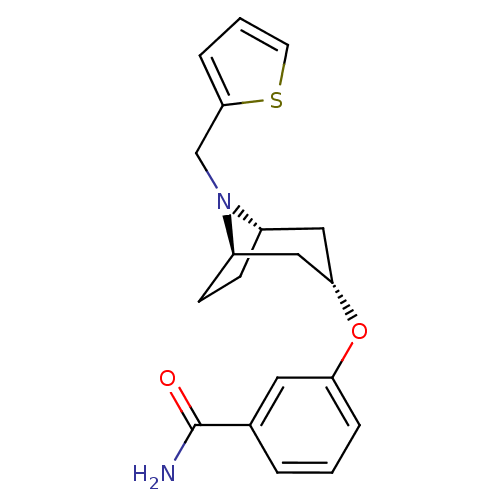
(CHEMBL1257577 | endo-3-(8-(thiophen-2-ylmethyl)-8-...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3Cc2cccs2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C19H22N2O2S/c20-19(22)13-3-1-4-16(9-13)23-17-10-14-6-7-15(11-17)21(14)12-18-5-2-8-24-18/h1-5,8-9,14-15,17H,6-7,10-12H2,(H2,20,22)/t14-,15+,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50130561

((R)-N-((S)-1-((3R,4R)-4-(3-hydroxyphenyl)-3,4-dime...)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C28H39N3O2/c1-19(2)26(30-27(33)25-14-21-8-5-6-9-22(21)16-29-25)18-31-13-12-28(4,20(3)17-31)23-10-7-11-24(32)15-23/h5-11,15,19-20,25-26,29,32H,12-14,16-18H2,1-4H3,(H,30,33)/t20-,25+,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327249
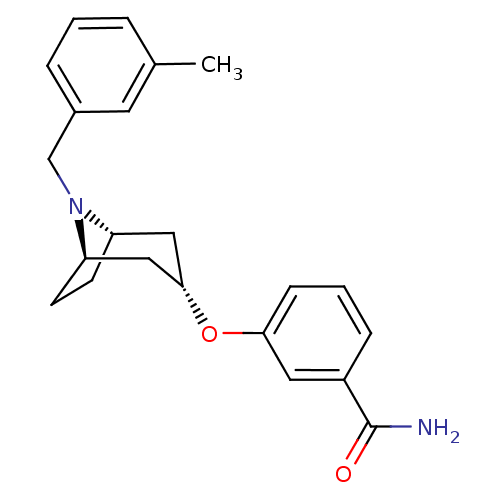
(CHEMBL1257937 | endo-3-(8-(3-methylbenzyl)-8-azabi...)Show SMILES Cc1cccc(CN2[C@H]3CC[C@@H]2C[C@H](C3)Oc2cccc(c2)C(N)=O)c1 |r,TLB:6:7:13.12.14:10.9| Show InChI InChI=1S/C22H26N2O2/c1-15-4-2-5-16(10-15)14-24-18-8-9-19(24)13-21(12-18)26-20-7-3-6-17(11-20)22(23)25/h2-7,10-11,18-19,21H,8-9,12-14H2,1H3,(H2,23,25)/t18-,19+,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50325855

(CHEMBL1223951 | exo-3-((1R,3s,5S)-8-benzyl-8-azabi...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3Cc2ccccc2)c1 |r,TLB:17:16:15.9.10:13.12| Show InChI InChI=1S/C21H24N2O2/c22-21(24)16-7-4-8-19(11-16)25-20-12-17-9-10-18(13-20)23(17)14-15-5-2-1-3-6-15/h1-8,11,17-18,20H,9-10,12-14H2,(H2,22,24)/t17-,18+,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327250
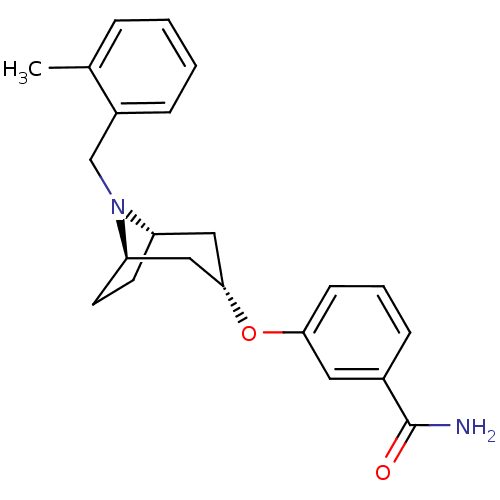
(CHEMBL1257938 | endo-3-(8-(2-methylbenzyl)-8-azabi...)Show SMILES Cc1ccccc1CN1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O |r,TLB:7:8:14.13.15:11.10| Show InChI InChI=1S/C22H26N2O2/c1-15-5-2-3-6-17(15)14-24-18-9-10-19(24)13-21(12-18)26-20-8-4-7-16(11-20)22(23)25/h2-8,11,18-19,21H,9-10,12-14H2,1H3,(H2,23,25)/t18-,19+,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327256
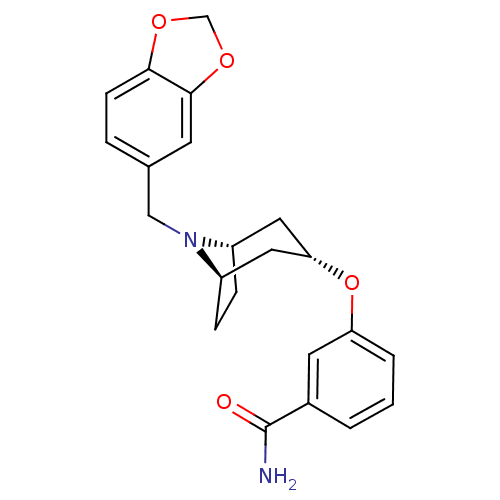
(CHEMBL1258280 | endo-3-(8-(benzo[d][1,3]dioxol-5-y...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3Cc2ccc3OCOc3c2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C22H24N2O4/c23-22(25)15-2-1-3-18(9-15)28-19-10-16-5-6-17(11-19)24(16)12-14-4-7-20-21(8-14)27-13-26-20/h1-4,7-9,16-17,19H,5-6,10-13H2,(H2,23,25)/t16-,17+,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327264
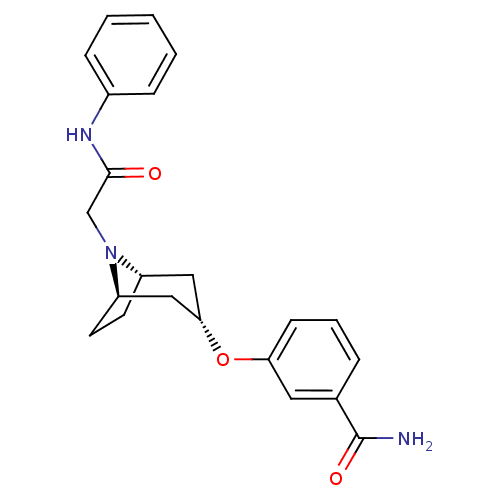
(CHEMBL1258503 | endo-3-(8-(2-oxo-2-(phenylamino)et...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3CC(=O)Nc2ccccc2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C22H25N3O3/c23-22(27)15-5-4-8-19(11-15)28-20-12-17-9-10-18(13-20)25(17)14-21(26)24-16-6-2-1-3-7-16/h1-8,11,17-18,20H,9-10,12-14H2,(H2,23,27)(H,24,26)/t17-,18+,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327268
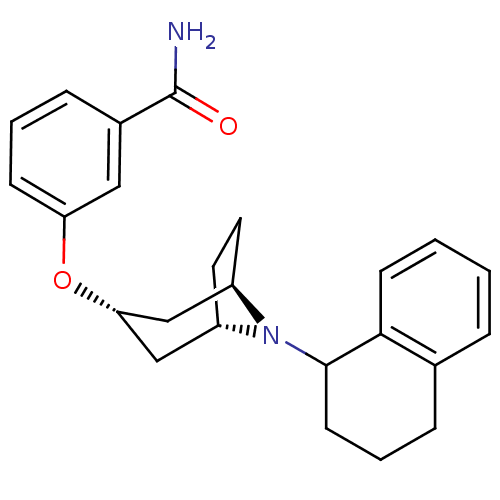
(CHEMBL1258725 | endo-3-(8-(1,2,3,4-tetrahydronapht...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3C2CCCc3ccccc23)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C24H28N2O2/c25-24(27)17-7-3-8-20(13-17)28-21-14-18-11-12-19(15-21)26(18)23-10-4-6-16-5-1-2-9-22(16)23/h1-3,5,7-9,13,18-19,21,23H,4,6,10-12,14-15H2,(H2,25,27)/t18-,19+,21-,23? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327251
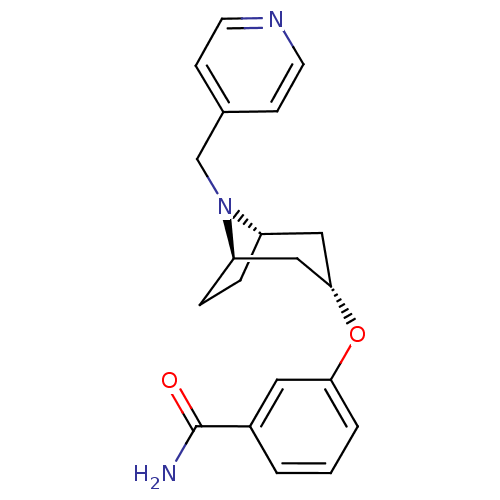
(CHEMBL1258047 | endo-3-(8-(pyridin-4-ylmethyl)-8-a...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3Cc2ccncc2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C20H23N3O2/c21-20(24)15-2-1-3-18(10-15)25-19-11-16-4-5-17(12-19)23(16)13-14-6-8-22-9-7-14/h1-3,6-10,16-17,19H,4-5,11-13H2,(H2,21,24)/t16-,17+,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327266
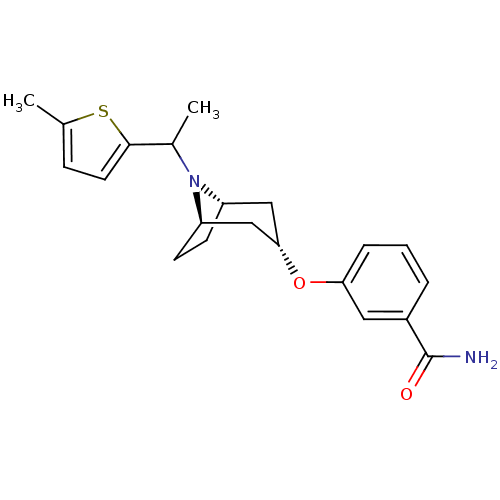
(CHEMBL1258616 | endo-3-(8-(1-(5-methylthiophen-2-y...)Show SMILES CC(N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O)c1ccc(C)s1 |r,TLB:1:2:8.7.9:5.4| Show InChI InChI=1S/C21H26N2O2S/c1-13-6-9-20(26-13)14(2)23-16-7-8-17(23)12-19(11-16)25-18-5-3-4-15(10-18)21(22)24/h3-6,9-10,14,16-17,19H,7-8,11-12H2,1-2H3,(H2,22,24)/t14?,16-,17+,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327274
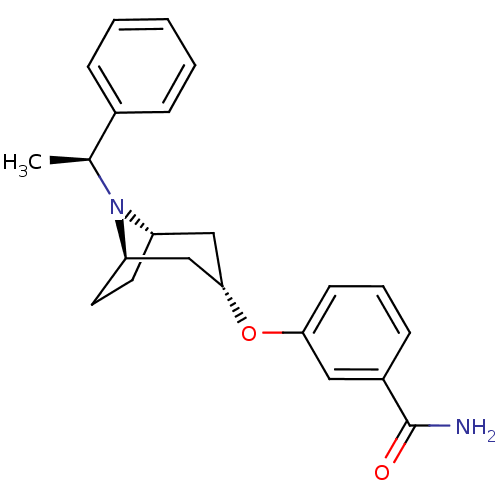
(CHEMBL1257227 | endo-3-(8-((S)-1-phenylethyl)-8-az...)Show SMILES C[C@H](N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O)c1ccccc1 |r,TLB:1:2:8.7.9:5.4| Show InChI InChI=1S/C22H26N2O2/c1-15(16-6-3-2-4-7-16)24-18-10-11-19(24)14-21(13-18)26-20-9-5-8-17(12-20)22(23)25/h2-9,12,15,18-19,21H,10-11,13-14H2,1H3,(H2,23,25)/t15-,18-,19+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327253
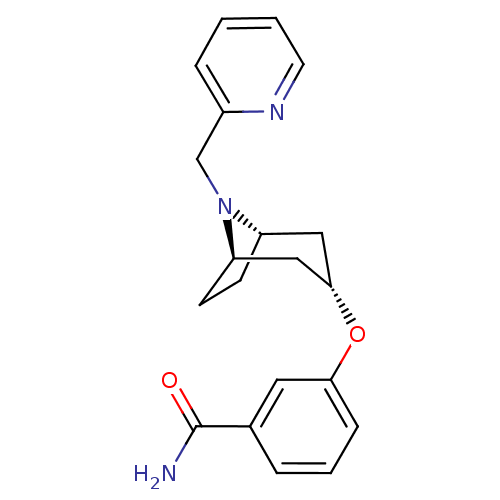
(CHEMBL1258164 | endo-3-(8-(pyridin-2-ylmethyl)-8-a...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3Cc2ccccn2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C20H23N3O2/c21-20(24)14-4-3-6-18(10-14)25-19-11-16-7-8-17(12-19)23(16)13-15-5-1-2-9-22-15/h1-6,9-10,16-17,19H,7-8,11-13H2,(H2,21,24)/t16-,17+,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327255
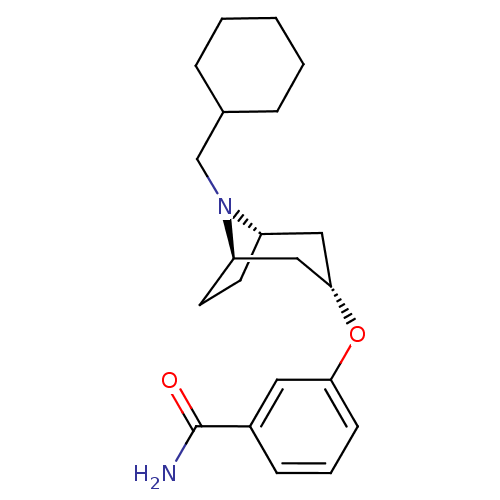
(CHEMBL1258279 | endo-3-(8-(cyclohexylmethyl)-8-aza...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3CC2CCCCC2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C21H30N2O2/c22-21(24)16-7-4-8-19(11-16)25-20-12-17-9-10-18(13-20)23(17)14-15-5-2-1-3-6-15/h4,7-8,11,15,17-18,20H,1-3,5-6,9-10,12-14H2,(H2,22,24)/t17-,18+,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327245
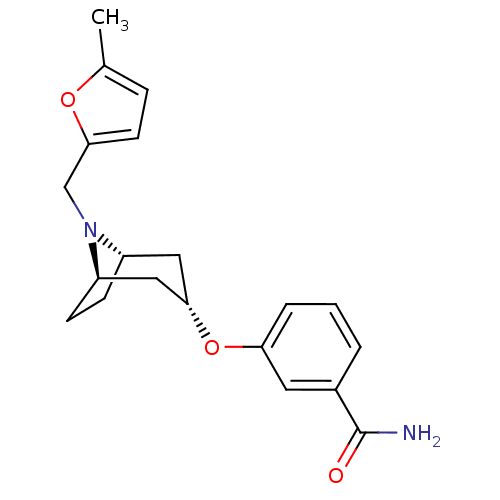
(CHEMBL1255595 | endo-3-(8-((5-methylfuran-2-yl)met...)Show SMILES Cc1ccc(CN2[C@H]3CC[C@@H]2C[C@H](C3)Oc2cccc(c2)C(N)=O)o1 |r,TLB:5:6:12.11.13:9.8| Show InChI InChI=1S/C20H24N2O3/c1-13-5-8-18(24-13)12-22-15-6-7-16(22)11-19(10-15)25-17-4-2-3-14(9-17)20(21)23/h2-5,8-9,15-16,19H,6-7,10-12H2,1H3,(H2,21,23)/t15-,16+,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327275
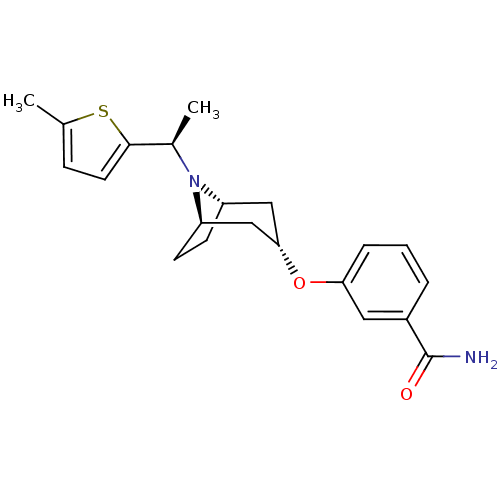
(CHEMBL1257347 | endo-3-(8-((R)-1-(5-methylthiophen...)Show SMILES C[C@@H](N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O)c1ccc(C)s1 |r,TLB:1:2:8.7.9:5.4| Show InChI InChI=1S/C21H26N2O2S/c1-13-6-9-20(26-13)14(2)23-16-7-8-17(23)12-19(11-16)25-18-5-3-4-15(10-18)21(22)24/h3-6,9-10,14,16-17,19H,7-8,11-12H2,1-2H3,(H2,22,24)/t14-,16-,17+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327277
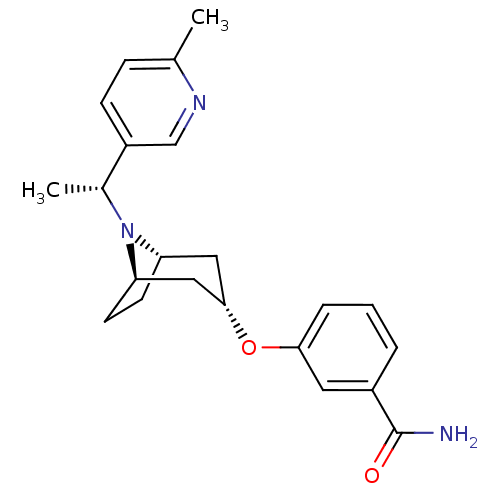
(CHEMBL1257458 | endo-3-(8-((R)-1-(6-methylpyridin-...)Show SMILES C[C@@H](N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O)c1ccc(C)nc1 |r,TLB:1:2:8.7.9:5.4| Show InChI InChI=1S/C22H27N3O2/c1-14-6-7-17(13-24-14)15(2)25-18-8-9-19(25)12-21(11-18)27-20-5-3-4-16(10-20)22(23)26/h3-7,10,13,15,18-19,21H,8-9,11-12H2,1-2H3,(H2,23,26)/t15-,18-,19+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327279

(CHEMBL1257579 | endo-3-(8-((R)-1-(6-chloropyridin-...)Show SMILES C[C@@H](N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O)c1ccc(Cl)nc1 |r,TLB:1:2:8.7.9:5.4| Show InChI InChI=1S/C21H24ClN3O2/c1-13(15-5-8-20(22)24-12-15)25-16-6-7-17(25)11-19(10-16)27-18-4-2-3-14(9-18)21(23)26/h2-5,8-9,12-13,16-17,19H,6-7,10-11H2,1H3,(H2,23,26)/t13-,16-,17+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327252

(CHEMBL1258048 | endo-3-(8-(pyridin-3-ylmethyl)-8-a...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3Cc2cccnc2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C20H23N3O2/c21-20(24)15-4-1-5-18(9-15)25-19-10-16-6-7-17(11-19)23(16)13-14-3-2-8-22-12-14/h1-5,8-9,12,16-17,19H,6-7,10-11,13H2,(H2,21,24)/t16-,17+,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327272
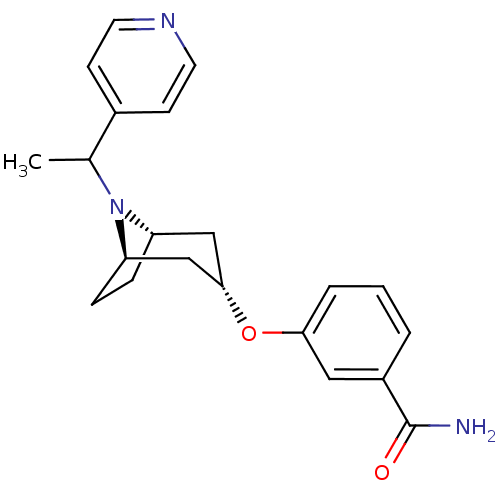
(CHEMBL1258962 | endo-3-(8-(1-(pyridin-4-yl)ethyl)-...)Show SMILES CC(N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O)c1ccncc1 |r,TLB:1:2:8.7.9:5.4| Show InChI InChI=1S/C21H25N3O2/c1-14(15-7-9-23-10-8-15)24-17-5-6-18(24)13-20(12-17)26-19-4-2-3-16(11-19)21(22)25/h2-4,7-11,14,17-18,20H,5-6,12-13H2,1H3,(H2,22,25)/t14?,17-,18+,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327276
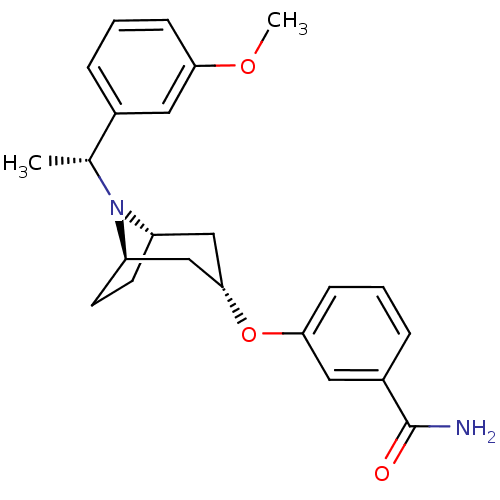
(CHEMBL1257348 | endo-3-(8-((R)-1-(3-methoxyphenyl)...)Show SMILES COc1cccc(c1)[C@@H](C)N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O |r,TLB:8:10:16.15.17:13.12| Show InChI InChI=1S/C23H28N2O3/c1-15(16-5-3-7-20(11-16)27-2)25-18-9-10-19(25)14-22(13-18)28-21-8-4-6-17(12-21)23(24)26/h3-8,11-12,15,18-19,22H,9-10,13-14H2,1-2H3,(H2,24,26)/t15-,18-,19+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327270
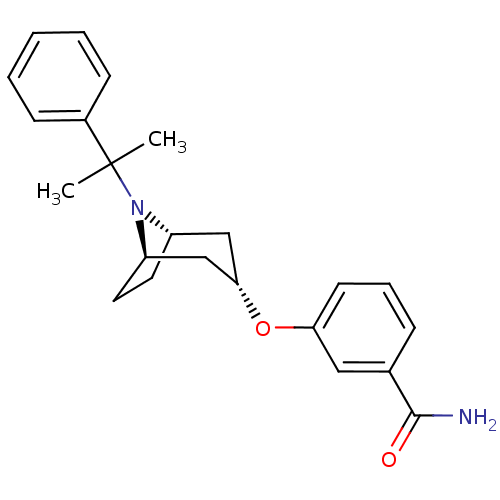
(CHEMBL1258846 | endo-3-(8-(2-phenylpropan-2-yl)-8-...)Show SMILES CC(C)(N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O)c1ccccc1 |r,TLB:1:3:9.8.10:6.5| Show InChI InChI=1S/C23H28N2O2/c1-23(2,17-8-4-3-5-9-17)25-18-11-12-19(25)15-21(14-18)27-20-10-6-7-16(13-20)22(24)26/h3-10,13,18-19,21H,11-12,14-15H2,1-2H3,(H2,24,26)/t18-,19+,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327271
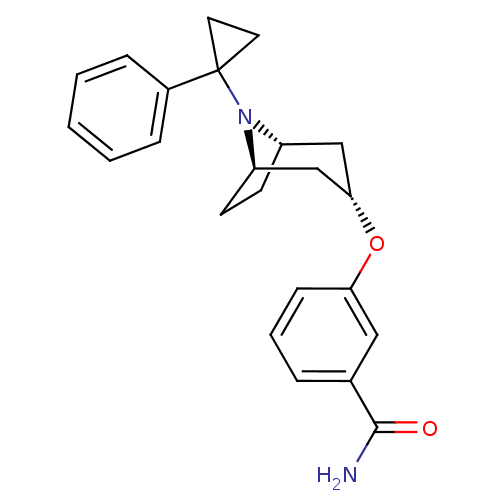
(CHEMBL1258961 | endo-3-(8-(1-phenylcyclopropyl)-8-...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3C2(CC2)c2ccccc2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C23H26N2O2/c24-22(26)16-5-4-8-20(13-16)27-21-14-18-9-10-19(15-21)25(18)23(11-12-23)17-6-2-1-3-7-17/h1-8,13,18-19,21H,9-12,14-15H2,(H2,24,26)/t18-,19+,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327267

(CHEMBL1258724 | endo-3-(8-(1-o-tolylethyl)-8-azabi...)Show SMILES CC(N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O)c1ccccc1C |r,TLB:1:2:8.7.9:5.4| Show InChI InChI=1S/C23H28N2O2/c1-15-6-3-4-9-22(15)16(2)25-18-10-11-19(25)14-21(13-18)27-20-8-5-7-17(12-20)23(24)26/h3-9,12,16,18-19,21H,10-11,13-14H2,1-2H3,(H2,24,26)/t16?,18-,19+,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327265
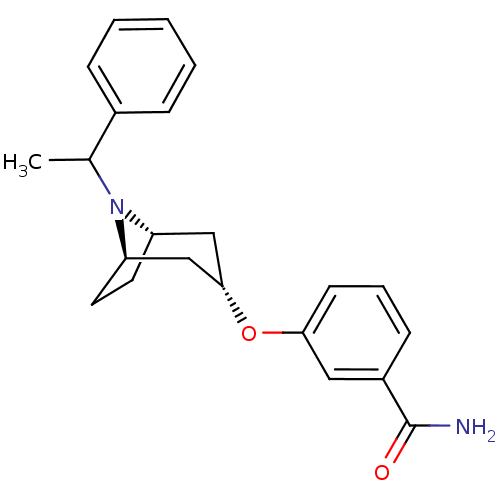
(CHEMBL1258615 | endo-3-(8-(1-phenylethyl)-8-azabic...)Show SMILES CC(N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O)c1ccccc1 |r,TLB:1:2:8.7.9:5.4| Show InChI InChI=1S/C22H26N2O2/c1-15(16-6-3-2-4-7-16)24-18-10-11-19(24)14-21(13-18)26-20-9-5-8-17(12-20)22(23)25/h2-9,12,15,18-19,21H,10-11,13-14H2,1H3,(H2,23,25)/t15?,18-,19+,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327261

(CHEMBL1258391 | endo-3-(8-(4-(methylsulfonylmethyl...)Show SMILES CS(=O)(=O)Cc1ccc(CN2[C@H]3CC[C@@H]2C[C@H](C3)Oc2cccc(c2)C(N)=O)cc1 |r,TLB:9:10:16.15.17:13.12| Show InChI InChI=1S/C23H28N2O4S/c1-30(27,28)15-17-7-5-16(6-8-17)14-25-19-9-10-20(25)13-22(12-19)29-21-4-2-3-18(11-21)23(24)26/h2-8,11,19-20,22H,9-10,12-15H2,1H3,(H2,24,26)/t19-,20+,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327254
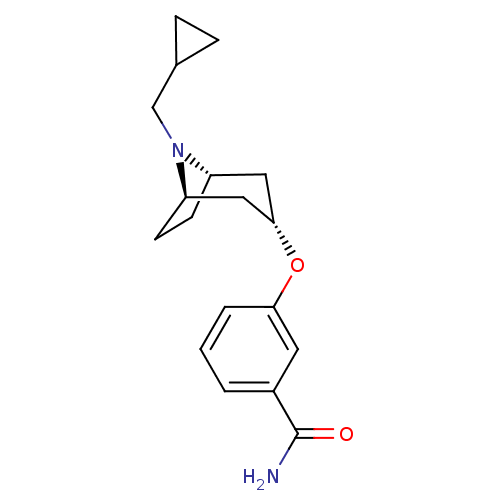
(CHEMBL1258166 | endo-3-(8-(cyclopropylmethyl)-8-az...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3CC2CC2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C18H24N2O2/c19-18(21)13-2-1-3-16(8-13)22-17-9-14-6-7-15(10-17)20(14)11-12-4-5-12/h1-3,8,12,14-15,17H,4-7,9-11H2,(H2,19,21)/t14-,15+,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50327256

(CHEMBL1258280 | endo-3-(8-(benzo[d][1,3]dioxol-5-y...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3Cc2ccc3OCOc3c2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C22H24N2O4/c23-22(25)15-2-1-3-18(9-15)28-19-10-16-5-6-17(11-19)24(16)12-14-4-7-20-21(8-14)27-13-26-20/h1-4,7-9,16-17,19H,5-6,10-13H2,(H2,23,25)/t16-,17+,19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327273
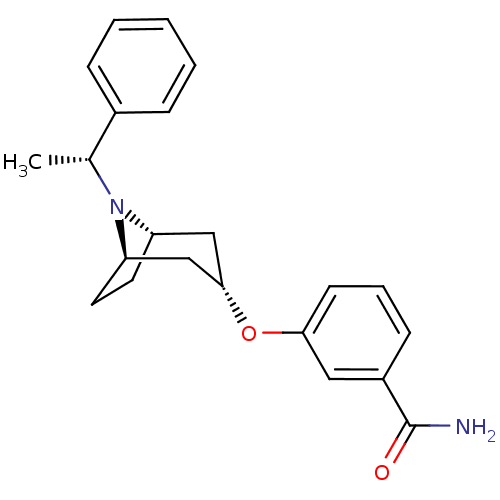
(CHEMBL1257226 | endo-3-(8-((R)-1-phenylethyl)-8-az...)Show SMILES C[C@@H](N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O)c1ccccc1 |r,TLB:1:2:8.7.9:5.4| Show InChI InChI=1S/C22H26N2O2/c1-15(16-6-3-2-4-7-16)24-18-10-11-19(24)14-21(13-18)26-20-9-5-8-17(12-20)22(23)25/h2-9,12,15,18-19,21H,10-11,13-14H2,1H3,(H2,23,25)/t15-,18-,19+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327278
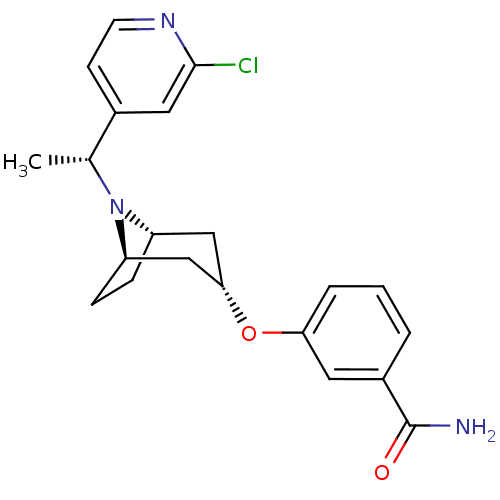
(CHEMBL1257459 | endo-3-(8-((R)-1-(2-chloropyridin-...)Show SMILES C[C@@H](N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O)c1ccnc(Cl)c1 |r,TLB:1:2:8.7.9:5.4| Show InChI InChI=1S/C21H24ClN3O2/c1-13(14-7-8-24-20(22)10-14)25-16-5-6-17(25)12-19(11-16)27-18-4-2-3-15(9-18)21(23)26/h2-4,7-10,13,16-17,19H,5-6,11-12H2,1H3,(H2,23,26)/t13-,16-,17+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327269

(CHEMBL1258845 | endo-3-(8-cyclohexyl-8-azabicyclo[...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3C2CCCCC2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C20H28N2O2/c21-20(23)14-5-4-8-18(11-14)24-19-12-16-9-10-17(13-19)22(16)15-6-2-1-3-7-15/h4-5,8,11,15-17,19H,1-3,6-7,9-10,12-13H2,(H2,21,23)/t16-,17+,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50327244

(3-Endo-(8-((5-methylthiophen-2-yl)methyl)-8-azabic...)Show SMILES Cc1ccc(CN2[C@H]3CC[C@@H]2C[C@H](C3)Oc2cccc(c2)C(N)=O)s1 |r,TLB:5:6:12.11.13:9.8| Show InChI InChI=1S/C20H24N2O2S/c1-13-5-8-19(25-13)12-22-15-6-7-16(22)11-18(10-15)24-17-4-2-3-14(9-17)20(21)23/h2-5,8-9,15-16,18H,6-7,10-12H2,1H3,(H2,21,23)/t15-,16+,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human delta opioid receptor assessed as inhibition of SNC80-stimulated [35S]GTPgammaS binding |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327263
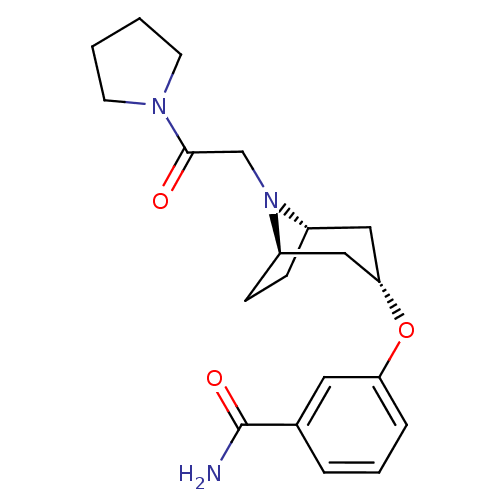
(CHEMBL1258502 | endo-3-(8-(2-oxo-2-(pyrrolidin-1-y...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3CC(=O)N2CCCC2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C20H27N3O3/c21-20(25)14-4-3-5-17(10-14)26-18-11-15-6-7-16(12-18)23(15)13-19(24)22-8-1-2-9-22/h3-5,10,15-16,18H,1-2,6-9,11-13H2,(H2,21,25)/t15-,16+,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327262
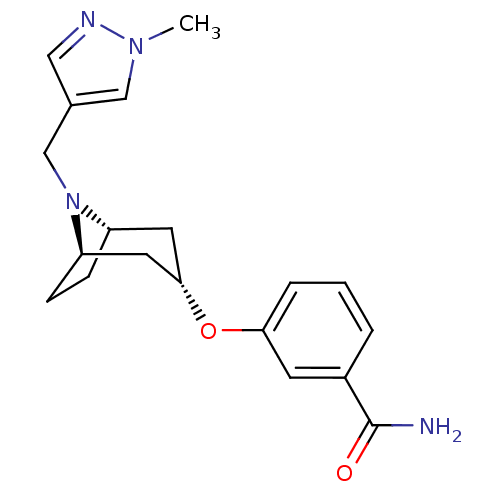
(CHEMBL1258392 | endo-3-(8-((1-methyl-1H-pyrazol-4-...)Show SMILES Cn1cc(CN2[C@H]3CC[C@@H]2C[C@H](C3)Oc2cccc(c2)C(N)=O)cn1 |r,TLB:4:5:11.10.12:8.7| Show InChI InChI=1S/C19H24N4O2/c1-22-11-13(10-21-22)12-23-15-5-6-16(23)9-18(8-15)25-17-4-2-3-14(7-17)19(20)24/h2-4,7,10-11,15-16,18H,5-6,8-9,12H2,1H3,(H2,20,24)/t15-,16+,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327260
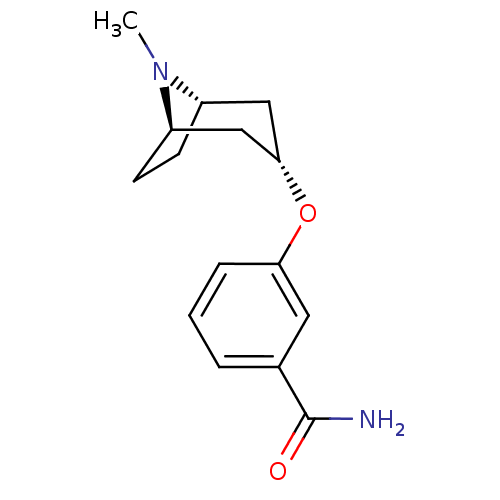
(CHEMBL1258165 | endo-3-(8-methyl-8-azabicyclo[3.2....)Show SMILES CN1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O |r,TLB:0:1:7.6.8:4.3| Show InChI InChI=1S/C15H20N2O2/c1-17-11-5-6-12(17)9-14(8-11)19-13-4-2-3-10(7-13)15(16)18/h2-4,7,11-12,14H,5-6,8-9H2,1H3,(H2,16,18)/t11-,12+,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50327261

(CHEMBL1258391 | endo-3-(8-(4-(methylsulfonylmethyl...)Show SMILES CS(=O)(=O)Cc1ccc(CN2[C@H]3CC[C@@H]2C[C@H](C3)Oc2cccc(c2)C(N)=O)cc1 |r,TLB:9:10:16.15.17:13.12| Show InChI InChI=1S/C23H28N2O4S/c1-30(27,28)15-17-7-5-16(6-8-17)14-25-19-9-10-20(25)13-22(12-19)29-21-4-2-3-18(11-21)23(24)26/h2-8,11,19-20,22H,9-10,12-15H2,1H3,(H2,24,26)/t19-,20+,22- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50130561

((R)-N-((S)-1-((3R,4R)-4-(3-hydroxyphenyl)-3,4-dime...)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C28H39N3O2/c1-19(2)26(30-27(33)25-14-21-8-5-6-9-22(21)16-29-25)18-31-13-12-28(4,20(3)17-31)23-10-7-11-24(32)15-23/h5-11,15,19-20,25-26,29,32H,12-14,16-18H2,1-4H3,(H,30,33)/t20-,25+,26+,28+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50327276

(CHEMBL1257348 | endo-3-(8-((R)-1-(3-methoxyphenyl)...)Show SMILES COc1cccc(c1)[C@@H](C)N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O |r,TLB:8:10:16.15.17:13.12| Show InChI InChI=1S/C23H28N2O3/c1-15(16-5-3-7-20(11-16)27-2)25-18-9-10-19(25)14-22(13-18)28-21-8-4-6-17(12-21)23(24)26/h3-8,11-12,15,18-19,22H,9-10,13-14H2,1-2H3,(H2,24,26)/t15-,18-,19+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50327248

(CHEMBL1257821 | endo-3-(8-(4-methylbenzyl)-8-azabi...)Show SMILES Cc1ccc(CN2[C@H]3CC[C@@H]2C[C@H](C3)Oc2cccc(c2)C(N)=O)cc1 |r,TLB:5:6:12.11.13:9.8| Show InChI InChI=1S/C22H26N2O2/c1-15-5-7-16(8-6-15)14-24-18-9-10-19(24)13-21(12-18)26-20-4-2-3-17(11-20)22(23)25/h2-8,11,18-19,21H,9-10,12-14H2,1H3,(H2,23,25)/t18-,19+,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data