

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043804 (5-(2-Amino-3-phenyl-propionylamino)-3-isobutyl-7-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant full length stromelysin (FLS) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043804 (5-(2-Amino-3-phenyl-propionylamino)-3-isobutyl-7-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant stromelysin catalytic domain (SCD) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043807 ((S)-2-Benzyloxycarbonylamino-3-(1H-indol-3-yl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant stromelysin catalytic domain (SCD) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043807 ((S)-2-Benzyloxycarbonylamino-3-(1H-indol-3-yl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant full length stromelysin (FLS) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043801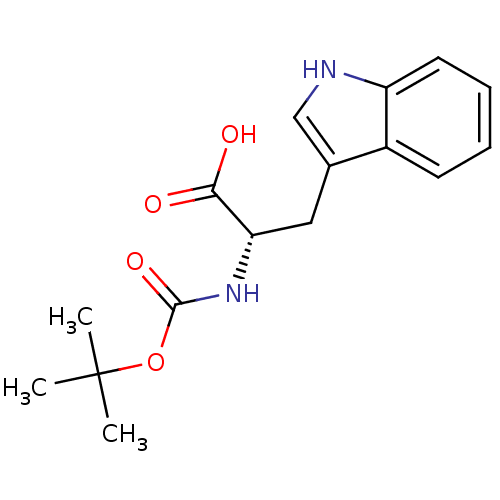 ((S)-2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant stromelysin catalytic domain (SCD) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043805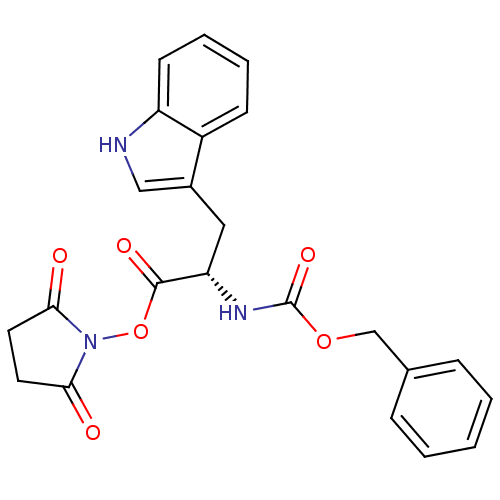 ((S)-2-Benzyloxycarbonylamino-3-(1H-indol-3-yl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant stromelysin catalytic domain (SCD) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043803 ((S)-2-Benzyloxycarbonylamino-3-(4-hydroxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant full length stromelysin (FLS) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043803 ((S)-2-Benzyloxycarbonylamino-3-(4-hydroxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant stromelysin catalytic domain (SCD) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043813 ((S)-2-tert-Butoxycarbonylamino-3-(1-formyl-1H-indo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant stromelysin catalytic domain (SCD) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043796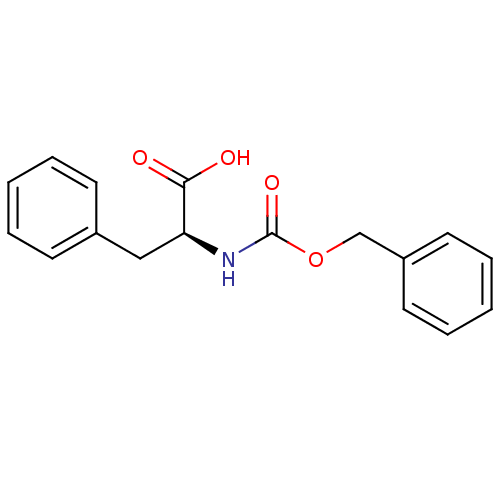 ((S)-2-Benzyloxycarbonylamino-3-phenyl-propionic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant stromelysin catalytic domain (SCD) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043806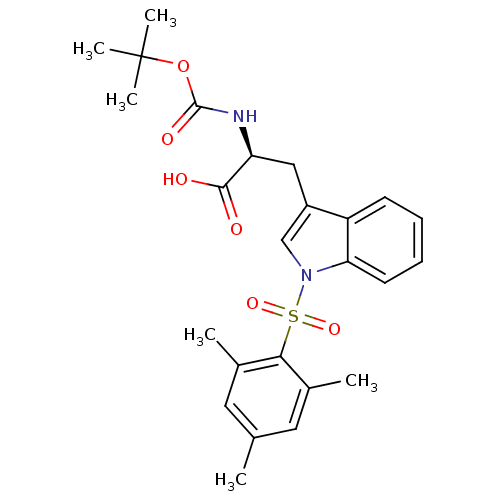 ((S)-2-tert-Butoxycarbonylamino-3-[1-(2,4,6-trimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant stromelysin catalytic domain (SCD) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043809 ((R)-2-Benzyloxycarbonylamino-3-(1H-indol-3-yl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant stromelysin catalytic domain (SCD) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043796 ((S)-2-Benzyloxycarbonylamino-3-phenyl-propionic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant full length stromelysin (FLS) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043812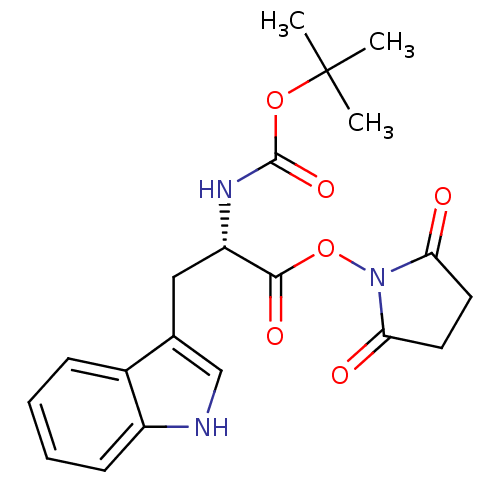 ((S)-2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant stromelysin catalytic domain (SCD) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043800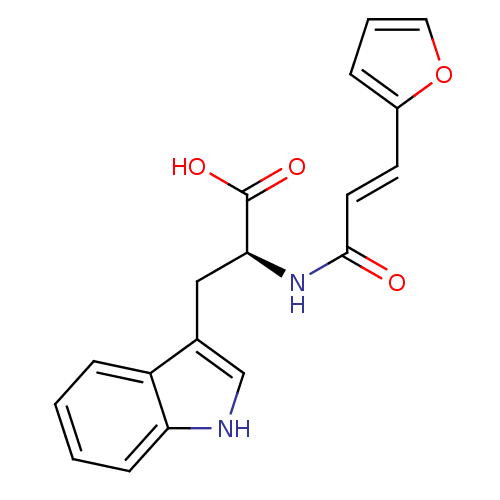 ((S)-2-((E)-3-Furan-2-yl-acryloylamino)-3-(1H-indol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant stromelysin catalytic domain (SCD) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043809 ((R)-2-Benzyloxycarbonylamino-3-(1H-indol-3-yl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant full length stromelysin (FLS) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043797 ((R)-2-Benzyloxycarbonylamino-3-(4-hydroxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant stromelysin catalytic domain (SCD) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043797 ((R)-2-Benzyloxycarbonylamino-3-(4-hydroxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant full length stromelysin (FLS) | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043804 (5-(2-Amino-3-phenyl-propionylamino)-3-isobutyl-7-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant full length stromelysin (FLS). | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043804 (5-(2-Amino-3-phenyl-propionylamino)-3-isobutyl-7-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of recombinant stromelysin catalytic domain. | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109692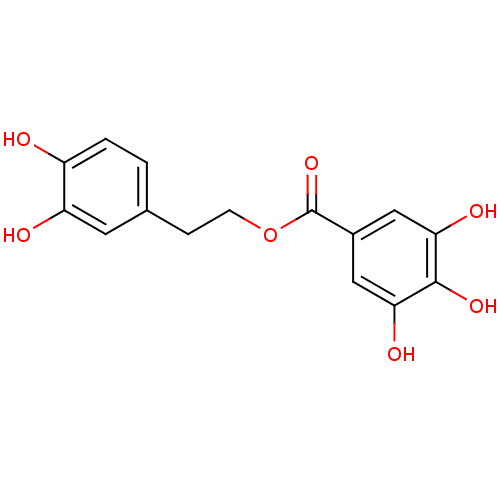 (3,4,5-Trihydroxy-benzoic acid 2-(3,4-dihydroxy-phe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase strand transfer using A17 double mutant HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50070942 ((-)-Epigallocatechin gallate | (-)-Epigallocatechi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Inhibition of polymerization in wild type HIV-1 RT with poly rC/dG12-18 template primer and [3H]dGTP | Bioorg Med Chem Lett 11: 2763-7 (2001) BindingDB Entry DOI: 10.7270/Q2X92BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50153015 ((-)-Epicatechin-3-gallate | (-)-epicatechin 3-O-ga...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Inhibition of polymerization in wild type HIV-1 RT with poly rC/dG12-18 template primer and [3H]dGTP | Bioorg Med Chem Lett 11: 2763-7 (2001) BindingDB Entry DOI: 10.7270/Q2X92BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109689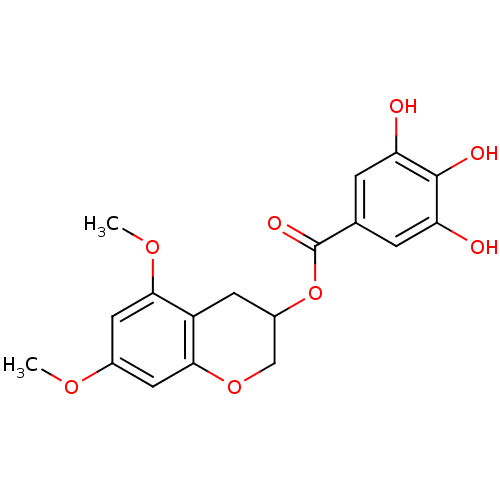 (3,4,5-Trihydroxy-benzoic acid 5,7-dimethoxy-chroma...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase polymerization using A17 double mutant HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50105589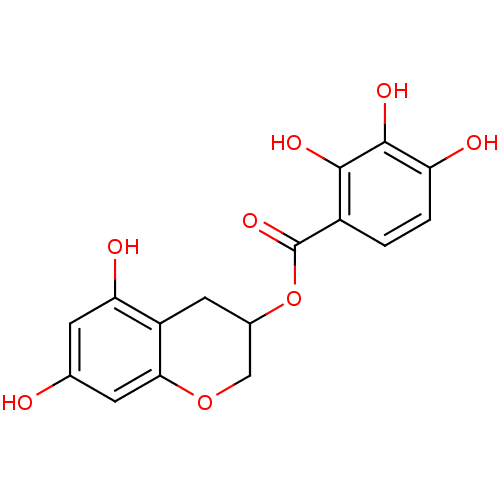 (2,3,4-Trihydroxy-benzoic acid 5,7-dihydroxy-chroma...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Inhibitory concentration against polymerization in A17 double mutant HIV-1 RT using a template primer of poly rC/dG12-18 and [3H]dGTP | Bioorg Med Chem Lett 11: 2763-7 (2001) BindingDB Entry DOI: 10.7270/Q2X92BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109688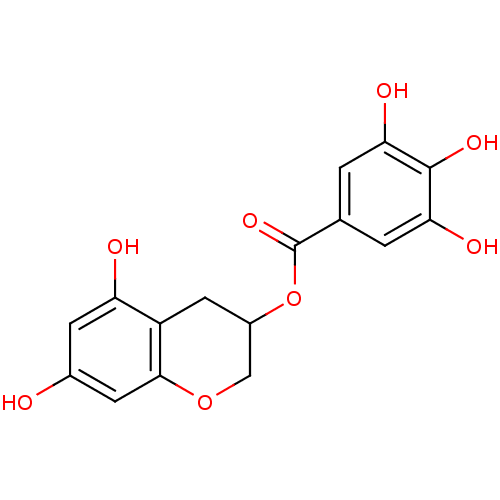 (3,4,5-Trihydroxy-benzoic acid 5,7-dihydroxy-chroma...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase polymerization using A17 double mutant HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043807 ((S)-2-Benzyloxycarbonylamino-3-(1H-indol-3-yl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of recombinant stromelysin catalytic domain. | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109694 (3,4,5-Trihydroxy-benzoic acid chroman-3-yl ester |...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase strand transfer using A17 double mutant HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109688 (3,4,5-Trihydroxy-benzoic acid 5,7-dihydroxy-chroma...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase polymerization using wild type HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50105589 (2,3,4-Trihydroxy-benzoic acid 5,7-dihydroxy-chroma...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Inhibition of polymerization in wild type HIV-1 RT with poly rC/dG12-18 template primer and [3H]dGTP | Bioorg Med Chem Lett 11: 2763-7 (2001) BindingDB Entry DOI: 10.7270/Q2X92BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109690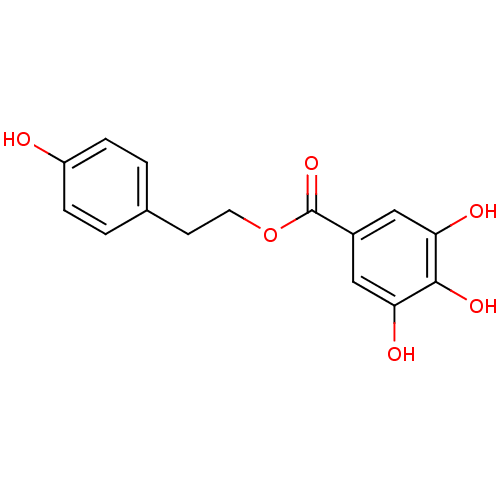 (3,4,5-Trihydroxy-benzoic acid 2-(4-hydroxy-phenyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase strand transfer using wild type HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109688 (3,4,5-Trihydroxy-benzoic acid 5,7-dihydroxy-chroma...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase polymerization using wild type HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109694 (3,4,5-Trihydroxy-benzoic acid chroman-3-yl ester |...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase strand transfer using wild type HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109688 (3,4,5-Trihydroxy-benzoic acid 5,7-dihydroxy-chroma...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase polymerization using A17 double mutant HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109692 (3,4,5-Trihydroxy-benzoic acid 2-(3,4-dihydroxy-phe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase polymerization using A17 double mutant HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50105592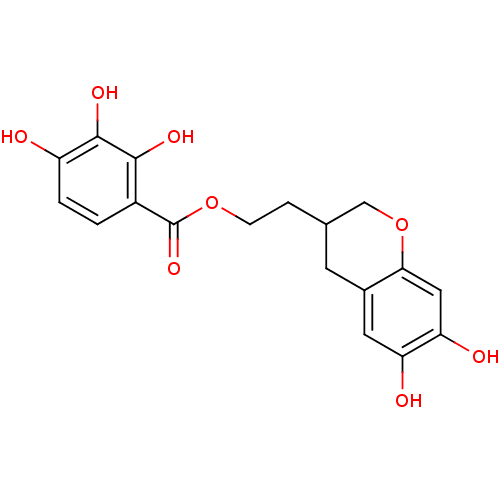 (2,3,4-Trihydroxy-benzoic acid 2-(6,7-dihydroxy-chr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Inhibitory concentration against polymerization in A17 double mutant HIV-1 RT using a template primer of poly rC/dG12-18 and [3H]dGTP | Bioorg Med Chem Lett 11: 2763-7 (2001) BindingDB Entry DOI: 10.7270/Q2X92BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109689 (3,4,5-Trihydroxy-benzoic acid 5,7-dimethoxy-chroma...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase strand transfer using wild type HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109695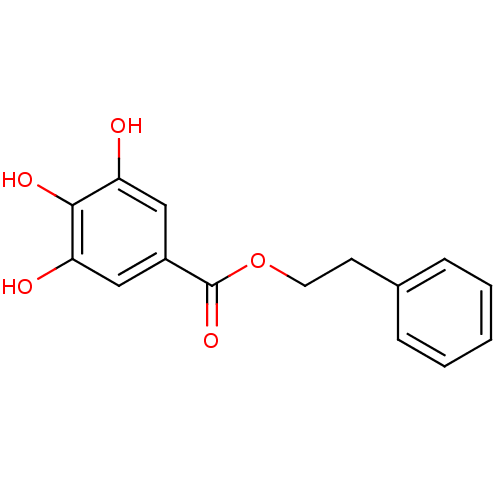 (3,4,5-Trihydroxy-benzoic acid phenethyl ester | CH...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase polymerization using A17 double mutant HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043807 ((S)-2-Benzyloxycarbonylamino-3-(1H-indol-3-yl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant full length stromelysin (FLS). | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109696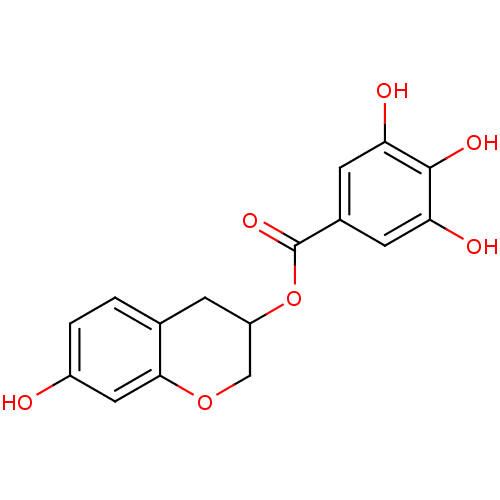 (3,4,5-Trihydroxy-benzoic acid 7-hydroxy-chroman-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase polymerization using A17 double mutant HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109690 (3,4,5-Trihydroxy-benzoic acid 2-(4-hydroxy-phenyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase strand transfer using A17 double mutant HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109697 (3,4,5-Trihydroxy-benzoic acid 5-hydroxy-chroman-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase strand transfer using wild type HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043801 ((S)-2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of recombinant stromelysin catalytic domain. | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109692 (3,4,5-Trihydroxy-benzoic acid 2-(3,4-dihydroxy-phe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase strand transfer using wild type HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109692 (3,4,5-Trihydroxy-benzoic acid 2-(3,4-dihydroxy-phe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase polymerization using wild type HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50105592 (2,3,4-Trihydroxy-benzoic acid 2-(6,7-dihydroxy-chr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Inhibition of polymerization in wild type HIV-1 RT with poly rC/dG12-18 template primer and [3H]dGTP | Bioorg Med Chem Lett 11: 2763-7 (2001) BindingDB Entry DOI: 10.7270/Q2X92BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043805 ((S)-2-Benzyloxycarbonylamino-3-(1H-indol-3-yl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of recombinant stromelysin catalytic domain. | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043803 ((S)-2-Benzyloxycarbonylamino-3-(4-hydroxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant full length stromelysin (FLS). | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50043803 ((S)-2-Benzyloxycarbonylamino-3-(4-hydroxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of recombinant stromelysin catalytic domain. | J Med Chem 37: 206-9 (1994) BindingDB Entry DOI: 10.7270/Q2X34WH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50109696 (3,4,5-Trihydroxy-benzoic acid 7-hydroxy-chroman-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase polymerization using wild type HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 108 total ) | Next | Last >> |