

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200636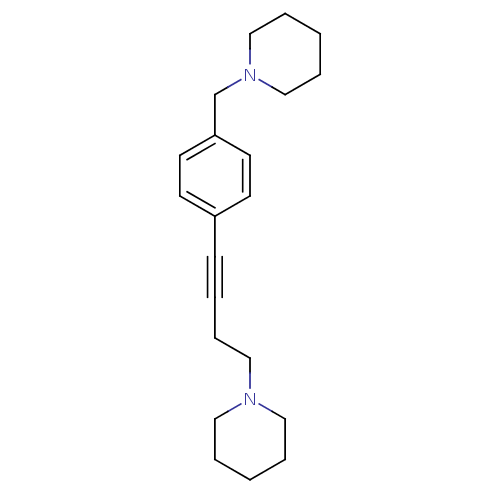 (1-(4-(4-(piperidin-1-ylmethyl)phenyl)but-3-ynyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414797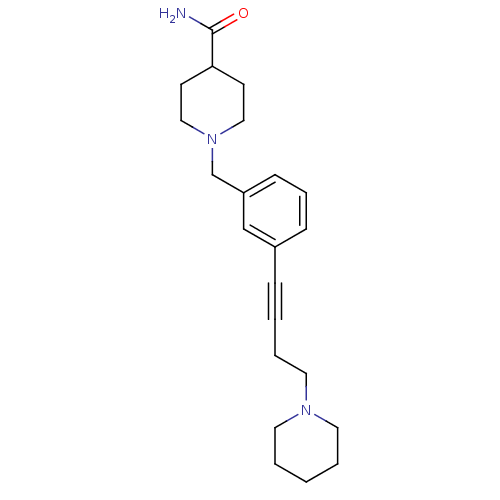 (CHEMBL582977) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414804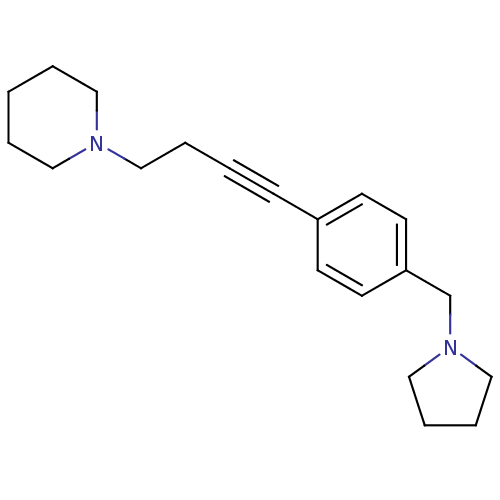 (CHEMBL574712) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414811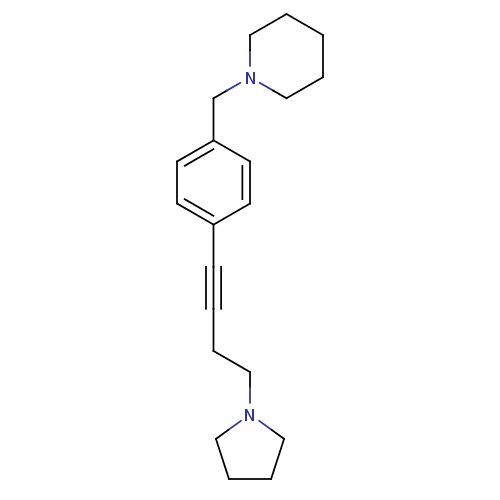 (CHEMBL574721) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to the human histamine H3 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414798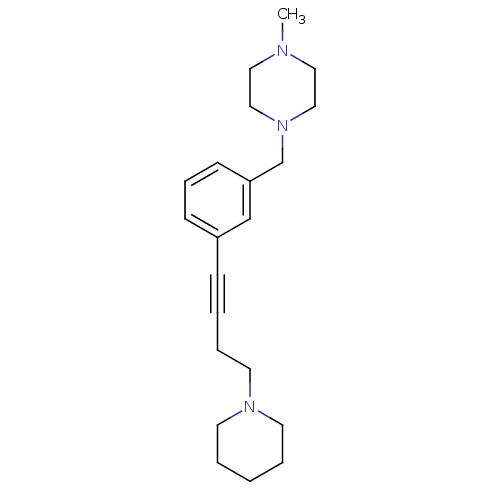 (CHEMBL575172) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198598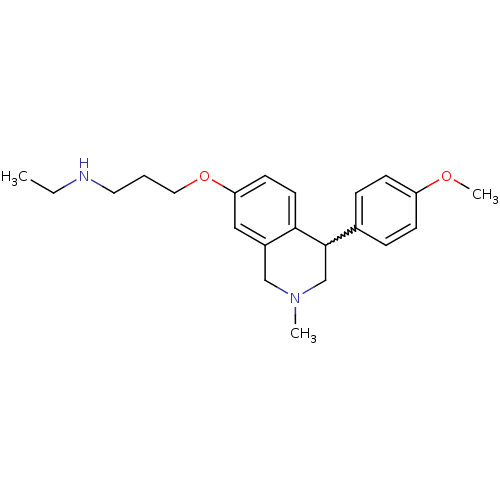 (CHEMBL396945 | N-ethyl-3-(4-(4-methoxyphenyl)-2-me...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414800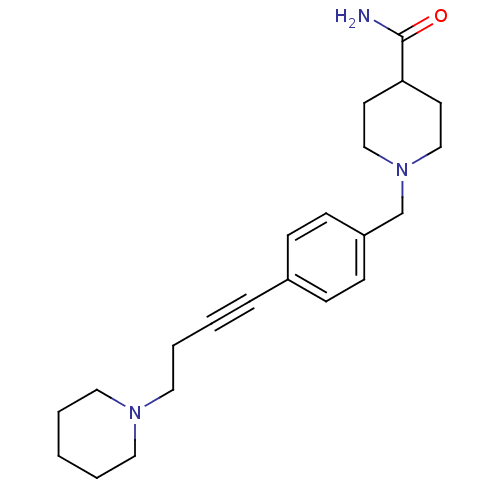 (CHEMBL583182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414792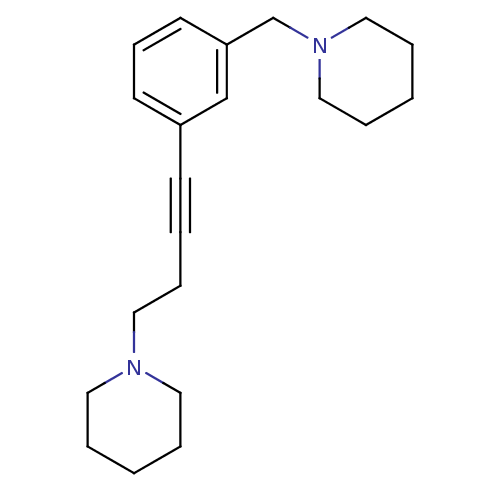 (CHEMBL573817) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414813 (CHEMBL573328) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414799 (CHEMBL573815) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414806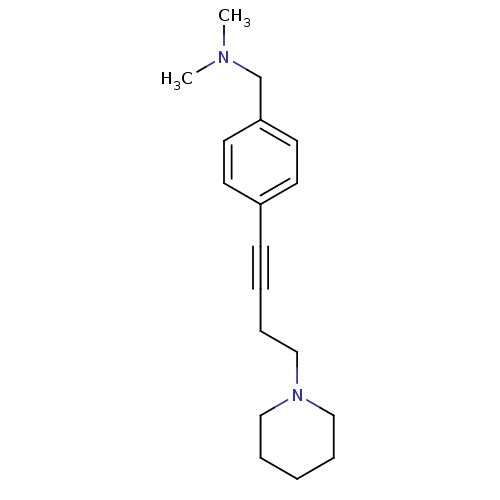 (CHEMBL572845) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414803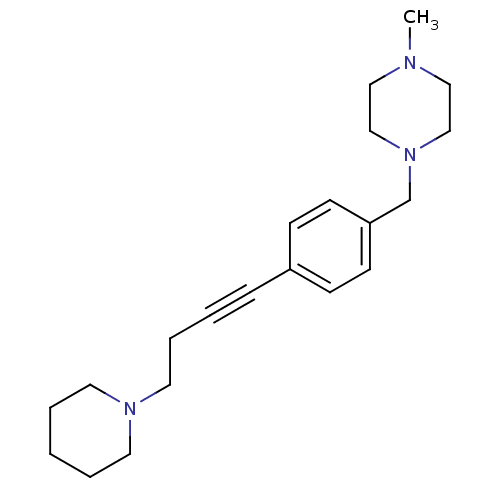 (CHEMBL573321) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM396409 (3-[2-(Azetidin-3-yloxy)-4-chloro-phenoxymethyl]-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Receptor binding was performed using membrane fractions prepared from the HEK-293 cell line recombinantly expressing rat 5-HT7 receptors (NCBI access... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q26T0Q0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50011237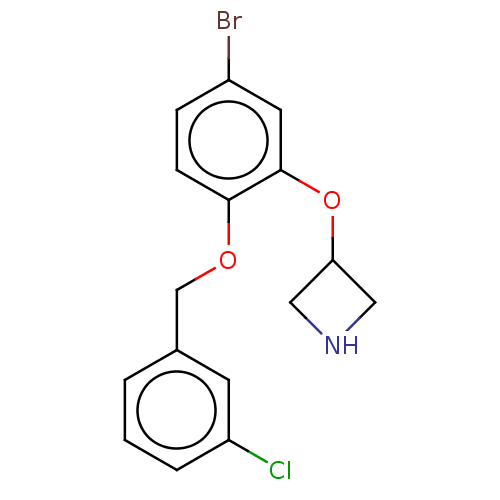 (CHEMBL3260334 | US9981909, Example 2) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Receptor binding was performed using membrane fractions prepared from the HEK-293 cell line recombinantly expressing rat 5-HT7 receptors (NCBI access... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q26T0Q0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198609 (7-(3-(azetidin-1-yl)propoxy)-4-(4-methoxyphenyl)-2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50255824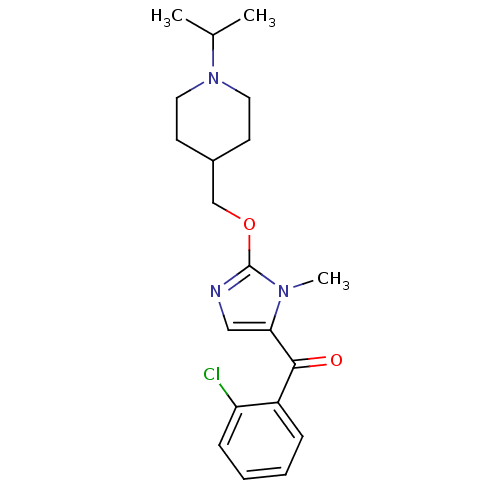 ((2-chlorophenyl)(2-((1-isopropylpiperidin-4-yl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 19: 903-7 (2009) Article DOI: 10.1016/j.bmcl.2008.11.114 BindingDB Entry DOI: 10.7270/Q2X63MTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50255823 ((2-((1-isopropylpiperidin-4-yl)methoxy)-1-methyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 19: 903-7 (2009) Article DOI: 10.1016/j.bmcl.2008.11.114 BindingDB Entry DOI: 10.7270/Q2X63MTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM396607 (3-[5-Bromo-2-(5-trifluoromethyl-furan-2-ylmethoxy)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Receptor binding was performed using membrane fractions prepared from the HEK-293 cell line recombinantly expressing rat 5-HT7 receptors (NCBI access... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q26T0Q0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414805 (CHEMBL574711) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50217569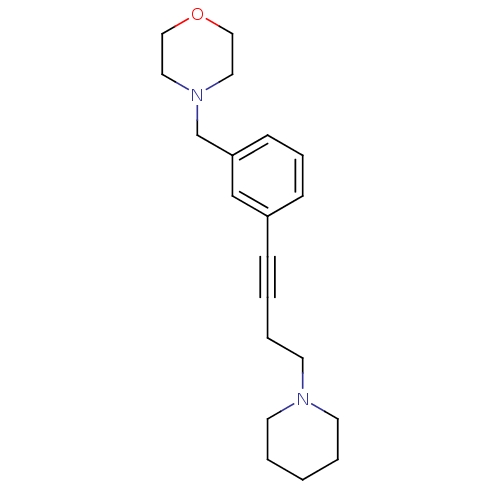 (4-(3-(4-(piperidin-1-yl)but-1-ynyl)benzyl)morpholi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198585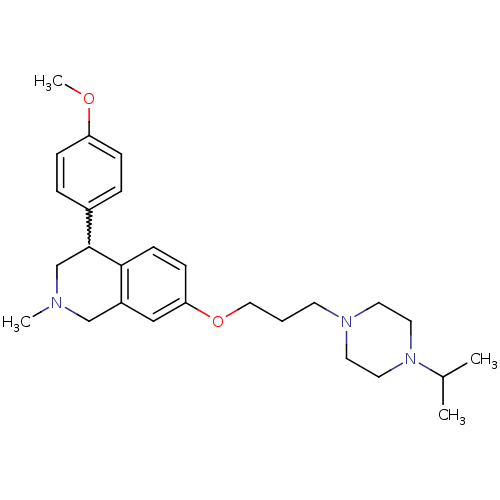 (7-(3-(4-isopropylpiperazin-1-yl)propoxy)-4-(4-meth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414812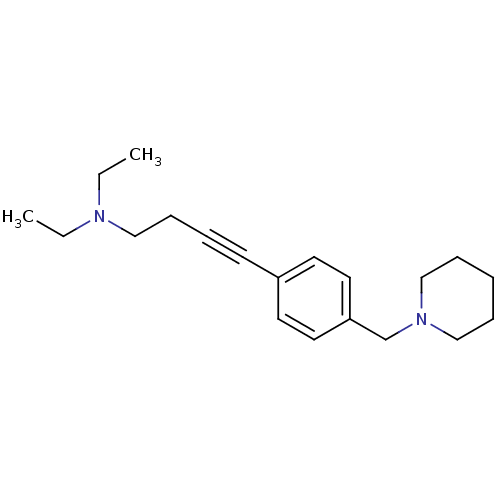 (CHEMBL574720) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198599 (1-(3-(4-(4-methoxyphenyl)-2-methyl-1,2,3,4-tetrahy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198607 (7-(3-(4-ethylpiperazin-1-yl)propoxy)-4-(4-methoxyp...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198593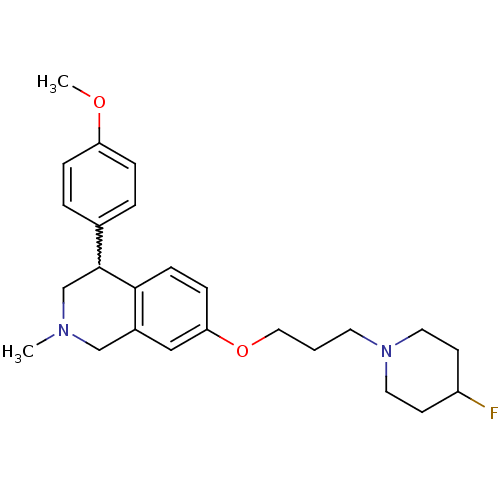 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(4-methoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at rat SERT | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(4-methoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50198596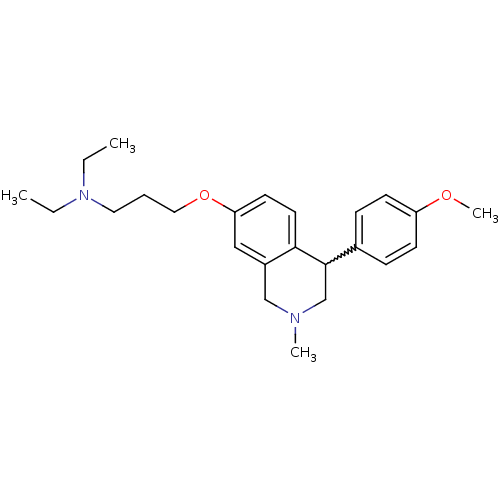 (CHEMBL427845 | N,N-diethyl-3-(4-(4-methoxyphenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(4-methoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at rat SERT | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(4-methoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at rat SERT | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414793 (CHEMBL573090) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50198585 (7-(3-(4-isopropylpiperazin-1-yl)propoxy)-4-(4-meth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to human SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414802 (CHEMBL584182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM396570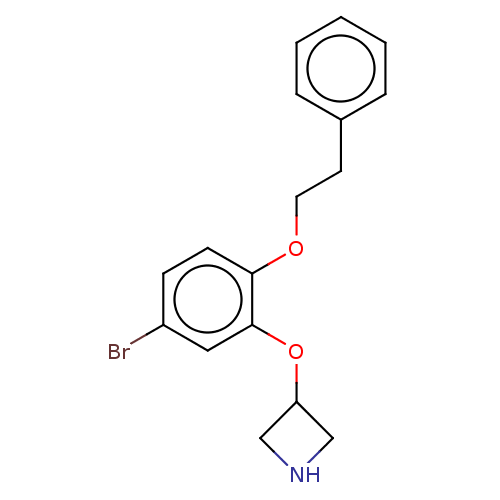 (3-(5-Bromo-2-phenethyloxy-phenoxy)-azetidine | US9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Receptor binding was performed using membrane fractions prepared from the HEK-293 cell line recombinantly expressing rat 5-HT7 receptors (NCBI access... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q26T0Q0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM396633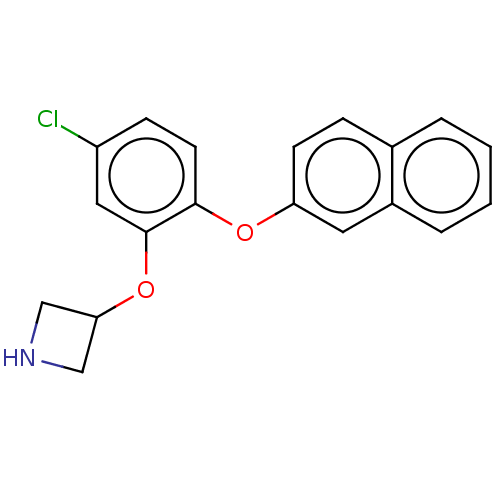 (3-[5-Chloro-2-(naphthalen-2-yloxy)-phenoxy]-azetid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Receptor binding was performed using membrane fractions prepared from the HEK-293 cell line recombinantly expressing rat 5-HT7 receptors (NCBI access... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q26T0Q0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198604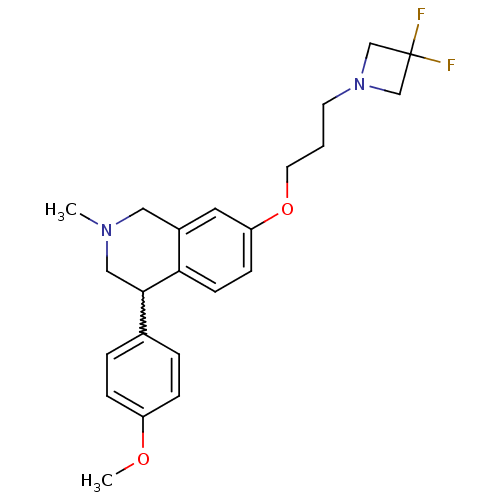 (7-(3-(3,3-difluoroazetidin-1-yl)propoxy)-4-(4-meth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM30130 (CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to human SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198590 (4-(4-methoxyphenyl)-2-methyl-7-(3-(piperidin-1-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50198593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(4-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50199601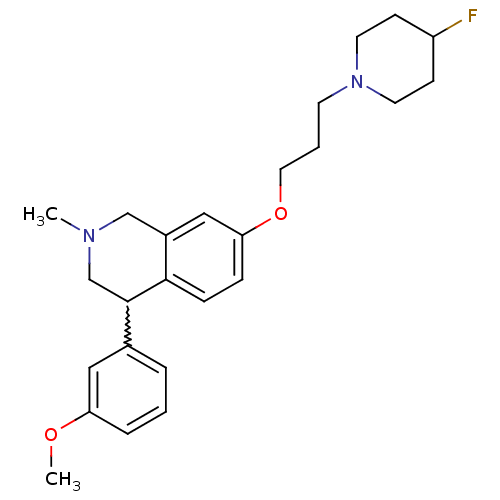 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(3-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at human histamine H3 receptor | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50199600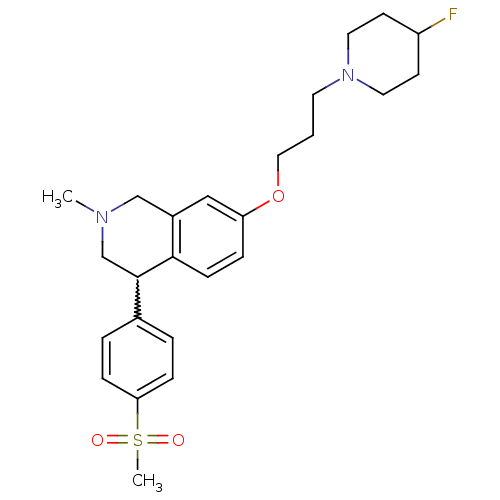 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-2-methyl-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at human histamine H3 receptor | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50199593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-2-methyl-4-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at rat SERT | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50198593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(4-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at human histamine H3 receptor | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50198607 (7-(3-(4-ethylpiperazin-1-yl)propoxy)-4-(4-methoxyp...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to human SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198602 ((1-(3-(4-(4-methoxyphenyl)-2-methyl-1,2,3,4-tetrah...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50255928 ((4-chlorophenyl)(2-(1-isopropylpiperidin-4-ylthio)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 19: 903-7 (2009) Article DOI: 10.1016/j.bmcl.2008.11.114 BindingDB Entry DOI: 10.7270/Q2X63MTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50199593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-2-methyl-4-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at rat SERT | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50198590 (4-(4-methoxyphenyl)-2-methyl-7-(3-(piperidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at human histamine H3 receptor | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM396384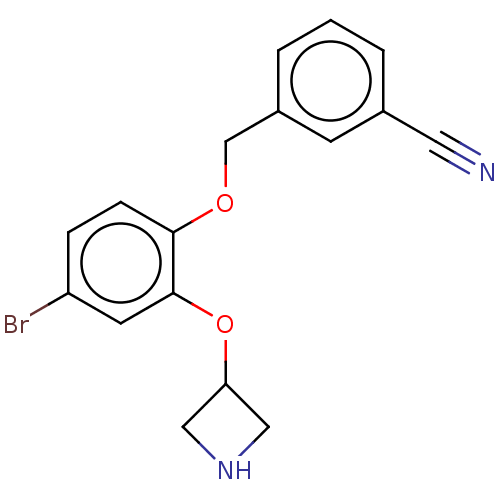 (3-[2-(Azetidin-3-yloxy)-4-bromo-phenoxymethyl]-ben...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Receptor binding was performed using membrane fractions prepared from the HEK-293 cell line recombinantly expressing rat 5-HT7 receptors (NCBI access... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q26T0Q0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50240620 (1-(4-chlorobenzyl)-1-(7-(pyrrolidin-1-yl)heptyl)gu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding affinity to guinea pig histamine H3 receptor | Bioorg Med Chem Lett 19: 903-7 (2009) Article DOI: 10.1016/j.bmcl.2008.11.114 BindingDB Entry DOI: 10.7270/Q2X63MTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1131 total ) | Next | Last >> |