

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166868 (CHEMBL3797635) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166873 (CHEMBL3800456) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166871 (CHEMBL3797841) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166866 (CHEMBL3798156) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166866 (CHEMBL3798156) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 assessed as enzyme ina... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166869 (CHEMBL3799744) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166890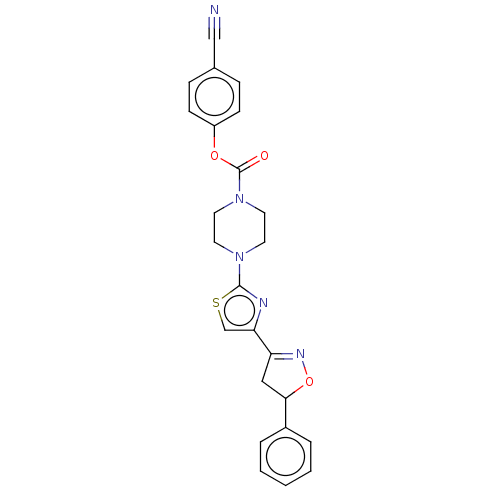 (CHEMBL3797809) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166867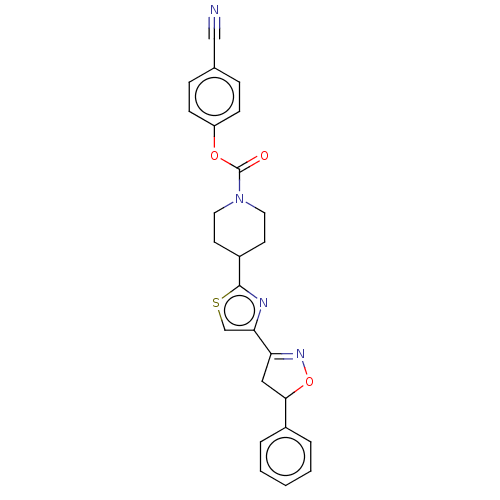 (CHEMBL3798476) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 assessed as enzyme ina... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166867 (CHEMBL3798476) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166910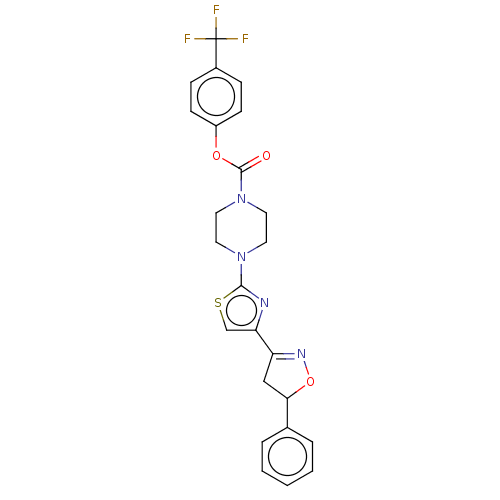 (CHEMBL3798355) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166911 (CHEMBL3799516) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166878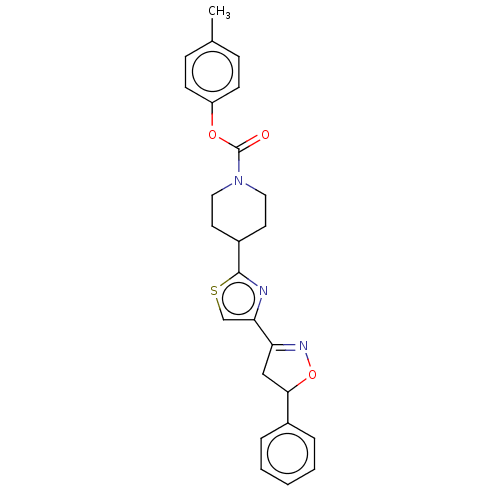 (CHEMBL3800599) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166891 (CHEMBL3799008) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166870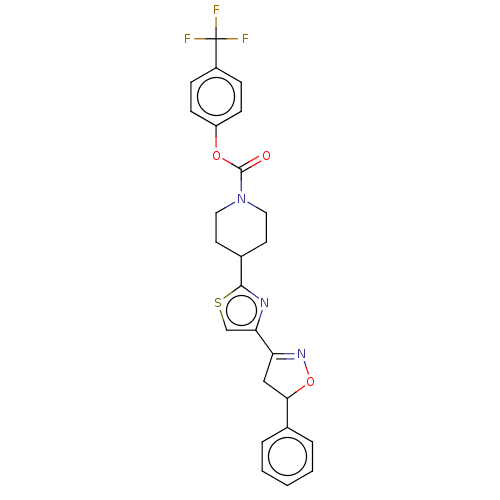 (CHEMBL3797226) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166875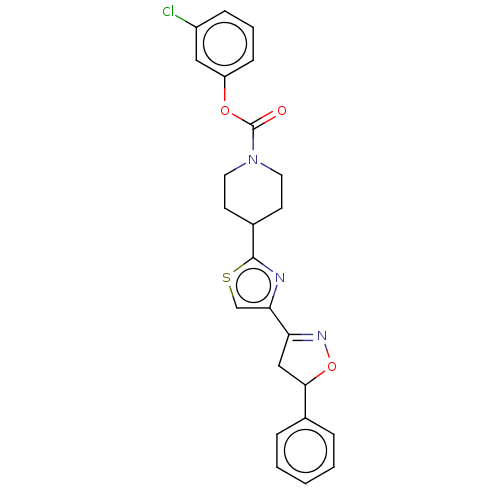 (CHEMBL3797377) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50379543 (CHEMBL2012752) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329261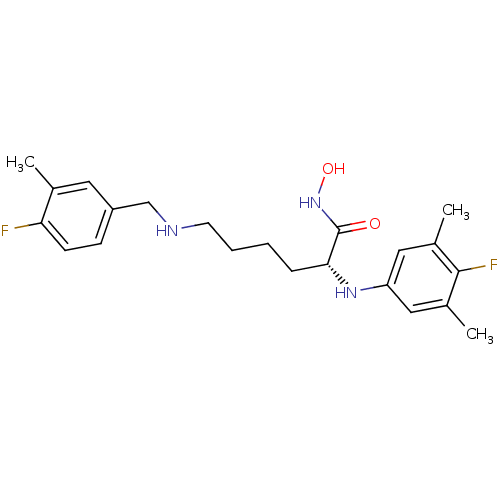 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate after 4 hrs by FRET assay | Bioorg Med Chem Lett 21: 2030-3 (2011) Article DOI: 10.1016/j.bmcl.2011.02.010 BindingDB Entry DOI: 10.7270/Q24M94VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329261 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate by FRET assay | Bioorg Med Chem Lett 20: 6850-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.058 BindingDB Entry DOI: 10.7270/Q2222V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166877 (CHEMBL3798594) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 assessed as enzyme ina... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166877 (CHEMBL3798594) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085734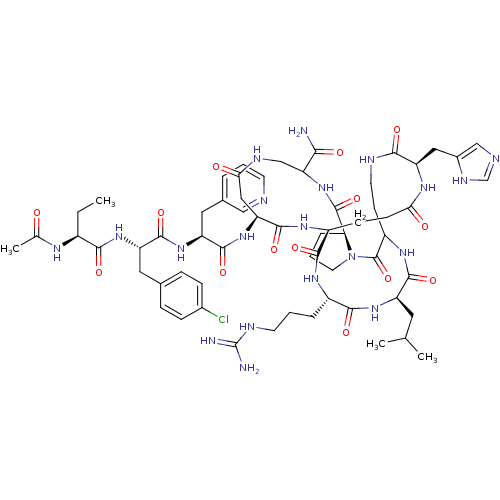 (CHEMBL407628 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50379542 (CHEMBL2012753) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085807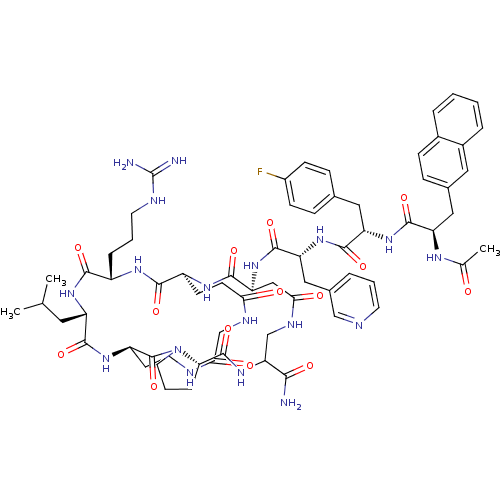 (CHEMBL425966 | Gonadotropin Releasing Hormone anal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Affinity for rat gonadotrophin releasing hormone (GnRH) receptor using HEK-293 cells transfected with rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 807-18 (2000) BindingDB Entry DOI: 10.7270/Q28P5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085777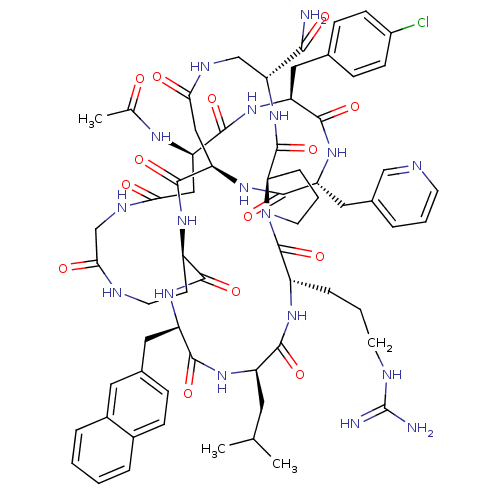 (CHEMBL386166 | Gonadotropin Releasing Hormone anal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Affinity for rat gonadotrophin releasing hormone (GnRH) receptor using HEK-293 cells transfected with rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 807-18 (2000) BindingDB Entry DOI: 10.7270/Q28P5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50340754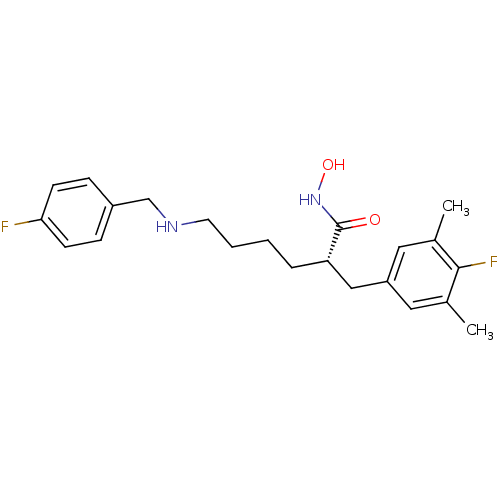 ((S)-2-(4-fluoro-3,5-dimethylbenzyl)-6-(4-fluoroben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50340754 ((S)-2-(4-fluoro-3,5-dimethylbenzyl)-6-(4-fluoroben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate after 4 hrs by FRET assay | Bioorg Med Chem Lett 21: 2030-3 (2011) Article DOI: 10.1016/j.bmcl.2011.02.010 BindingDB Entry DOI: 10.7270/Q24M94VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50166889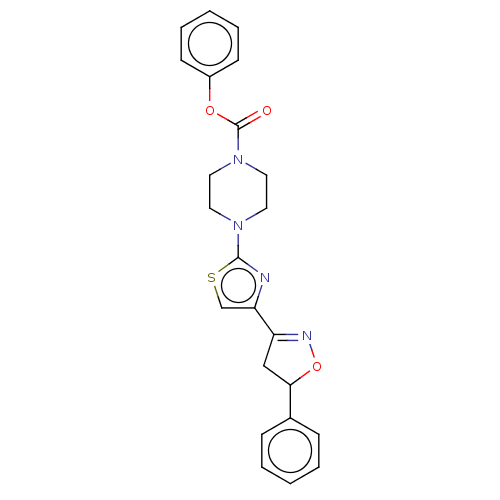 (CHEMBL3799633) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E.I. Du Pont de Nemours and Company Curated by ChEMBL | Assay Description Inhibition of MBP-fused human recombinant FAAH with truncated N-terminal transmembrane domain expressed in Escherichia coli T7 using D-AMC substrate ... | Bioorg Med Chem Lett 26: 2965-2973 (2016) Article DOI: 10.1016/j.bmcl.2016.02.061 BindingDB Entry DOI: 10.7270/Q2M32XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085791 (CHEMBL265240 | Gonadotropin Releasing Hormone anal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Affinity for rat gonadotrophin releasing hormone (GnRH) receptor using HEK-293 cells transfected with rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 807-18 (2000) BindingDB Entry DOI: 10.7270/Q28P5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085715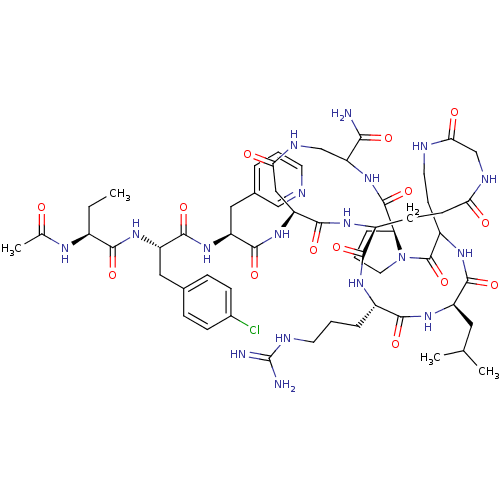 (CHEMBL266300 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50379536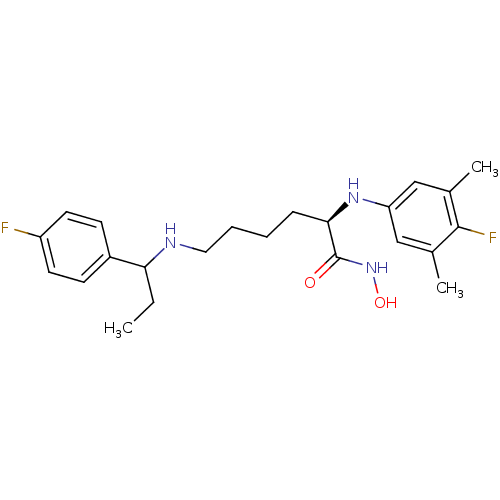 (CHEMBL2012836) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085710 (CHEMBL414756 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085719 (CHEMBL438543 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085718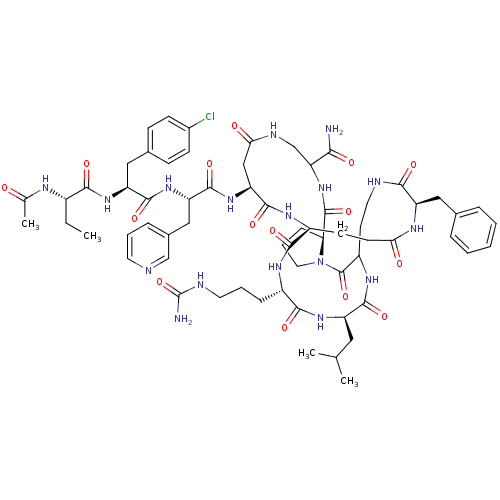 (CHEMBL410820 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085799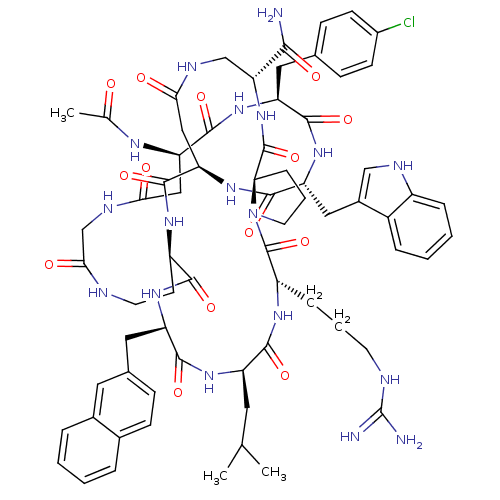 (CHEMBL439083 | Gonadotropin Releasing Hormone anal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Affinity for rat gonadotrophin releasing hormone (GnRH) receptor using HEK-293 cells transfected with rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 807-18 (2000) BindingDB Entry DOI: 10.7270/Q28P5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085797 (CHEMBL407361 | Gonadotropin Releasing Hormone anal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Affinity for rat gonadotrophin releasing hormone (GnRH) receptor using HEK-293 cells transfected with rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 807-18 (2000) BindingDB Entry DOI: 10.7270/Q28P5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085720 (CHEMBL404930 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50379541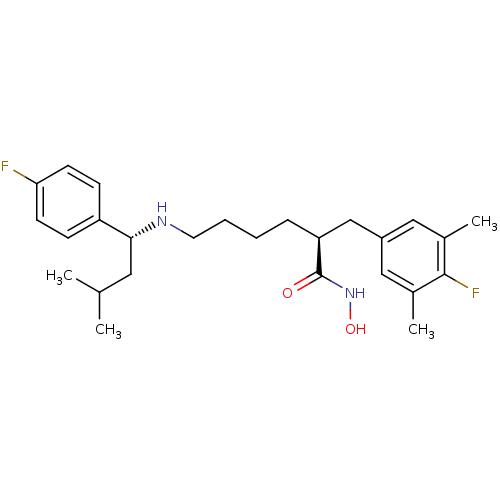 (CHEMBL2012832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085737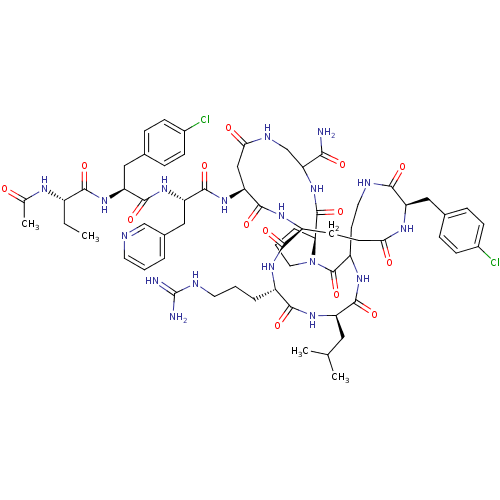 (CHEMBL438597 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085783 (CHEMBL438897 | Gonadotropin Releasing Hormone anal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Affinity for rat gonadotrophin releasing hormone (GnRH) receptor using HEK-293 cells transfected with rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 807-18 (2000) BindingDB Entry DOI: 10.7270/Q28P5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329265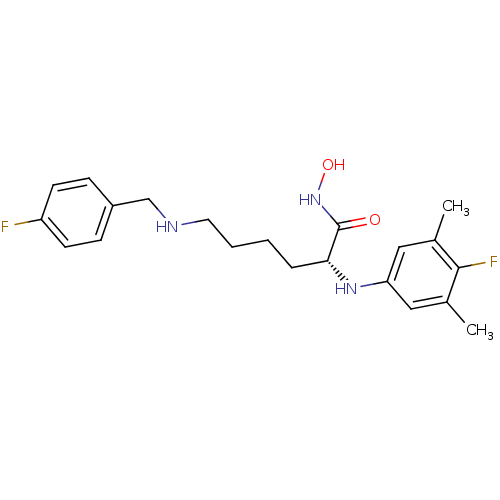 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329265 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate after 4 hrs by FRET assay | Bioorg Med Chem Lett 21: 2030-3 (2011) Article DOI: 10.1016/j.bmcl.2011.02.010 BindingDB Entry DOI: 10.7270/Q24M94VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329265 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate by FRET assay | Bioorg Med Chem Lett 20: 6850-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.058 BindingDB Entry DOI: 10.7270/Q2222V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085771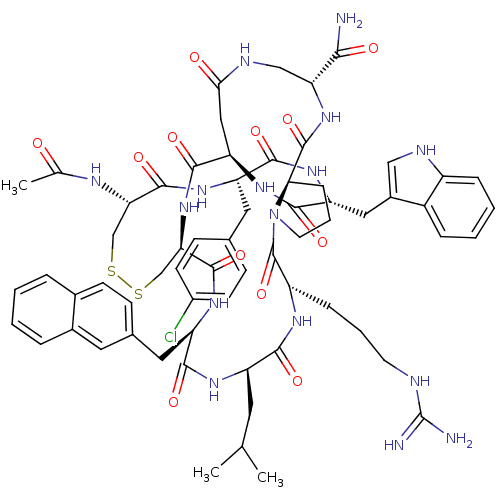 (CHEMBL436657 | Gonadotropin Releasing Hormone anal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Affinity for rat gonadotrophin releasing hormone (GnRH) receptor using HEK-293 cells transfected with rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 807-18 (2000) BindingDB Entry DOI: 10.7270/Q28P5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50340755 ((S)-2-(4-fluoro-3-methylbenzyl)-6-(4-fluorobenzyla...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate after 4 hrs by FRET assay | Bioorg Med Chem Lett 21: 2030-3 (2011) Article DOI: 10.1016/j.bmcl.2011.02.010 BindingDB Entry DOI: 10.7270/Q24M94VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085810 (CHEMBL406809 | Gonadotropin Releasing Hormone anal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Affinity of the compound for rat Gonadotropin-releasing hormone receptor membrane evaluated using HEK-293 cells transfected with rat Gonadotropin-rel... | J Med Chem 43: 807-18 (2000) BindingDB Entry DOI: 10.7270/Q28P5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085768 (CHEMBL268538 | Gonadotropin Releasing Hormone anal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Affinity for rat gonadotrophin releasing hormone (GnRH) receptor using HEK-293 cells transfected with rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 807-18 (2000) BindingDB Entry DOI: 10.7270/Q28P5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085732 (CHEMBL406094 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50379533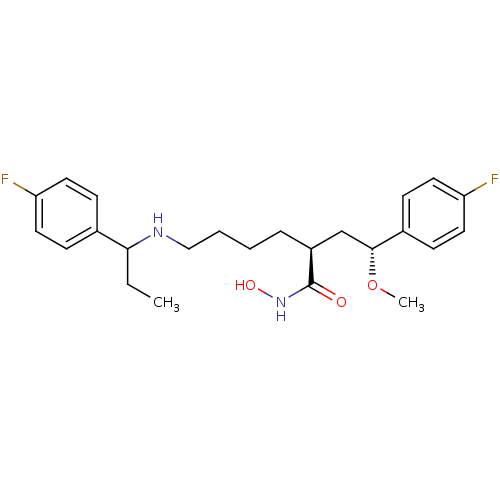 (CHEMBL2012838) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085735 (CHEMBL412681 | Cyclo (5-8) [Ac-D Nal, D Cpa, D Pal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329270 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-N-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate by FRET assay | Bioorg Med Chem Lett 20: 6850-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.058 BindingDB Entry DOI: 10.7270/Q2222V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2089 total ) | Next | Last >> |