

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]T3 from recombinant thyroid hormone receptor alpha (unknown origin) expressed in sf9 cells by scintillation proximity assay | Proc Natl Acad Sci U S A 104: 15490-5 (2007) Article DOI: 10.1073/pnas.0702759104 BindingDB Entry DOI: 10.7270/Q2JW8DPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18869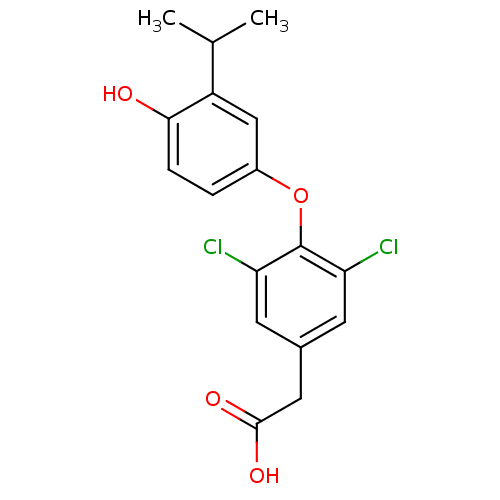 (2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine His-tagged human recombinant TRbeta1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]Spiprone from human dopamine D2 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50505284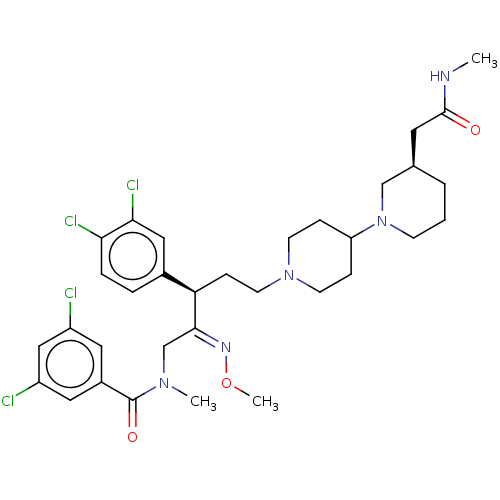 (CHEMBL4576324) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [125I][MePhe]NKB from human NK3 receptor expressed in CHO cells membranes | J Med Chem 62: 8881-8914 (2019) Article DOI: 10.1021/acs.jmedchem.9b00017 BindingDB Entry DOI: 10.7270/Q27084PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50115668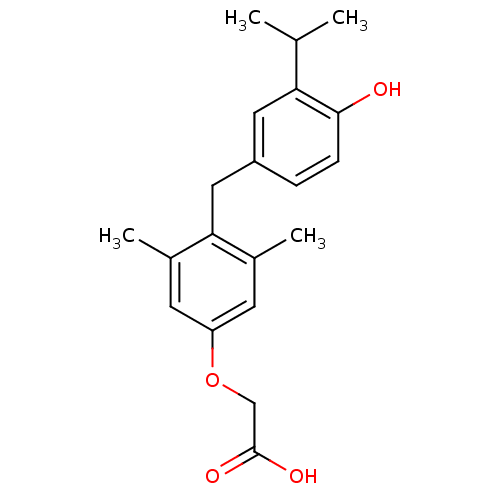 (3,5-dimethyl-4-(4'-hydroxy-3'-isopropylbenzyl)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine His-tagged human recombinant TRbeta1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine from His-tagged human recombinant TRalpha1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18869 (2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]T3 from recombinant thyroid hormone receptor beta (unknown origin) expressed in sf9 cells by scintillation proximity assay | Proc Natl Acad Sci U S A 104: 15490-5 (2007) Article DOI: 10.1073/pnas.0702759104 BindingDB Entry DOI: 10.7270/Q2JW8DPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50505284 (CHEMBL4576324) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]NKA from human NK2 receptor expressed in CHO cells membranes | J Med Chem 62: 8881-8914 (2019) Article DOI: 10.1021/acs.jmedchem.9b00017 BindingDB Entry DOI: 10.7270/Q27084PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50343621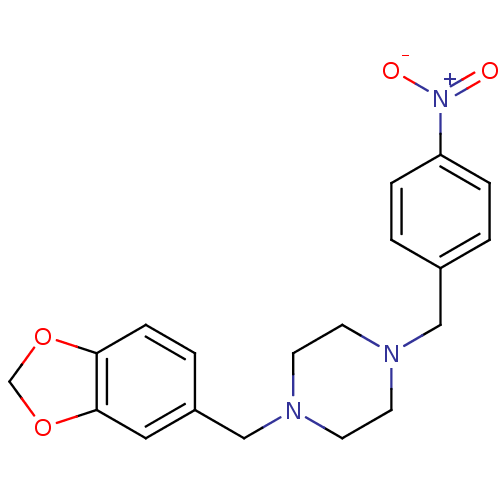 (1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-nitrobenzyl)pi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay | Bioorg Med Chem 19: 2911-7 (2011) Article DOI: 10.1016/j.bmc.2011.03.037 BindingDB Entry DOI: 10.7270/Q2QJ7HN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50396980 (CHEMBL2171169) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter | J Med Chem 55: 8066-74 (2012) Article DOI: 10.1021/jm300917h BindingDB Entry DOI: 10.7270/Q2TD9ZGG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50036402 (CHEMBL46882 | N-[3,5-dimethyl-4-(4'-hydroxy-3'-iso...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine His-tagged human recombinant TRbeta1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | 0.5 | -54.0 | 2.56 | n/a | n/a | n/a | n/a | n/a | 30 |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES US Patent | Assay Description Different concentrations (10^−5 M-10^−11 M) of the compound of the invention and corresponding isotope receptor ligand as well as recepto... | US Patent US9359372 (2016) BindingDB Entry DOI: 10.7270/Q2736PSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159251 (US9034874, 2.2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50036402 (CHEMBL46882 | N-[3,5-dimethyl-4-(4'-hydroxy-3'-iso...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine from His-tagged human recombinant TRalpha1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]T3 from recombinant thyroid hormone receptor beta (unknown origin) expressed in sf9 cells by scintillation proximity assay | Proc Natl Acad Sci U S A 104: 15490-5 (2007) Article DOI: 10.1073/pnas.0702759104 BindingDB Entry DOI: 10.7270/Q2JW8DPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50259004 (1'-[4-Fluorobenzyl]-3H-spiro[[2]benzofuran-1,4'pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals Beijing Normal University Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from rat brain sigma1 receptor by competitive binding assay | J Med Chem 56: 3478-91 (2013) Article DOI: 10.1021/jm301734g BindingDB Entry DOI: 10.7270/Q28W3FN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50058020 (4-[1-((E)-3-Iodo-allyl)-piperidin-4-ylmethoxy]-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals Beijing Normal University Curated by ChEMBL | Assay Description Binding affinity to human brain sigma1 receptor | J Med Chem 56: 3478-91 (2013) Article DOI: 10.1021/jm301734g BindingDB Entry DOI: 10.7270/Q28W3FN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine His-tagged human recombinant TRbeta1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50396979 (CHEMBL2171170) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter | J Med Chem 55: 8066-74 (2012) Article DOI: 10.1021/jm300917h BindingDB Entry DOI: 10.7270/Q2TD9ZGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50343619 (1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-bromobenzyl)pi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay | Bioorg Med Chem 19: 2911-7 (2011) Article DOI: 10.1016/j.bmc.2011.03.037 BindingDB Entry DOI: 10.7270/Q2QJ7HN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254013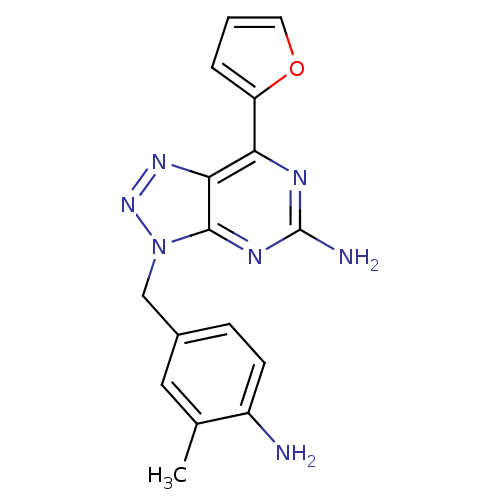 (3-(4-Amino-3-methylbenzyl)-7-(2-furyl)-3H-[1,2,3]t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114326 BindingDB Entry DOI: 10.7270/Q28D01B4 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50432550 (CHEMBL2347001) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals Beijing Normal University Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from rat brain sigma1 receptor by competitive binding assay | J Med Chem 56: 3478-91 (2013) Article DOI: 10.1021/jm301734g BindingDB Entry DOI: 10.7270/Q28W3FN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50343620 (1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-iodobenzyl)pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay | Bioorg Med Chem 19: 2911-7 (2011) Article DOI: 10.1016/j.bmc.2011.03.037 BindingDB Entry DOI: 10.7270/Q2QJ7HN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50274809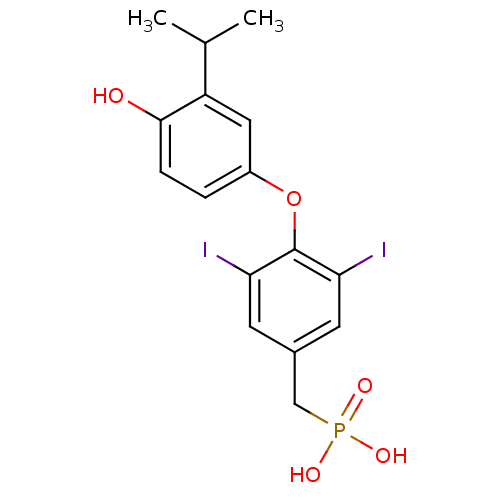 (3,5-Diiodo-4-(4'-hydroxy-3'-isopropylphenoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine His-tagged human recombinant TRbeta1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50343618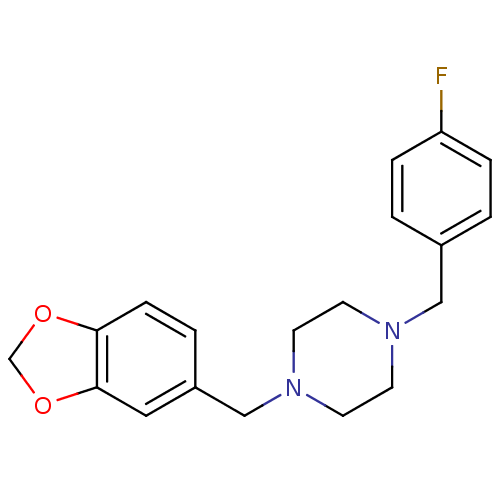 (1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-fluorobenzyl)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay | Bioorg Med Chem 19: 2911-7 (2011) Article DOI: 10.1016/j.bmc.2011.03.037 BindingDB Entry DOI: 10.7270/Q2QJ7HN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50580277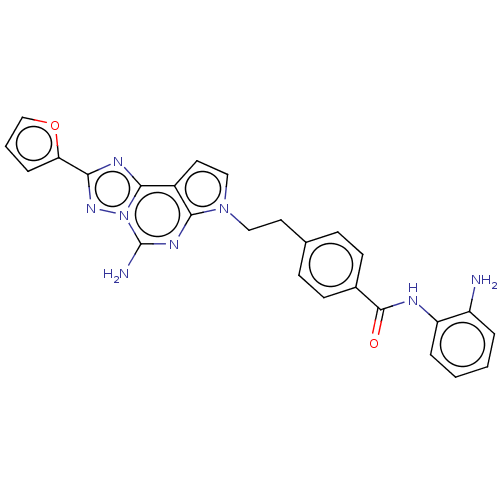 (CHEMBL5088258) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]SCH58261 from adenosine A2A receptor membrane (unknown origin) measured after 60 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01155 BindingDB Entry DOI: 10.7270/Q2R49VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor (unknown origin) expressed in HEK293 cells by liquid scintillation counter | Bioorg Med Chem 21: 856-68 (2013) Article DOI: 10.1016/j.bmc.2012.12.016 BindingDB Entry DOI: 10.7270/Q2JD4Z4M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159252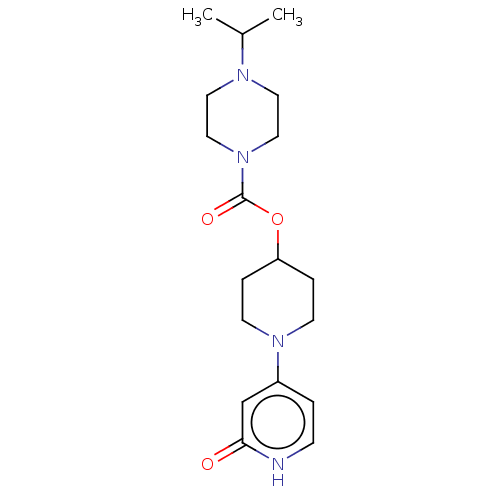 (US9034874, 2.3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50343622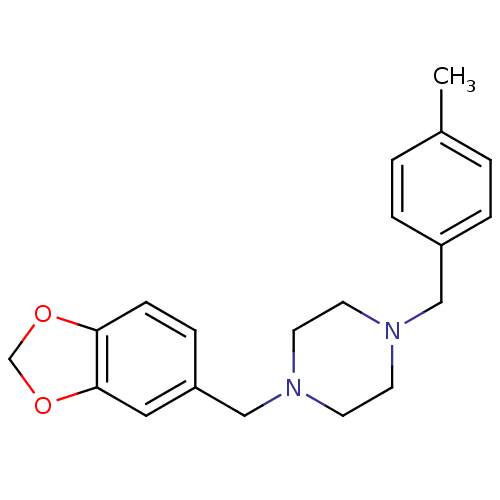 (1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-methylbenzyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University) Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay | Bioorg Med Chem 19: 2911-7 (2011) Article DOI: 10.1016/j.bmc.2011.03.037 BindingDB Entry DOI: 10.7270/Q2QJ7HN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50580282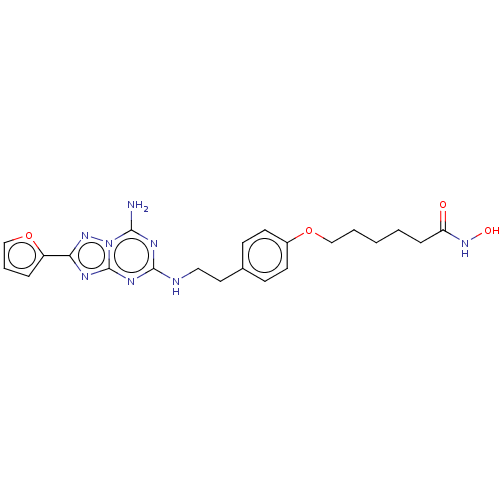 (CHEMBL5079065) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]SCH58261 from adenosine A2A receptor membrane (unknown origin) measured after 60 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01155 BindingDB Entry DOI: 10.7270/Q2R49VN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50274812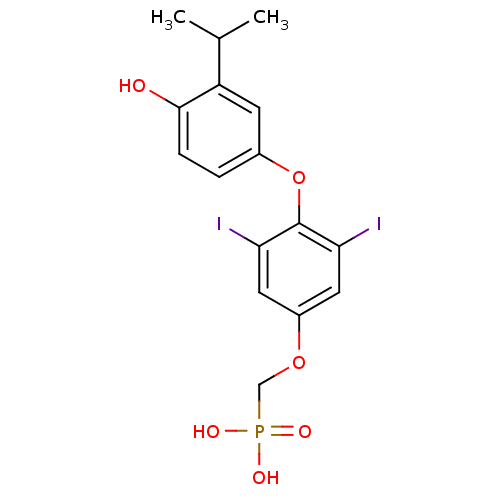 (CHEMBL457528 | [3,5-Diiodo-4-(4'-hydroxy-3'-isopro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine His-tagged human recombinant TRbeta1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50115668 (3,5-dimethyl-4-(4'-hydroxy-3'-isopropylbenzyl)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine from His-tagged human recombinant TRalpha1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159248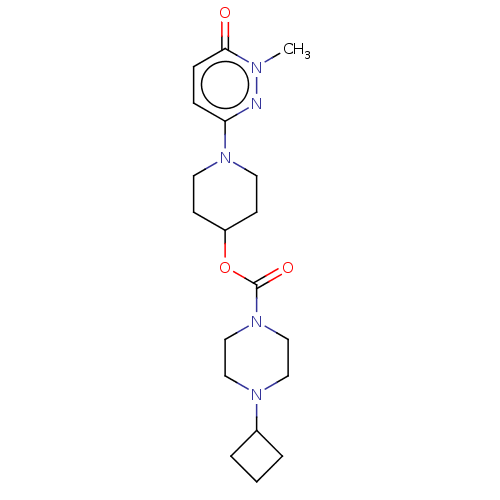 (US9034874, 1.5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159246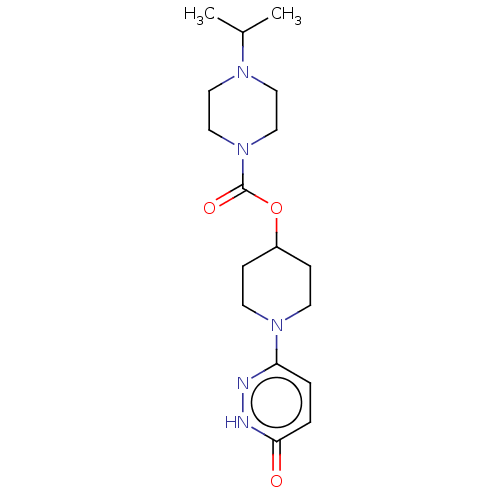 (US9034874, 1.3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in human HEK293 cells by liquid scintillation counter | Bioorg Med Chem 21: 856-68 (2013) Article DOI: 10.1016/j.bmc.2012.12.016 BindingDB Entry DOI: 10.7270/Q2JD4Z4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor (unknown origin) expressed in human HEK293 cells by liquid scintillation counter | Bioorg Med Chem 21: 856-68 (2013) Article DOI: 10.1016/j.bmc.2012.12.016 BindingDB Entry DOI: 10.7270/Q2JD4Z4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159253 (US9034874, 3.1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM86180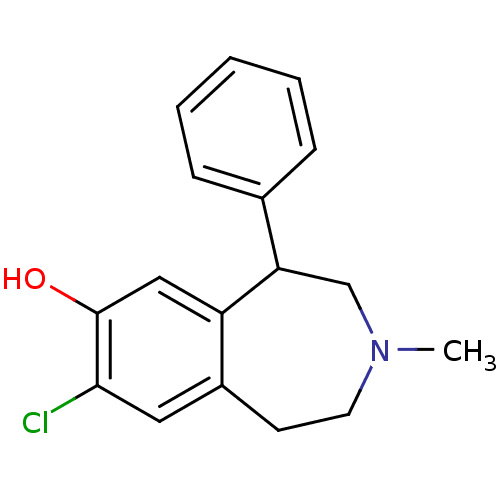 (CAS_87075-17-0 | NSC_5018 | SCH 23390 | SCH23390 |...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | US Patent | 1.24 | -51.7 | 2.52 | n/a | n/a | n/a | n/a | n/a | 30 |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES US Patent | Assay Description Different concentrations (10^−5 M-10^−11 M) of the compound of the invention and corresponding isotope receptor ligand as well as recepto... | US Patent US9359372 (2016) BindingDB Entry DOI: 10.7270/Q2736PSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50505284 (CHEMBL4576324) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]Sar SP from human NK1 receptor expressed in CHO cells membranes | J Med Chem 62: 8881-8914 (2019) Article DOI: 10.1021/acs.jmedchem.9b00017 BindingDB Entry DOI: 10.7270/Q27084PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159244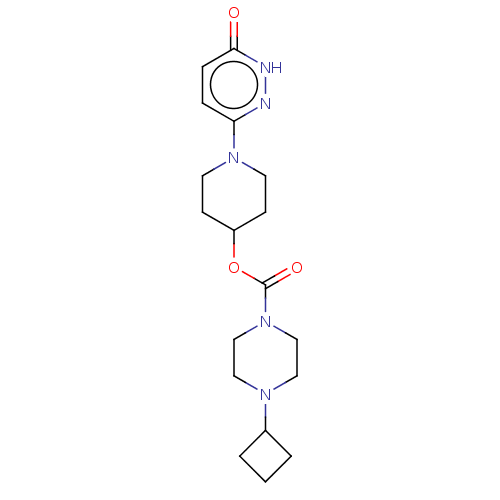 (US9034874, 1.1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50274846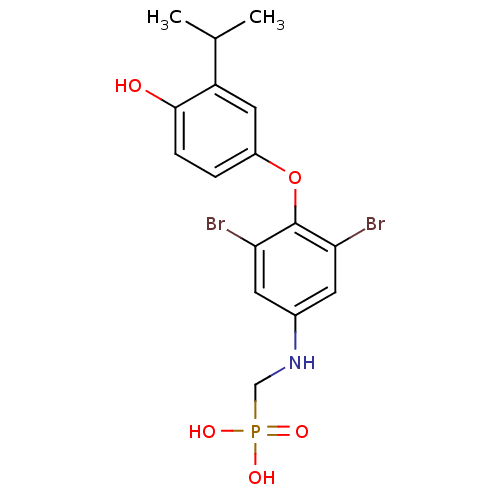 (CHEMBL511862 | [3,5-Dibromo-4-(4'-hydroxy-3'-isopr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine His-tagged human recombinant TRbeta1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159253 (US9034874, 3.1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Emopamil-binding protein-like (Homo sapiens (Human)) | BDBM50424041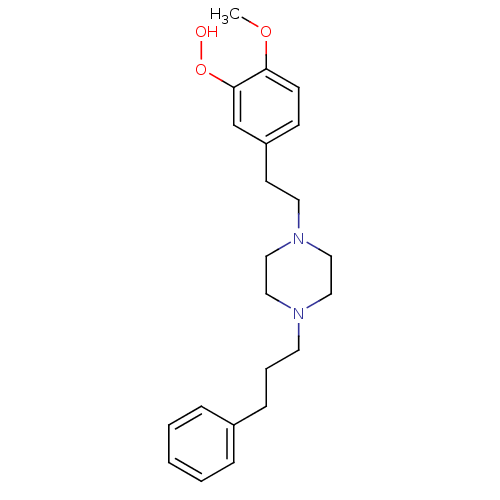 (CHEMBL2314420) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University) Curated by ChEMBL | Assay Description Binding affinity to emopamil binding protein (unknown origin) | Bioorg Med Chem 21: 215-22 (2012) Article DOI: 10.1016/j.bmc.2012.10.038 BindingDB Entry DOI: 10.7270/Q2VM4DKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50338990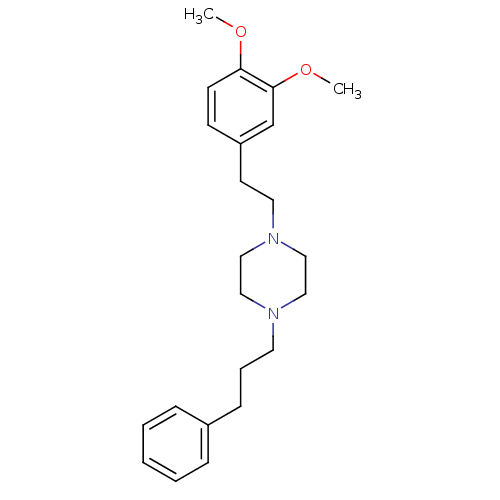 (1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals Beijing Normal University Curated by ChEMBL | Assay Description Binding affinity to EBP (unknown origin) | J Med Chem 56: 3478-91 (2013) Article DOI: 10.1021/jm301734g BindingDB Entry DOI: 10.7270/Q28W3FN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50338990 (1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University) Curated by ChEMBL | Assay Description Binding affinity to emopamil binding protein | Bioorg Med Chem 19: 2911-7 (2011) Article DOI: 10.1016/j.bmc.2011.03.037 BindingDB Entry DOI: 10.7270/Q2QJ7HN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50432555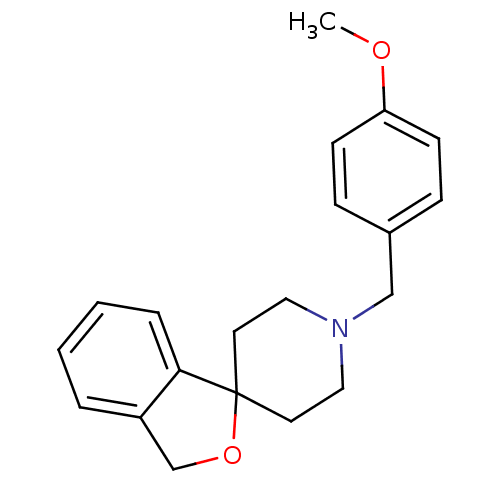 (CHEMBL2347002) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals Beijing Normal University Curated by ChEMBL | Assay Description Displacement of (+)-[3H]pentazocine from rat brain sigma1 receptor by competitive binding assay | J Med Chem 56: 3478-91 (2013) Article DOI: 10.1021/jm301734g BindingDB Entry DOI: 10.7270/Q28W3FN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50274809 (3,5-Diiodo-4-(4'-hydroxy-3'-isopropylphenoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]3,5,3'-triiodo-L-thyronine from His-tagged human recombinant TRalpha1 by scintillation proximity assay | J Med Chem 51: 7075-93 (2009) Article DOI: 10.1021/jm800824d BindingDB Entry DOI: 10.7270/Q21V5DT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50116743 (3,5-Dichloro-N-{(R)-3-(3,4-dichloro-phenyl)-5-(4-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]Sar SP from human NK1 receptor expressed in CHO cells membranes | J Med Chem 62: 8881-8914 (2019) Article DOI: 10.1021/acs.jmedchem.9b00017 BindingDB Entry DOI: 10.7270/Q27084PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7039 total ) | Next | Last >> |