 Found 188 hits with Last Name = 'joseph' and Initial = 'l'
Found 188 hits with Last Name = 'joseph' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50601872

(CHEMBL5174733)Show SMILES [11CH3]Oc1ccc2c(NC(=O)Nc3cccc(c3)C(F)(F)F)ccnc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072352
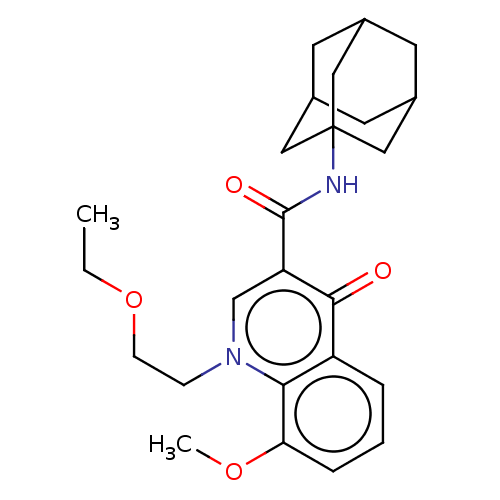
(CHEMBL3409318)Show SMILES CCOCCn1cc(C(=O)NC23CC4CC(CC(C4)C2)C3)c(=O)c2cccc(OC)c12 |TLB:18:13:20:17.19.16,18:17:20:12.13.14,THB:16:15:12:18.17.19,16:17:12:20.14.15| Show InChI InChI=1S/C25H32N2O4/c1-3-31-8-7-27-15-20(23(28)19-5-4-6-21(30-2)22(19)27)24(29)26-25-12-16-9-17(13-25)11-18(10-16)14-25/h4-6,15-18H,3,7-14H2,1-2H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50601855
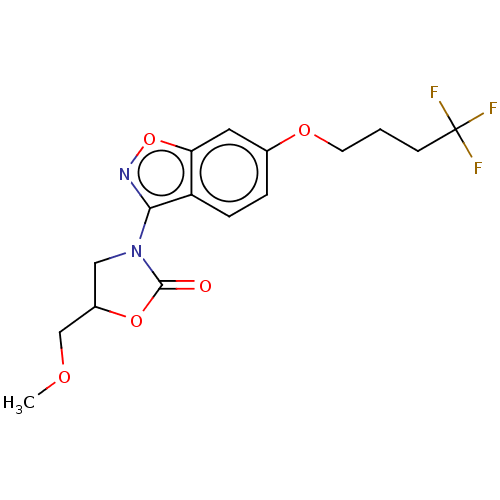
(CHEMBL5189391)Show SMILES COCC1CN(c2noc3cc(OCCCC(F)(F)F)ccc23)[11C](=O)O1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50601864

(CHEMBL5171821) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50601854

(CHEMBL4117628)Show SMILES COC(=O)c1c(NC(=O)C23CC4CC(CC(O)(C4)C2)C3)sc2CCCCc12 |TLB:16:15:12:19.9.10,7:9:12:17.14.15,THB:14:13:10:17.15.18,14:15:12.13.19:10,18:15:12:19.9.10,18:9:12:17.14.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50506752
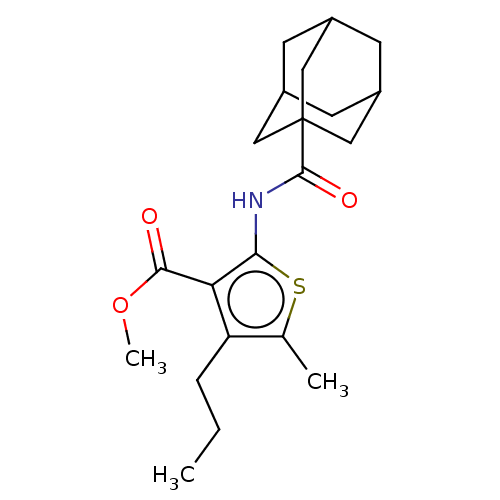
(CHEMBL4441693)Show SMILES CCCc1c(C)sc(NC(=O)C23CC4CC(CC(C4)C2)C3)c1C(=O)OC |TLB:9:11:14.13.18:16,THB:9:11:14:18.17.16,12:13:16:20.11.19,12:11:14.13.18:16,19:11:14:18.17.16,19:17:14:20.12.11| Show InChI InChI=1S/C21H29NO3S/c1-4-5-16-12(2)26-18(17(16)19(23)25-3)22-20(24)21-9-13-6-14(10-21)8-15(7-13)11-21/h13-15H,4-11H2,1-3H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50397745
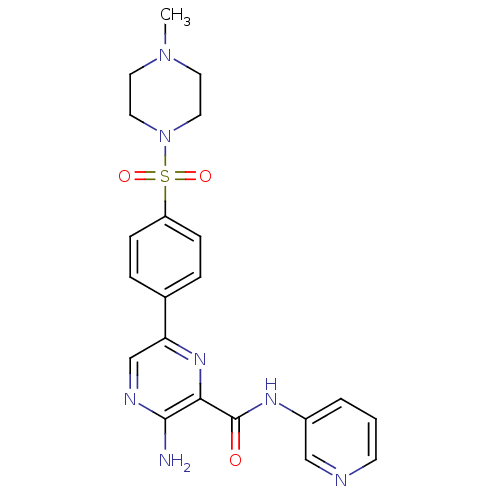
(CHEMBL2177161)Show SMILES CN1CCN(CC1)S(=O)(=O)c1ccc(cc1)-c1cnc(N)c(n1)C(=O)Nc1cccnc1 Show InChI InChI=1S/C21H23N7O3S/c1-27-9-11-28(12-10-27)32(30,31)17-6-4-15(5-7-17)18-14-24-20(22)19(26-18)21(29)25-16-3-2-8-23-13-16/h2-8,13-14H,9-12H2,1H3,(H2,22,24)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580260

(CHEMBL5093347)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CCC(CF)C2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50601865

(CHEMBL5180946)Show SMILES CCOc1cc2ccc(C(N)=O)c(Nc3ccc(F)cc3F)c2cc1N1CCN([11CH3])CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM8296
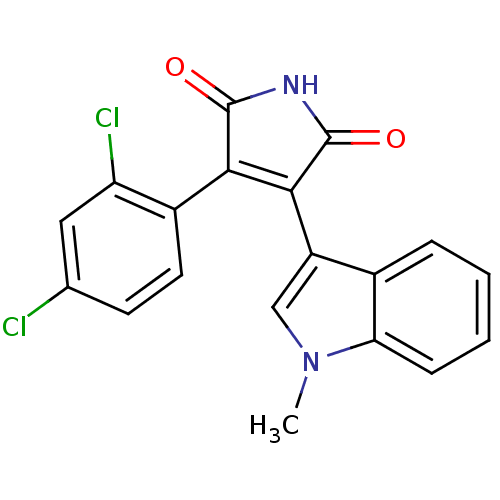
(3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccc(Cl)cc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50312837
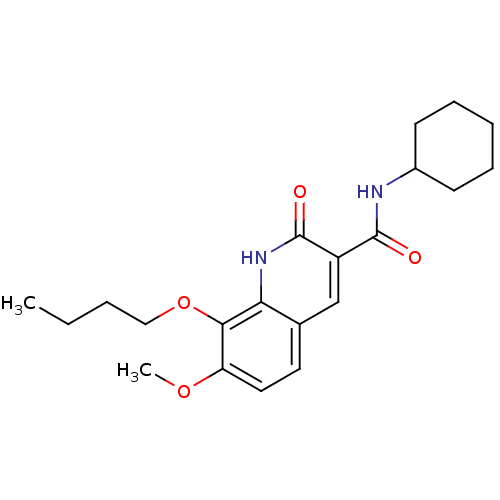
(CHEMBL1081610 | [11C]8-butoxy-N-cyclohexyl-7-metho...)Show SMILES CCCCOc1c(OC)ccc2cc(C(=O)NC3CCCCC3)c(=O)[nH]c12 Show InChI InChI=1S/C21H28N2O4/c1-3-4-12-27-19-17(26-2)11-10-14-13-16(21(25)23-18(14)19)20(24)22-15-8-6-5-7-9-15/h10-11,13,15H,3-9,12H2,1-2H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580262
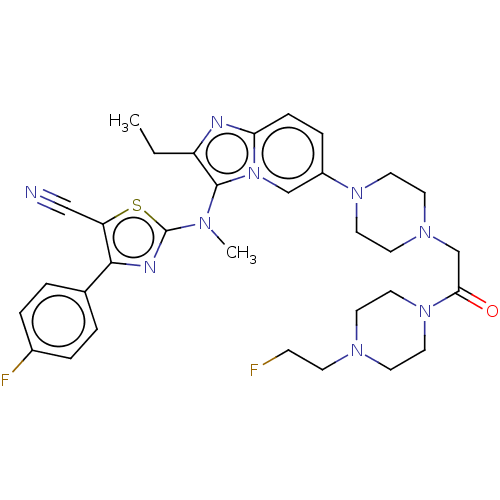
(CHEMBL5085520)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CCN(CCF)CC2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580256
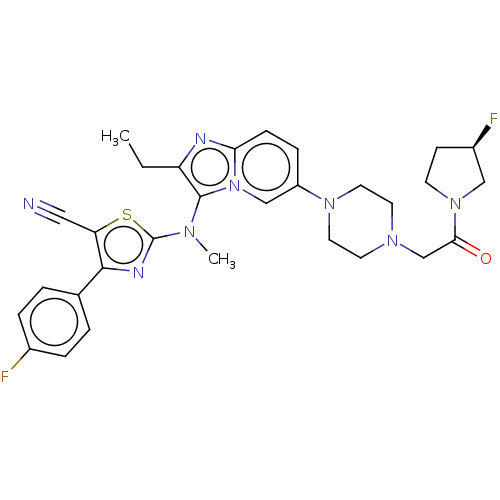
(CHEMBL5091502)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CC[C@@H](F)C2)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580261
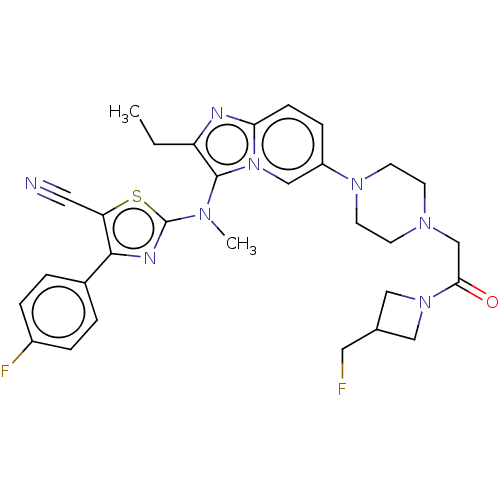
(CHEMBL5084424)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CC(CF)C2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580259

(CHEMBL5088520)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CCCC(F)C2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580255
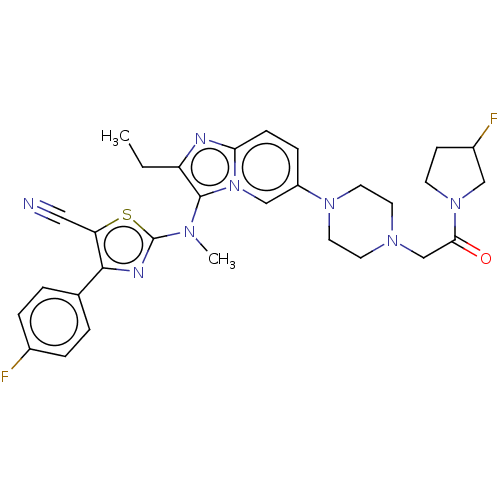
(CHEMBL5084058)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CCC(F)C2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580258
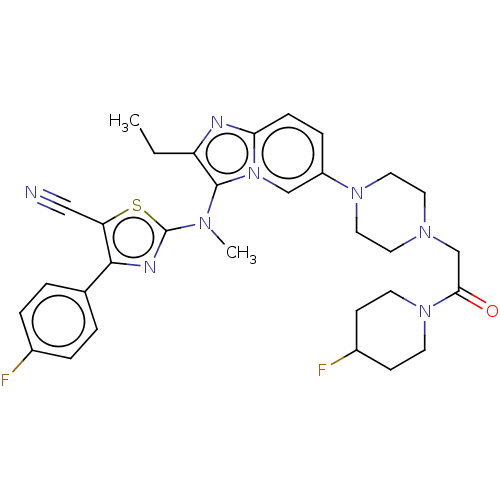
(CHEMBL5076928)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CCC(F)CC2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580263
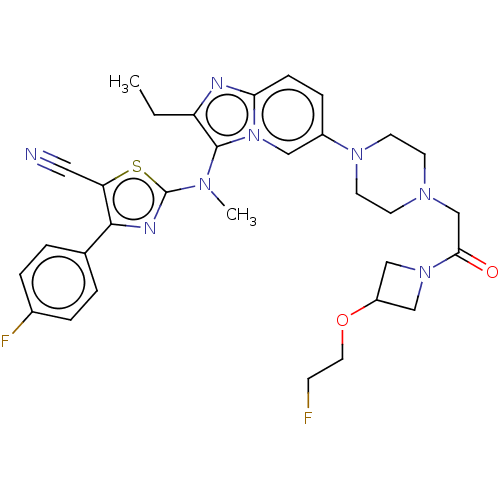
(CHEMBL5088371)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CC(C2)OCCF)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM21006
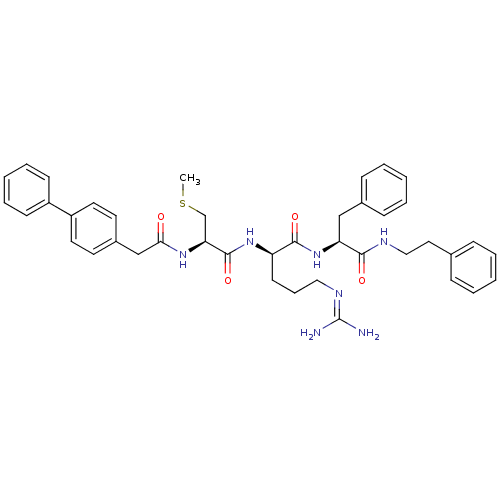
((2R)-5-[(diaminomethylidene)amino]-2-[(2R)-3-(meth...)Show SMILES [#6]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C41H49N7O4S/c1-53-28-36(46-37(49)27-31-19-21-33(22-20-31)32-16-9-4-10-17-32)40(52)47-34(18-11-24-45-41(42)43)39(51)48-35(26-30-14-7-3-8-15-30)38(50)44-25-23-29-12-5-2-6-13-29/h2-10,12-17,19-22,34-36H,11,18,23-28H2,1H3,(H,44,50)(H,46,49)(H,47,52)(H,48,51)(H4,42,43,45)/t34-,35+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | -44.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580262

(CHEMBL5085520)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CCN(CCF)CC2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20998
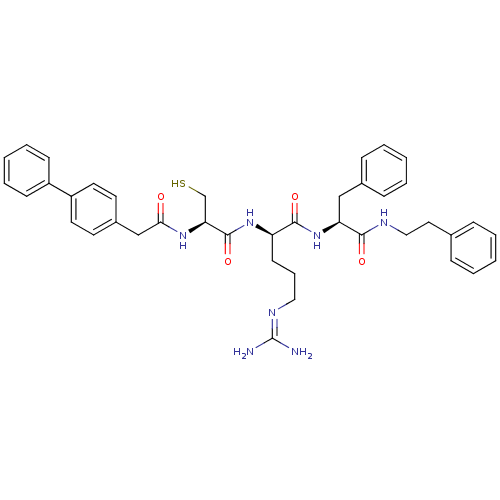
((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-pheny...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#16])-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C40H47N7O4S/c41-40(42)44-23-10-17-33(38(50)47-34(25-29-13-6-2-7-14-29)37(49)43-24-22-28-11-4-1-5-12-28)46-39(51)35(27-52)45-36(48)26-30-18-20-32(21-19-30)31-15-8-3-9-16-31/h1-9,11-16,18-21,33-35,52H,10,17,22-27H2,(H,43,49)(H,45,48)(H,46,51)(H,47,50)(H4,41,42,44)/t33-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | -43.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20997
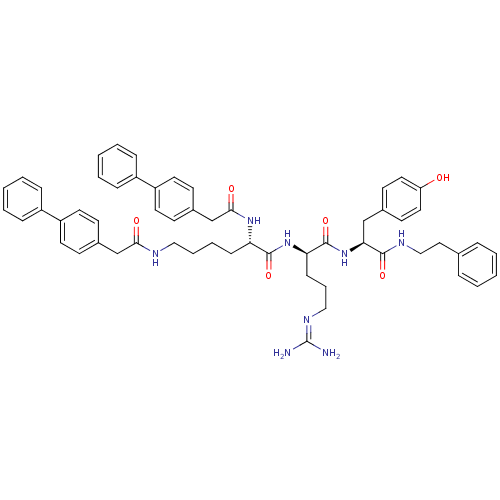
((2S)-N-[(1R)-4-[(diaminomethylidene)amino]-1-{[(1S...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C57H64N8O6/c58-57(59)62-35-12-20-50(56(71)65-51(37-41-25-31-48(66)32-26-41)54(69)61-36-33-40-13-4-1-5-14-40)64-55(70)49(63-53(68)39-43-23-29-47(30-24-43)45-17-8-3-9-18-45)19-10-11-34-60-52(67)38-42-21-27-46(28-22-42)44-15-6-2-7-16-44/h1-9,13-18,21-32,49-51,66H,10-12,19-20,33-39H2,(H,60,67)(H,61,69)(H,63,68)(H,64,70)(H,65,71)(H4,58,59,62)/t49-,50+,51-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | -43.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20999
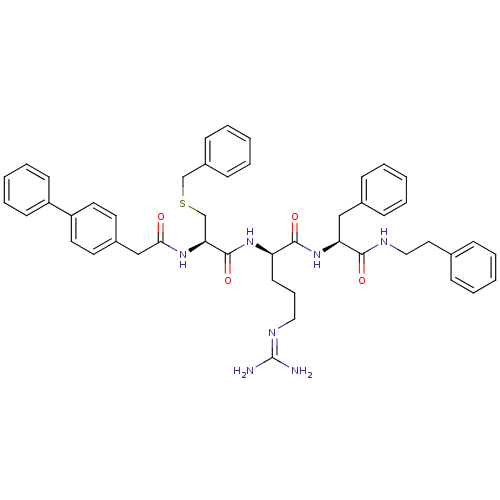
((2R)-2-[(2R)-3-(benzylsulfanyl)-2-[1-(4-phenylphen...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C47H53N7O4S/c48-47(49)51-28-13-22-40(45(57)54-41(30-35-16-7-2-8-17-35)44(56)50-29-27-34-14-5-1-6-15-34)53-46(58)42(33-59-32-37-18-9-3-10-19-37)52-43(55)31-36-23-25-39(26-24-36)38-20-11-4-12-21-38/h1-12,14-21,23-26,40-42H,13,22,27-33H2,(H,50,56)(H,52,55)(H,53,58)(H,54,57)(H4,48,49,51)/t40-,41+,42+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | -43.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM192943
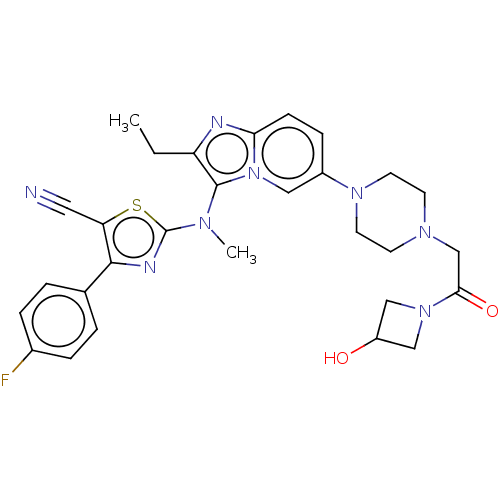
(US10526329, Compound 139 | US9670204, 138 2-((2-et...)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CC(O)C2)CC1 Show InChI InChI=1S/C29H31FN8O2S/c1-3-23-28(34(2)29-33-27(24(14-31)41-29)19-4-6-20(30)7-5-19)38-15-21(8-9-25(38)32-23)36-12-10-35(11-13-36)18-26(40)37-16-22(39)17-37/h4-9,15,22,39H,3,10-13,16-18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580257
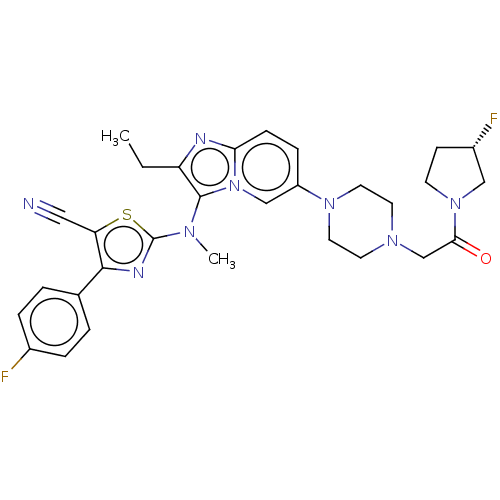
(CHEMBL5078471)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CC[C@H](F)C2)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580254
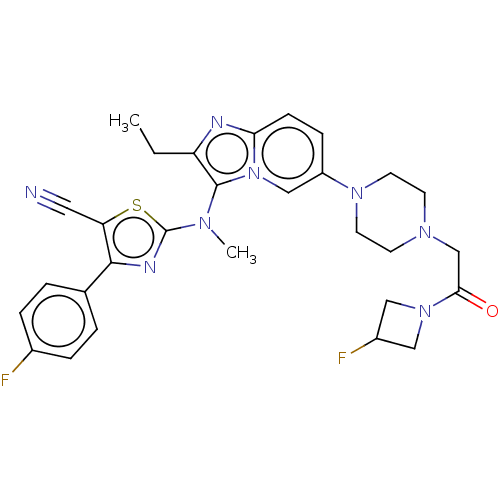
(CHEMBL5088215)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CC(F)C2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20993
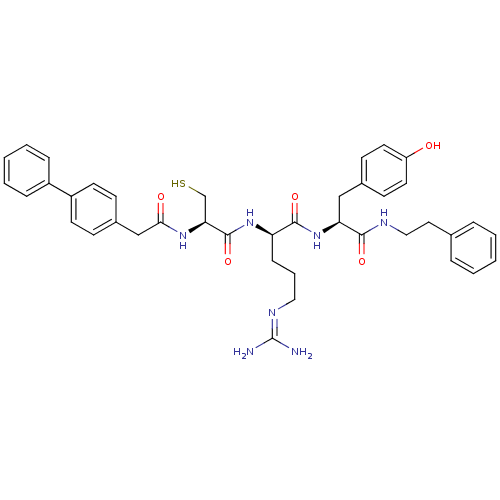
((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-(4-hy...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#16])-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C40H47N7O5S/c41-40(42)44-22-7-12-33(46-39(52)35(26-53)45-36(49)25-29-13-17-31(18-14-29)30-10-5-2-6-11-30)38(51)47-34(24-28-15-19-32(48)20-16-28)37(50)43-23-21-27-8-3-1-4-9-27/h1-6,8-11,13-20,33-35,48,53H,7,12,21-26H2,(H,43,50)(H,45,49)(H,46,52)(H,47,51)(H4,41,42,44)/t33-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 45 | -41.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50249570
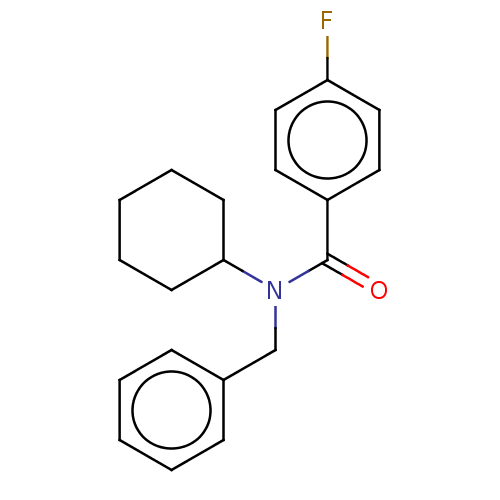
(CHEMBL4066660)Show InChI InChI=1S/C20H22FNO/c21-18-13-11-17(12-14-18)20(23)22(19-9-5-2-6-10-19)15-16-7-3-1-4-8-16/h1,3-4,7-8,11-14,19H,2,5-6,9-10,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20996
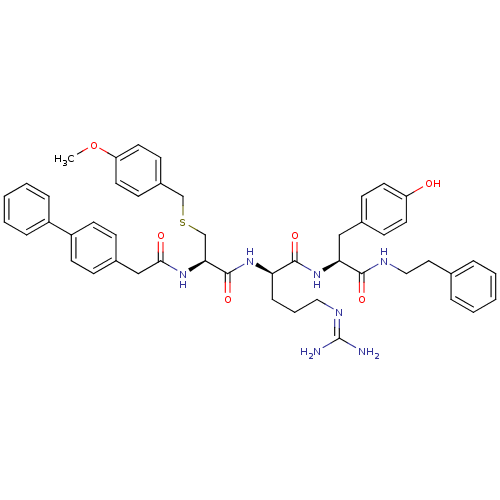
((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-(4-hy...)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-c2ccc(cc2)-c2ccccc2)-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6]-[#6]-c2ccccc2)cc1 |r| Show InChI InChI=1S/C48H55N7O6S/c1-61-40-24-18-36(19-25-40)31-62-32-43(53-44(57)30-35-14-20-38(21-15-35)37-11-6-3-7-12-37)47(60)54-41(13-8-27-52-48(49)50)46(59)55-42(29-34-16-22-39(56)23-17-34)45(58)51-28-26-33-9-4-2-5-10-33/h2-7,9-12,14-25,41-43,56H,8,13,26-32H2,1H3,(H,51,58)(H,53,57)(H,54,60)(H,55,59)(H4,49,50,52)/t41-,42+,43+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | -39.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20995
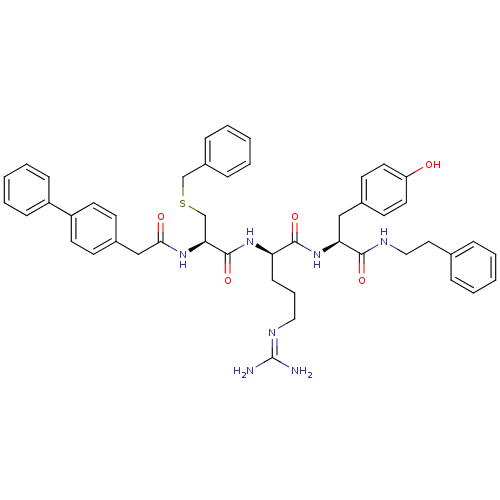
((2R)-2-[(2R)-3-(benzylsulfanyl)-2-[1-(4-phenylphen...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C47H53N7O5S/c48-47(49)51-27-10-17-40(45(58)54-41(29-34-20-24-39(55)25-21-34)44(57)50-28-26-33-11-4-1-5-12-33)53-46(59)42(32-60-31-36-13-6-2-7-14-36)52-43(56)30-35-18-22-38(23-19-35)37-15-8-3-9-16-37/h1-9,11-16,18-25,40-42,55H,10,17,26-32H2,(H,50,57)(H,52,56)(H,53,59)(H,54,58)(H4,48,49,51)/t40-,41+,42+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 155 | -38.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50285227
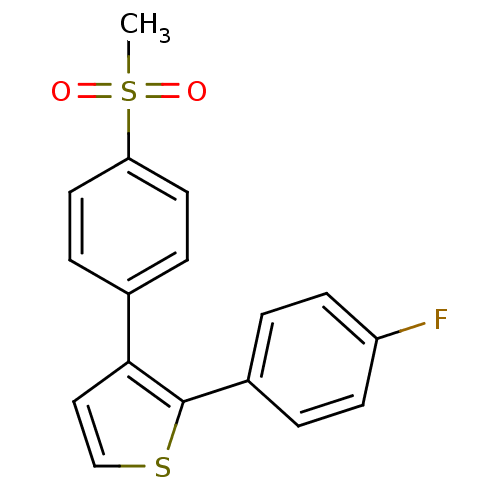
(2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-t...)Show InChI InChI=1S/C17H13FO2S2/c1-22(19,20)15-8-4-12(5-9-15)16-10-11-21-17(16)13-2-6-14(18)7-3-13/h2-11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM21005

((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-pheny...)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C42H51N7O4/c1-2-13-35(47-38(50)29-32-21-23-34(24-22-32)33-18-10-5-11-19-33)40(52)48-36(20-12-26-46-42(43)44)41(53)49-37(28-31-16-8-4-9-17-31)39(51)45-27-25-30-14-6-3-7-15-30/h3-11,14-19,21-24,35-37H,2,12-13,20,25-29H2,1H3,(H,45,51)(H,47,50)(H,48,52)(H,49,53)(H4,43,44,46)/t35-,36+,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 460 | -36.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM21000

((2R)-5-[(diaminomethylidene)amino]-2-[(2R)-3-{[(4-...)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-c2ccc(cc2)-c2ccccc2)-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6]-[#6]-c2ccccc2)cc1 |r| Show InChI InChI=1S/C48H55N7O5S/c1-60-40-25-21-37(22-26-40)32-61-33-43(53-44(56)31-36-19-23-39(24-20-36)38-16-9-4-10-17-38)47(59)54-41(18-11-28-52-48(49)50)46(58)55-42(30-35-14-7-3-8-15-35)45(57)51-29-27-34-12-5-2-6-13-34/h2-10,12-17,19-26,41-43H,11,18,27-33H2,1H3,(H,51,57)(H,53,56)(H,54,59)(H,55,58)(H4,49,50,52)/t41-,42+,43+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 464 | -36.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
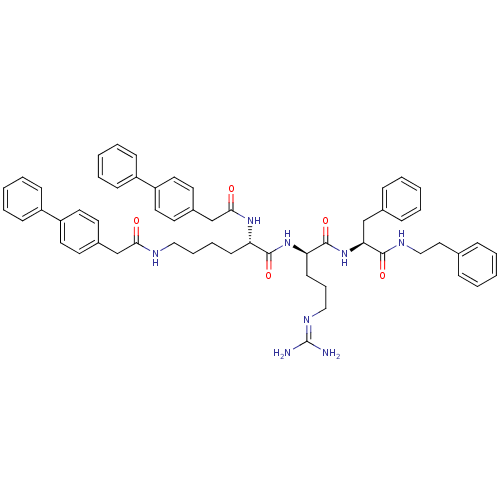
Procathepsin L
(Homo sapiens (Human)) | BDBM21001
((2S)-N-[(1R)-4-[(diaminomethylidene)amino]-1-{[(1S...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C57H64N8O5/c58-57(59)62-36-15-25-50(56(70)65-51(38-42-18-7-2-8-19-42)54(68)61-37-34-41-16-5-1-6-17-41)64-55(69)49(63-53(67)40-44-28-32-48(33-29-44)46-22-11-4-12-23-46)24-13-14-35-60-52(66)39-43-26-30-47(31-27-43)45-20-9-3-10-21-45/h1-12,16-23,26-33,49-51H,13-15,24-25,34-40H2,(H,60,66)(H,61,68)(H,63,67)(H,64,69)(H,65,70)(H4,58,59,62)/t49-,50+,51-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 511 | -35.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Rattus norvegicus (rat)) | BDBM15579

(CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...)Show InChI InChI=1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50601854

(CHEMBL4117628)Show SMILES COC(=O)c1c(NC(=O)C23CC4CC(CC(O)(C4)C2)C3)sc2CCCCc12 |TLB:16:15:12:19.9.10,7:9:12:17.14.15,THB:14:13:10:17.15.18,14:15:12.13.19:10,18:15:12:19.9.10,18:9:12:17.14.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50506752

(CHEMBL4441693)Show SMILES CCCc1c(C)sc(NC(=O)C23CC4CC(CC(C4)C2)C3)c1C(=O)OC |TLB:9:11:14.13.18:16,THB:9:11:14:18.17.16,12:13:16:20.11.19,12:11:14.13.18:16,19:11:14:18.17.16,19:17:14:20.12.11| Show InChI InChI=1S/C21H29NO3S/c1-4-5-16-12(2)26-18(17(16)19(23)25-3)22-20(24)21-9-13-6-14(10-21)8-15(7-13)11-21/h13-15H,4-11H2,1-3H3,(H,22,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM21002

((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-(4-hy...)Show SMILES [#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C41H49N7O5/c1-2-34(46-37(50)27-30-15-19-32(20-16-30)31-12-7-4-8-13-31)39(52)47-35(14-9-24-45-41(42)43)40(53)48-36(26-29-17-21-33(49)22-18-29)38(51)44-25-23-28-10-5-3-6-11-28/h3-8,10-13,15-22,34-36,49H,2,9,14,23-27H2,1H3,(H,44,51)(H,46,50)(H,47,52)(H,48,53)(H4,42,43,45)/t34-,35+,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | -29.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | BDBM50545375
(CHEMBL4527162)Show InChI InChI=1S/C22H23N5O/c28-22(24-15-18-7-3-1-4-8-18)27-13-11-26(12-14-27)20-16-23-17-25-21(20)19-9-5-2-6-10-19/h1-10,16-17H,11-15H2,(H,24,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity to full length human CYP46A1 expressed in Escherichia coli DH5alpha as cholesterol-24 hydroxylation using cholesterol as substrate i... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127068
BindingDB Entry DOI: 10.7270/Q2PK0KRK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM21003

((2R)-5-[(diaminomethylidene)amino]-2-[(2S)-2-forma...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#7+])-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C40H48N8O5/c41-26-35(46-36(50)25-29-13-17-31(18-14-29)30-10-5-2-6-11-30)39(53)47-33(12-7-22-45-40(42)43)38(52)48-34(24-28-15-19-32(49)20-16-28)37(51)44-23-21-27-8-3-1-4-9-27/h1-6,8-11,13-20,33-35,49H,7,12,21-26,41H2,(H,44,51)(H,46,50)(H,47,53)(H,48,52)(H4,42,43,45)/p+1/t33-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+4 | -28.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM21004

((3S)-3-{[(1R)-4-[(diaminomethylidene)amino]-1-{[(1...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8-])=O)-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C41H47N7O7/c42-41(43)45-22-7-12-33(39(54)48-34(24-28-15-19-32(49)20-16-28)38(53)44-23-21-27-8-3-1-4-9-27)47-40(55)35(26-37(51)52)46-36(50)25-29-13-17-31(18-14-29)30-10-5-2-6-11-30/h1-6,8-11,13-20,33-35,49H,7,12,21-26H2,(H,44,53)(H,46,50)(H,47,55)(H,48,54)(H,51,52)(H4,42,43,45)/p-1/t33-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.75E+4 | -24.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272095
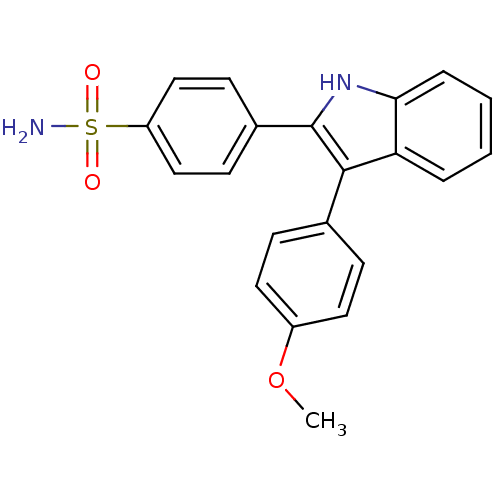
(4-(3-(4-methoxyphenyl)-1H-indol-2-yl)benzenesulfon...)Show SMILES COc1ccc(cc1)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H18N2O3S/c1-26-16-10-6-14(7-11-16)20-18-4-2-3-5-19(18)23-21(20)15-8-12-17(13-9-15)27(22,24)25/h2-13,23H,1H3,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
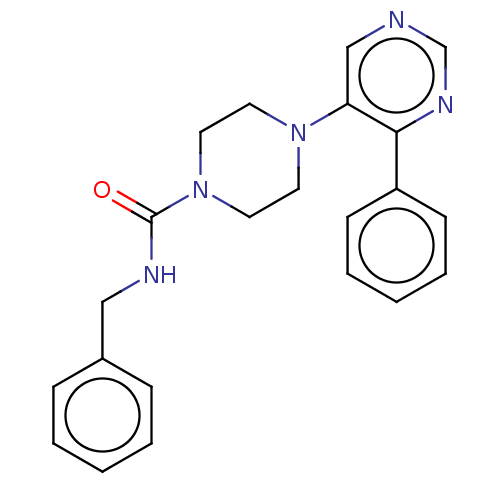
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50408487
(CHEMBL5281567)Show InChI InChI=1S/C18H21N3OS/c1-20(2)10-5-11-21-14-6-3-4-7-16(14)23-17-9-8-13(18(19)22)12-15(17)21/h3-4,6-9,12H,5,10-11H2,1-2H3,(H2,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretion |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50408486
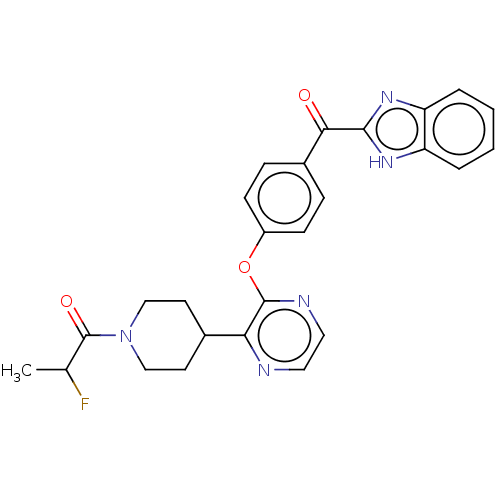
(CHEMBL5268340)Show InChI InChI=1S/C18H21ClN2S/c1-3-20(4-2)11-12-21-15-7-5-6-8-17(15)22-18-10-9-14(19)13-16(18)21/h5-10,13H,3-4,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretion |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50497974
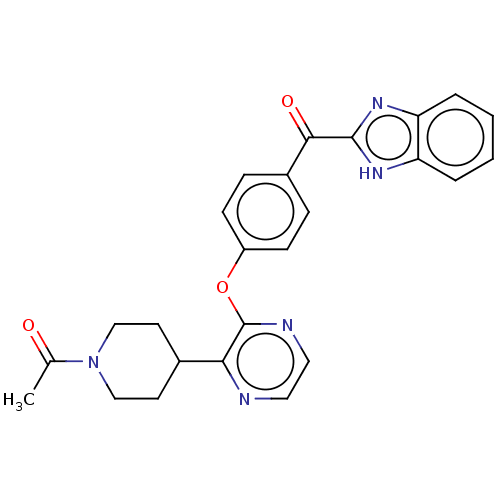
(CHEMBL3319209)Show SMILES CC(=O)N1CCC(CC1)c1nccnc1Oc1ccc(cc1)C(=O)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C25H23N5O3/c1-16(31)30-14-10-17(11-15-30)22-25(27-13-12-26-22)33-19-8-6-18(7-9-19)23(32)24-28-20-4-2-3-5-21(20)29-24/h2-9,12-13,17H,10-11,14-15H2,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretion |
Citation and Details
|
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50594973
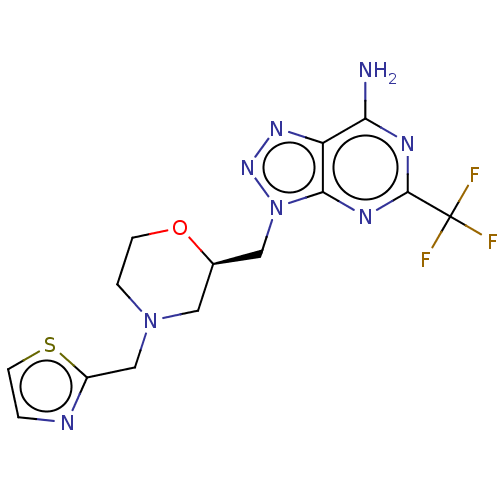
(CHEMBL5169811)Show SMILES Nc1nc(nc2n(C[C@H]3CN(Cc4nccs4)CCO3)nnc12)C(F)(F)F |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at AT1 receptor assessed inhibition of angiotensin-2-induced contraction of rabbit thoracic aortic rings |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50398017
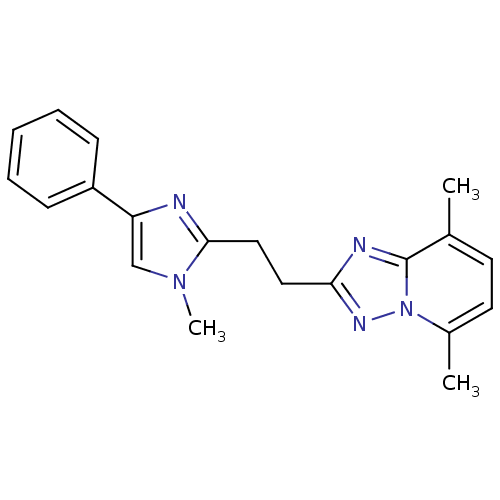
(CHEMBL2180411 | US9018217, 5,8-Dimethyl-2-[2-(1-me...)Show InChI InChI=1S/C20H21N5/c1-14-9-10-15(2)25-20(14)22-18(23-25)11-12-19-21-17(13-24(19)3)16-7-5-4-6-8-16/h4-10,13H,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of P-glycoprotein expressed in MDCK-MDR1 cells by calcein AM assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
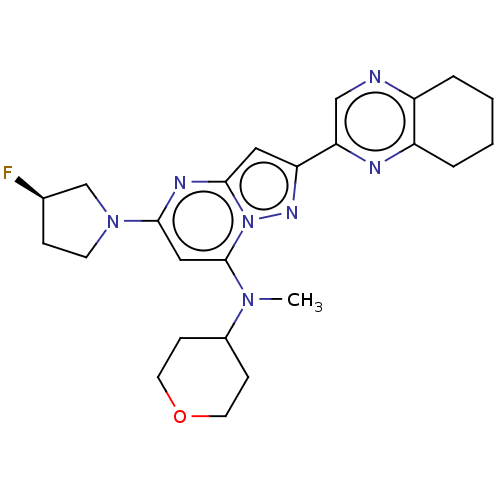
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50601869
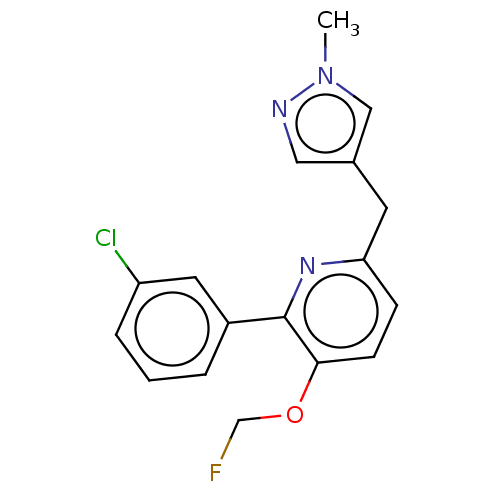
(CHEMBL5184286)Show SMILES [11CH3]n1cc(Cc2ccc(OCF)c(n2)-c2cccc(Cl)c2)cn1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50419436
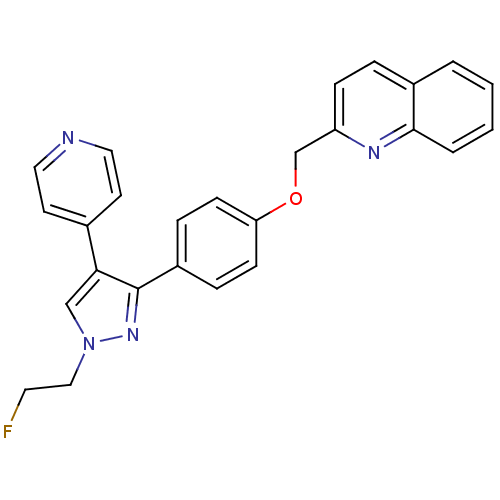
(CHEMBL1915747 | US9138494, JNJ-41510417)Show SMILES FCCn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C26H21FN4O/c27-13-16-31-17-24(19-11-14-28-15-12-19)26(30-31)21-6-9-23(10-7-21)32-18-22-8-5-20-3-1-2-4-25(20)29-22/h1-12,14-15,17H,13,16,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at CCK2 receptor in immature rat stomach assessed as pentagastrin-induced acid secretion |
Citation and Details
|
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50594973

(CHEMBL5169811)Show SMILES Nc1nc(nc2n(C[C@H]3CN(Cc4nccs4)CCO3)nnc12)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human EP1 receptor expressed in CHO cells receptor by NFTA reporter gene assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data