

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522827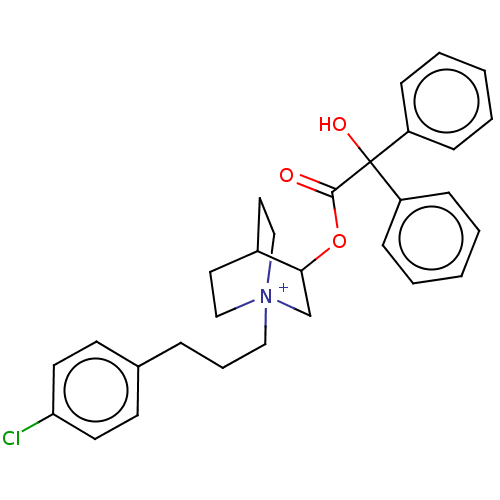 (CHEMBL4519930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571836 ((Z)-3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbon...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522833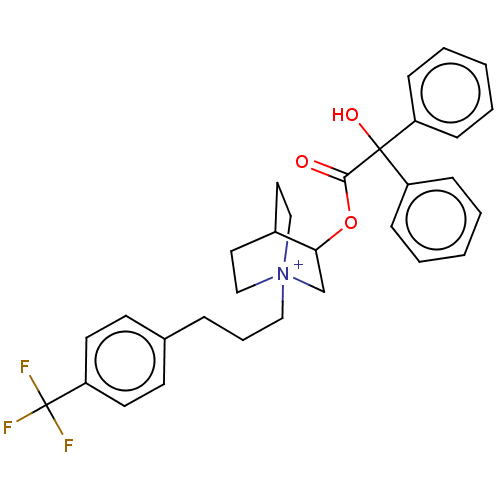 (CHEMBL4516342) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30369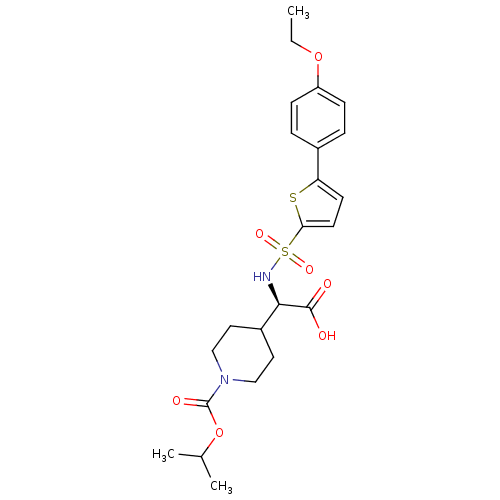 (piperidinyl glycine derivative, 24f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.190 | -54.9 | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50061649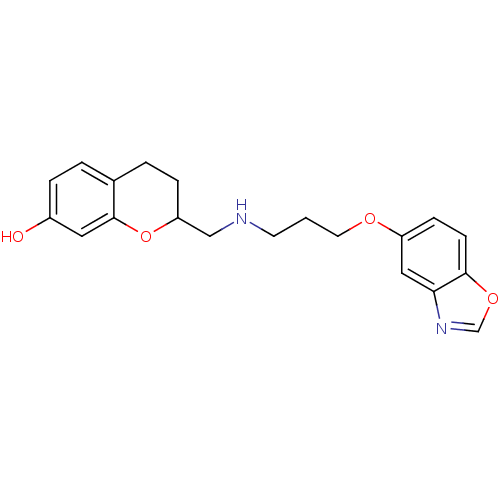 (2-{[3-(Benzooxazol-5-yloxy)-propylamino]-methyl}-c...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat hippocampal 5-hydroxytryptamine 1A receptor was determined using [3H]8-OH-DPAT as radioligand | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061682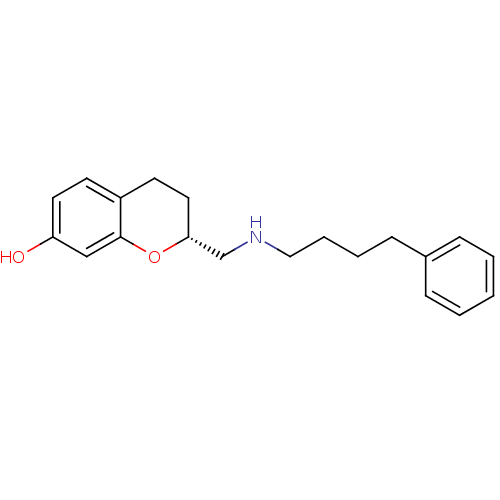 ((R)-2-[(4-Phenyl-butylamino)-methyl]-chroman-7-ol;...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262566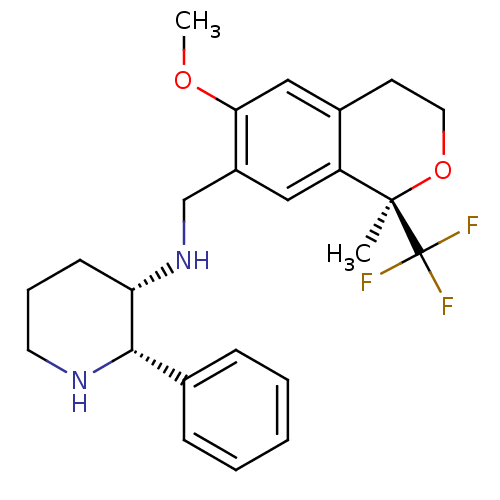 ((2S,3S)-3-[(1R)-6-Methoxy-1-methyl-1-trifluorometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061669 ((R)-2-(Benzylamino-methyl)-chroman-7-ol; oxalic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50239981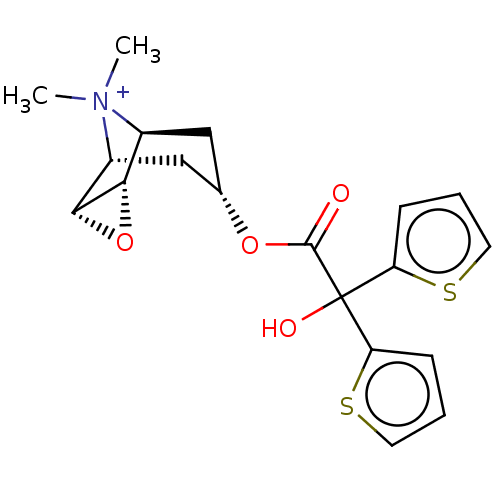 (Braltus | Spiriva | Spiriva Respimat | Tiotropium) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50061681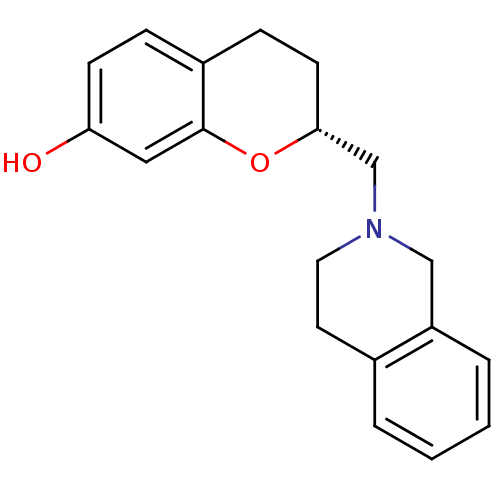 ((R)-2-(3,4-Dihydro-1H-isoquinolin-2-ylmethyl)-chro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Binding affinity at human Dopamine receptor D3 (hD3) using [3H]spiperone radioligand. | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061684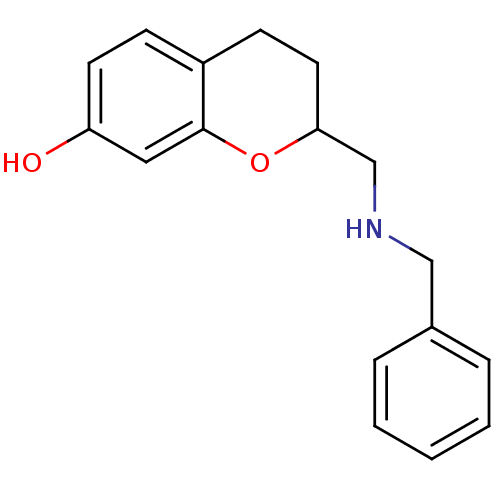 (2-(Benzylamino-methyl)-chroman-7-ol; oxalic acid |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma1 in human MDA-MB-468 cell membranes | Bioorg Med Chem Lett 27: 2216-2220 (2017) Article DOI: 10.1016/j.bmcl.2017.03.030 BindingDB Entry DOI: 10.7270/Q2P84F1X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11863 (4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.280 | -54.0 | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061665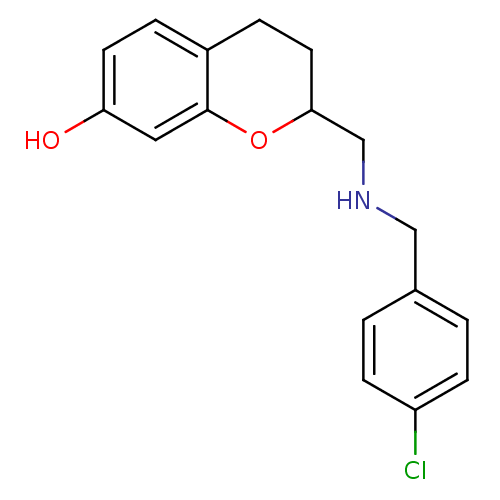 (2-[(4-Chloro-benzylamino)-methyl]-chroman-7-ol; ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50061646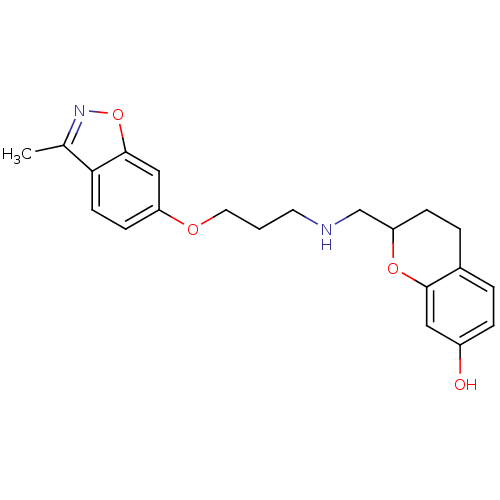 (2-{[3-(3-Methyl-benzo[d]isoxazol-6-yloxy)-propylam...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat hippocampal 5-hydroxytryptamine 1A receptor was determined using [3H]8-OH-DPAT as radioligand | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061671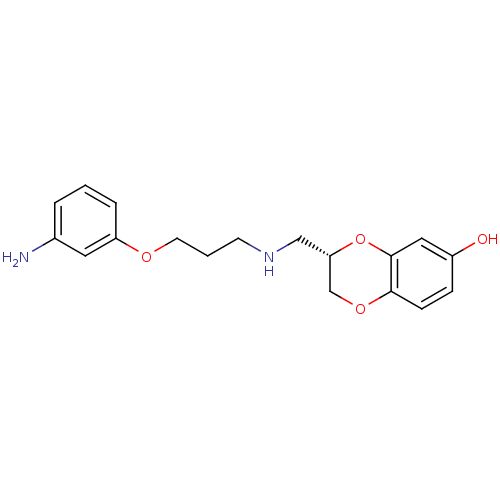 (3-{[3-(3-Amino-phenoxy)-propylamino]-methyl}-2,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061646 (2-{[3-(3-Methyl-benzo[d]isoxazol-6-yloxy)-propylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061636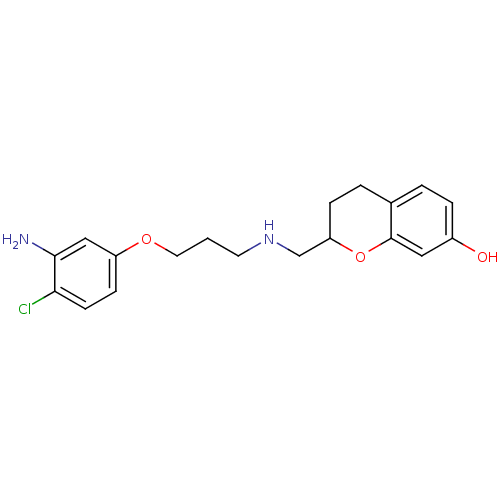 (2-{[3-(3-Amino-4-chloro-phenoxy)-propylamino]-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522832 (CHEMBL4483083) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522829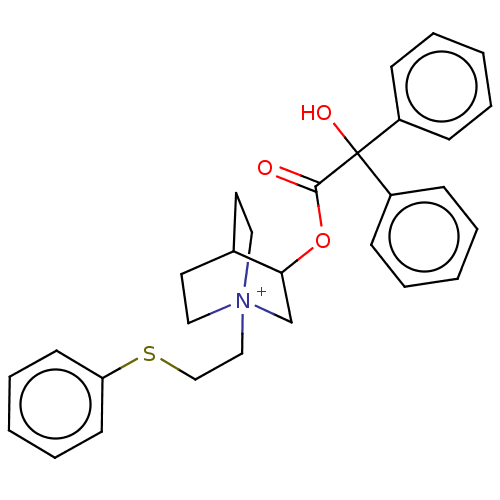 (CHEMBL4457734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061661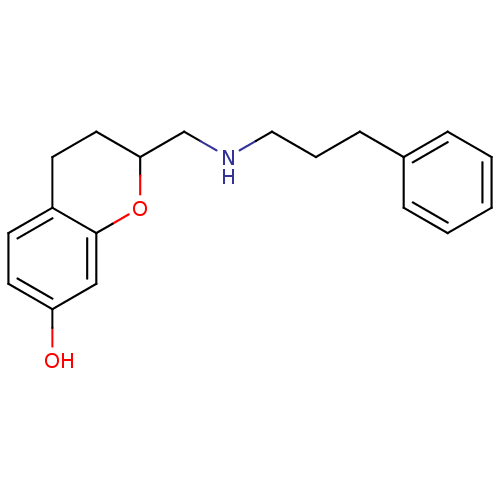 (2-[(3-Phenyl-propylamino)-methyl]-chroman-7-ol; ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061642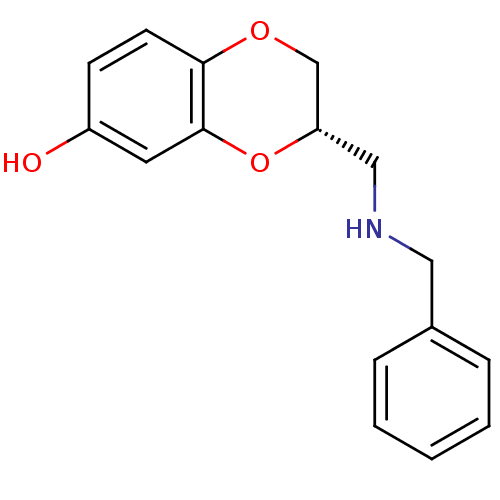 ((S)-3-(Benzylamino-methyl)-2,3-dihydro-benzo[1,4]d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061649 (2-{[3-(Benzooxazol-5-yloxy)-propylamino]-methyl}-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50061682 ((R)-2-[(4-Phenyl-butylamino)-methyl]-chroman-7-ol;...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat hippocampal 5-hydroxytryptamine 1A receptor was determined using [3H]8-OH-DPAT as radioligand | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296331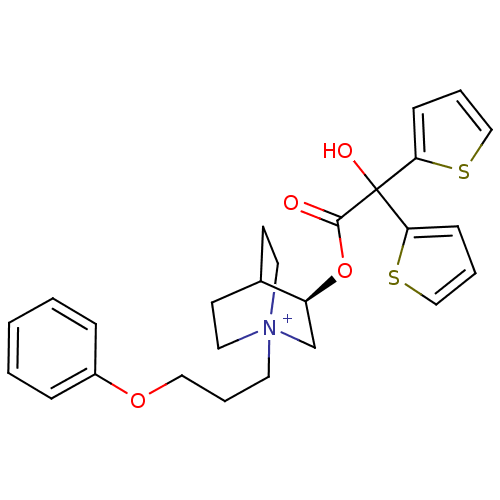 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522817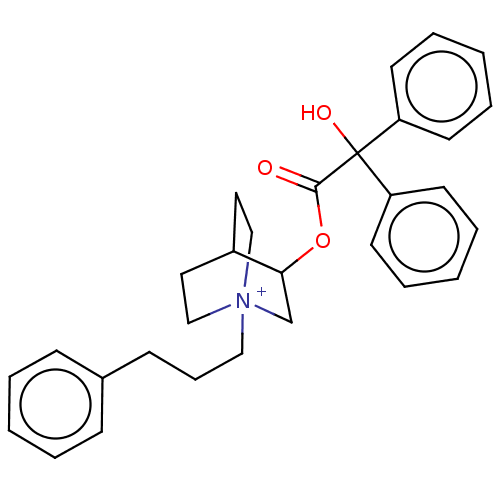 (CHEMBL4448944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50239981 (Braltus | Spiriva | Spiriva Respimat | Tiotropium) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522821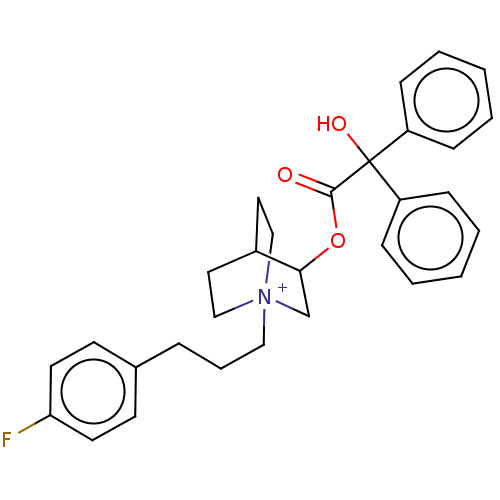 (CHEMBL4450188) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061673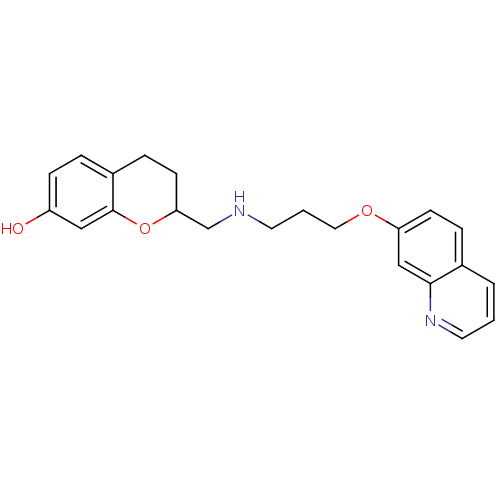 (2-{[3-(Quinolin-7-yloxy)-propylamino]-methyl}-chro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061662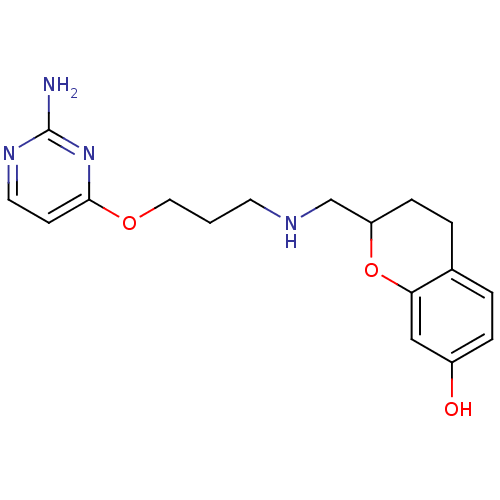 (2-{[3-(2-Amino-pyrimidin-4-yloxy)-propylamino]-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50061654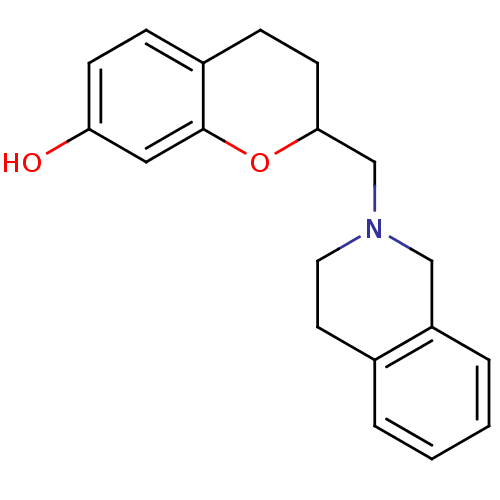 (2-(3,4-Dihydro-1H-isoquinolin-2-ylmethyl)-chroman-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Compound was tested to inhibit Dopa accumulation in Dopamine receptor D3 at 10 mg/kg, sc | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061631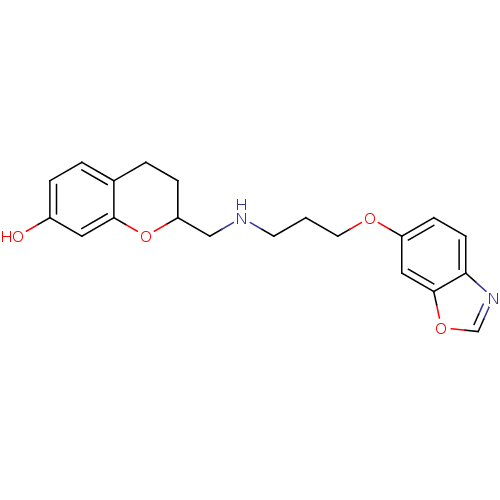 (2-{[3-(Benzooxazol-6-yloxy)-propylamino]-methyl}-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061638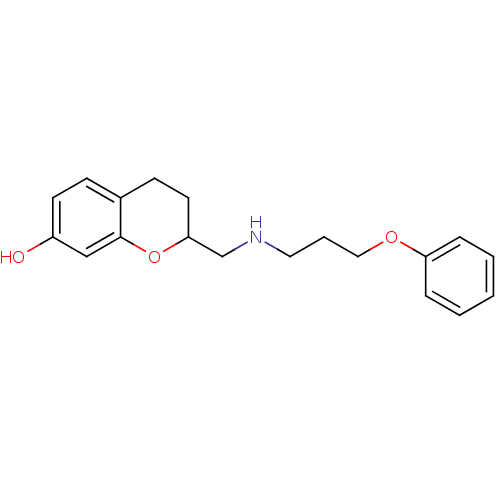 (2-[(3-Phenoxy-propylamino)-methyl]-chroman-7-ol; o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522827 (CHEMBL4519930) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571793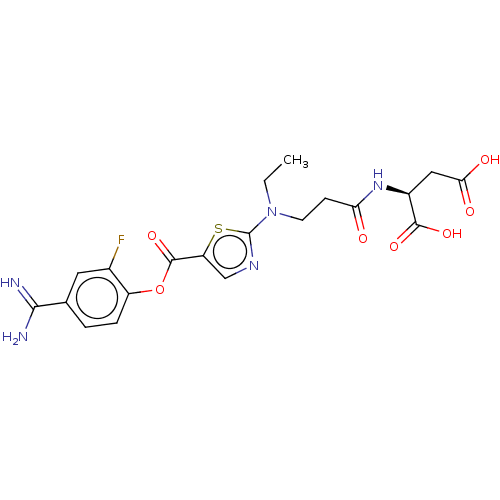 ((3-((5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30344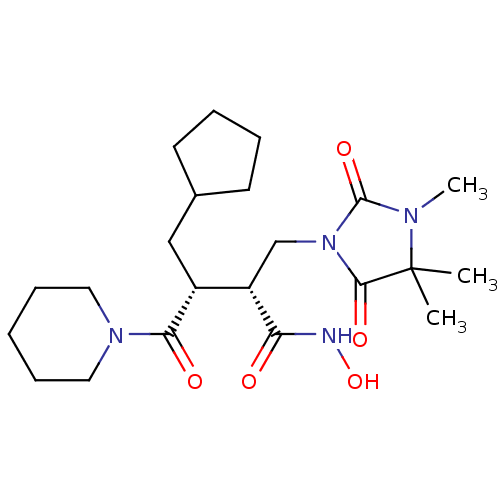 (Cipemastat | Trocade) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.530 | -52.4 | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571830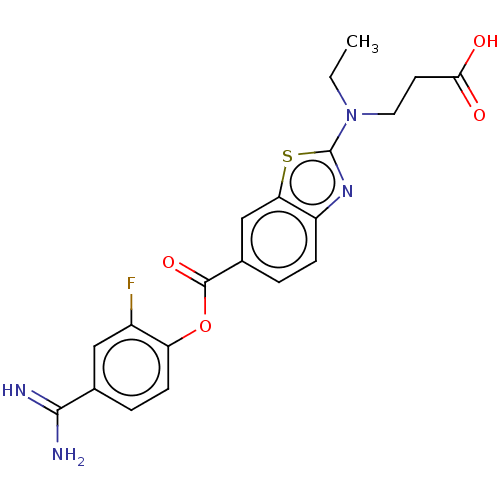 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296331 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571817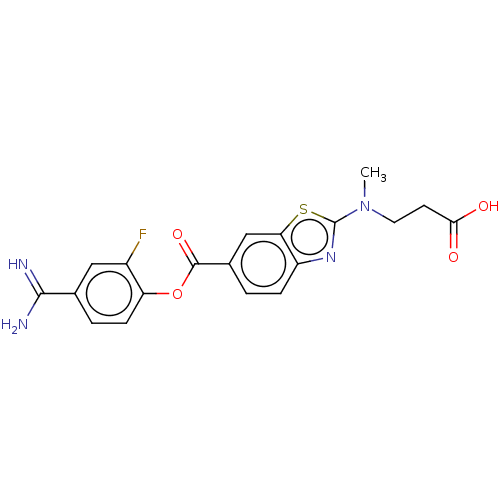 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571802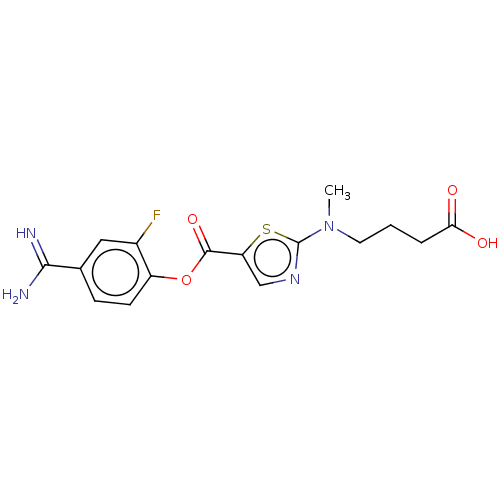 (4-((5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571797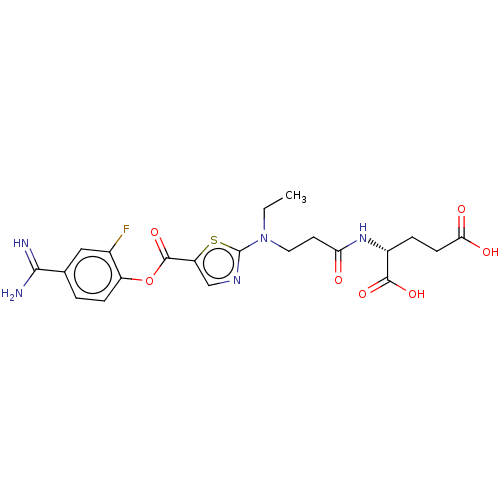 ((3-((5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571815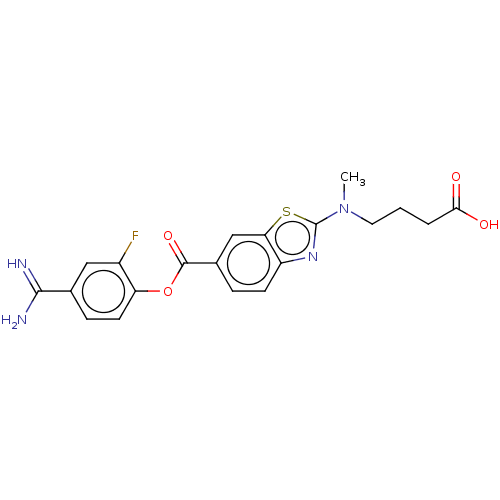 (4-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571814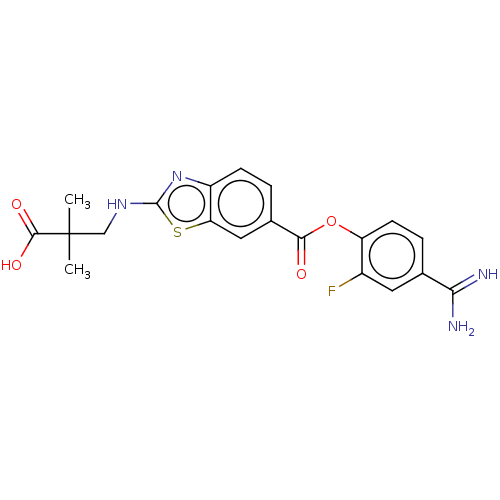 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (RAT) | BDBM50336889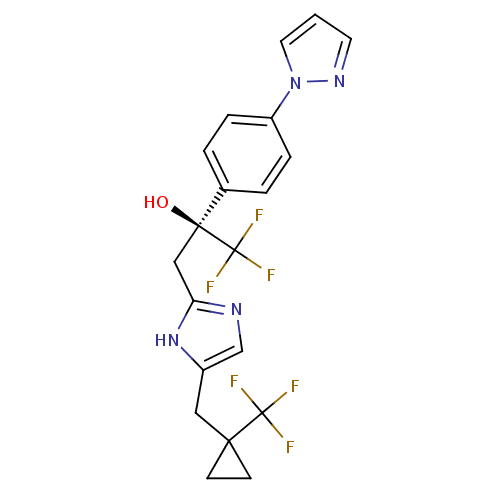 ((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]Bag-3 from rat BRS-3 | ACS Med Chem Lett 2: 43-47 (2011) Article DOI: 10.1021/ml100196d BindingDB Entry DOI: 10.7270/Q26D5T81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50061658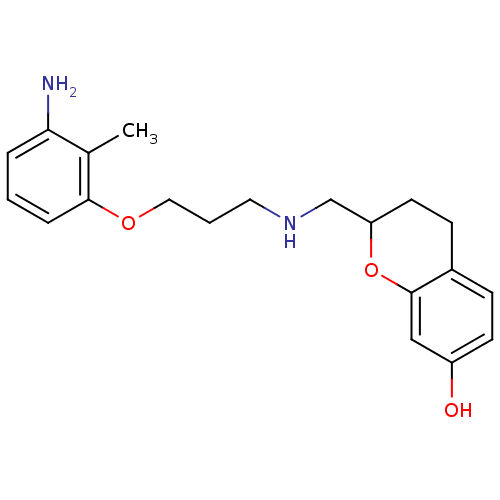 (2-{[3-(3-Amino-2-methyl-phenoxy)-propylamino]-meth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat hippocampal 5-hydroxytryptamine 1A receptor was determined using [3H]8-OH-DPAT as radioligand | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571772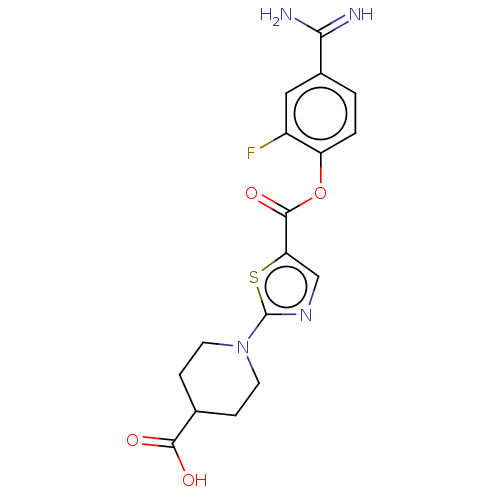 (1-(5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)th...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061633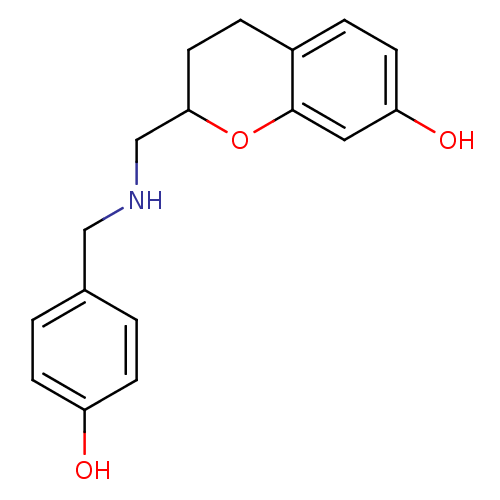 (2-[(4-Hydroxy-benzylamino)-methyl]-chroman-7-ol; o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061639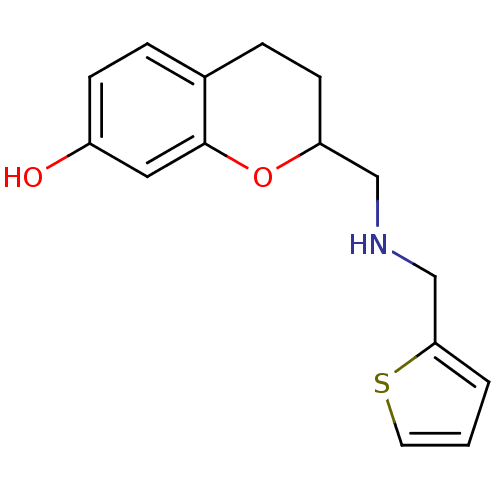 (2-{[(Thiophen-2-ylmethyl)-amino]-methyl}-chroman-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061632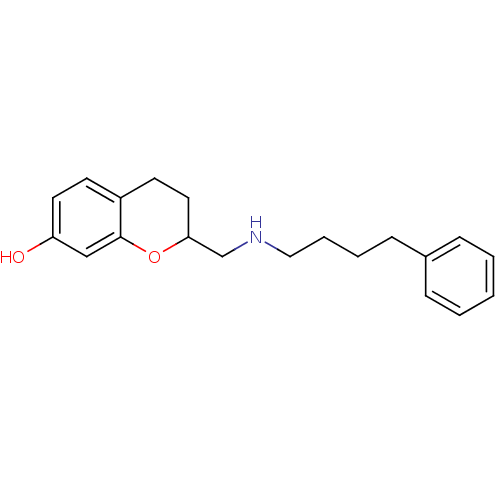 (2-[(4-Phenyl-butylamino)-methyl]-chroman-7-ol; oxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity to rat striatal Dopamine receptor D2 was determined using the agonist [3H]quinpirole to label the high affinity state (D2 h... | J Med Chem 40: 4235-56 (1998) Article DOI: 10.1021/jm9703653 BindingDB Entry DOI: 10.7270/Q2KP82TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (RAT) | BDBM50336889 ((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]Bag-3 from rat BRS-3 | ACS Med Chem Lett 2: 43-47 (2011) Article DOI: 10.1021/ml100196d BindingDB Entry DOI: 10.7270/Q26D5T81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7442 total ) | Next | Last >> |