 Found 525 hits with Last Name = 'karoutchi' and Initial = 'g'
Found 525 hits with Last Name = 'karoutchi' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743
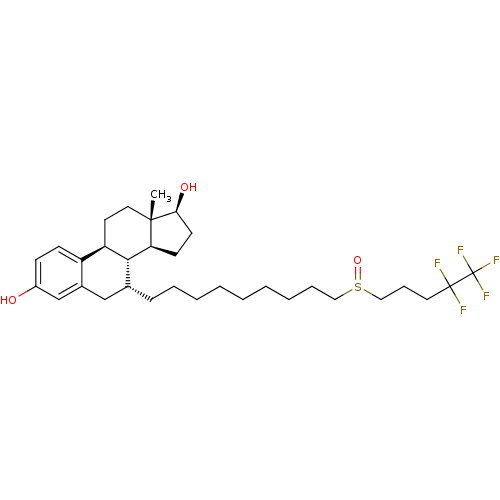
((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948
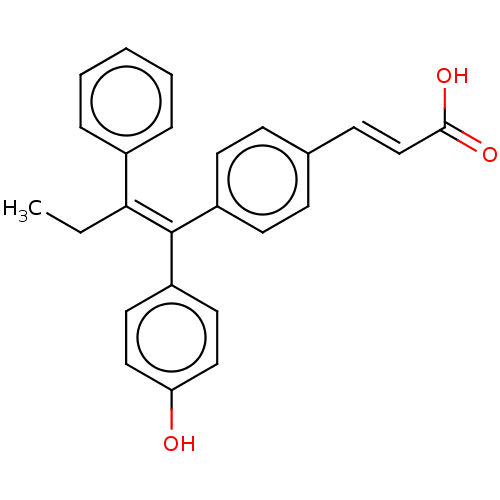
(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells in presence of 0.25 uM tamoxifen |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50263148
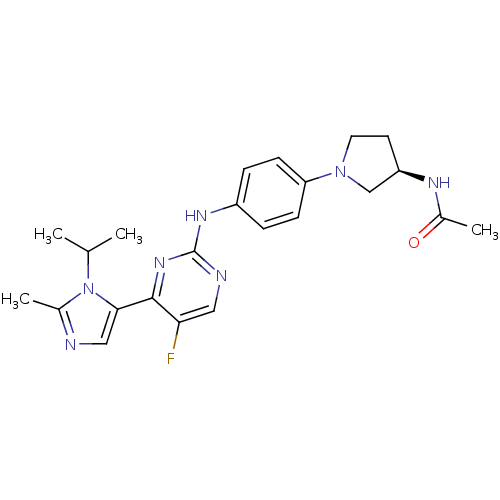
(CHEMBL476578 | N-((R)-1-(4-(5-fluoro-4-(1-isopropy...)Show SMILES CC(C)n1c(C)ncc1-c1nc(Nc2ccc(cc2)N2CC[C@H](C2)NC(C)=O)ncc1F |r| Show InChI InChI=1S/C23H28FN7O/c1-14(2)31-15(3)25-12-21(31)22-20(24)11-26-23(29-22)28-17-5-7-19(8-6-17)30-10-9-18(13-30)27-16(4)32/h5-8,11-12,14,18H,9-10,13H2,1-4H3,(H,27,32)(H,26,28,29)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
Bioorg Med Chem Lett 18: 4442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.027
BindingDB Entry DOI: 10.7270/Q24749PG |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20608
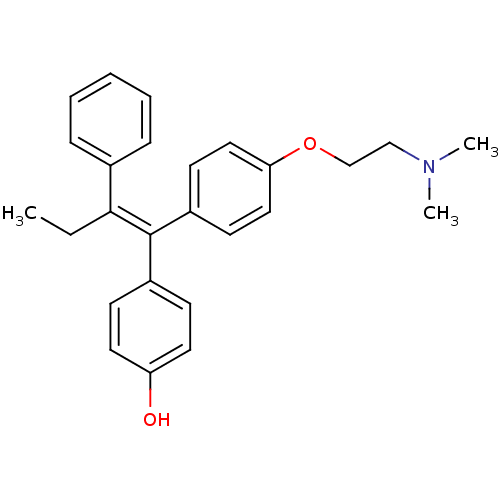
(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.309 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125055
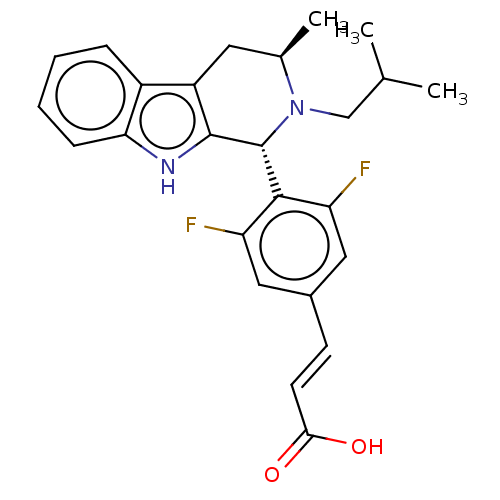
(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.417 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50263147
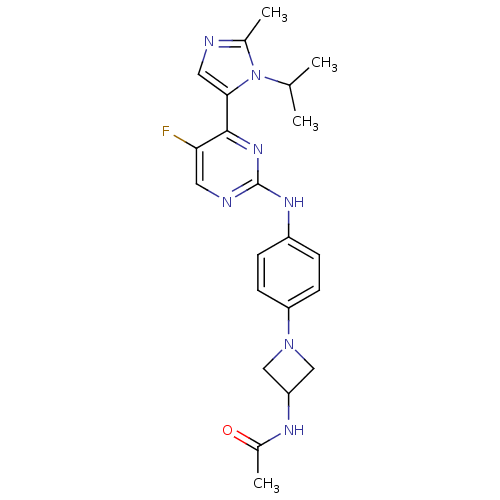
(CHEMBL476577 | N-(1-(4-(5-fluoro-4-(1-isopropyl-2-...)Show SMILES CC(C)n1c(C)ncc1-c1nc(Nc2ccc(cc2)N2CC(C2)NC(C)=O)ncc1F Show InChI InChI=1S/C22H26FN7O/c1-13(2)30-14(3)24-10-20(30)21-19(23)9-25-22(28-21)27-16-5-7-18(8-6-16)29-11-17(12-29)26-15(4)31/h5-10,13,17H,11-12H2,1-4H3,(H,26,31)(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
Bioorg Med Chem Lett 18: 4442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.027
BindingDB Entry DOI: 10.7270/Q24749PG |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125055

(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM20608

(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.813 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125055

(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414644
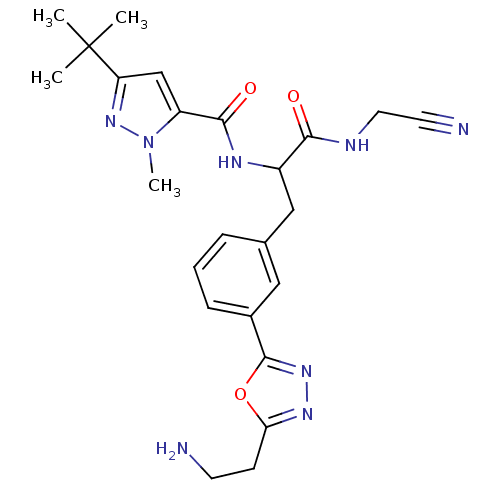
(CHEMBL555122)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CCN)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C24H30N8O3/c1-24(2,3)19-14-18(32(4)31-19)22(34)28-17(21(33)27-11-10-26)13-15-6-5-7-16(12-15)23-30-29-20(35-23)8-9-25/h5-7,12,14,17H,8-9,11,13,25H2,1-4H3,(H,27,33)(H,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414643

(CHEMBL557455)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CN)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C23H28N8O3/c1-23(2,3)18-12-17(31(4)30-18)21(33)27-16(20(32)26-9-8-24)11-14-6-5-7-15(10-14)22-29-28-19(13-25)34-22/h5-7,10,12,16H,9,11,13,25H2,1-4H3,(H,26,32)(H,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414641
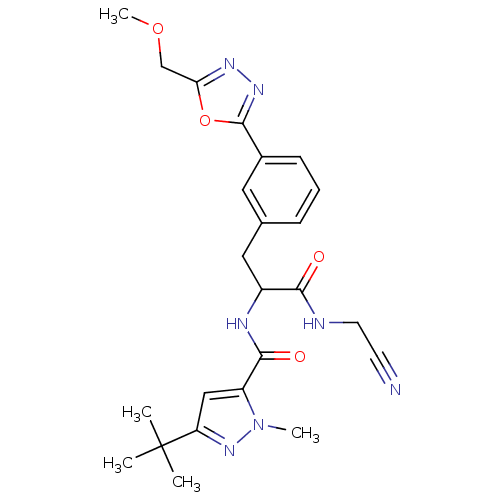
(CHEMBL554065)Show SMILES COCc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C24H29N7O4/c1-24(2,3)19-13-18(31(4)30-19)22(33)27-17(21(32)26-10-9-25)12-15-7-6-8-16(11-15)23-29-28-20(35-23)14-34-5/h6-8,11,13,17H,10,12,14H2,1-5H3,(H,26,32)(H,27,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125077

(CHEMBL3623001)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-16(2)15-27-17(3)14-21-20-6-4-5-7-22(20)26-24(21)25(27)19-11-8-18(9-12-19)10-13-23(28)29/h4-13,16-17,25-26H,14-15H2,1-3H3,(H,28,29)/b13-10+/t17-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50414636
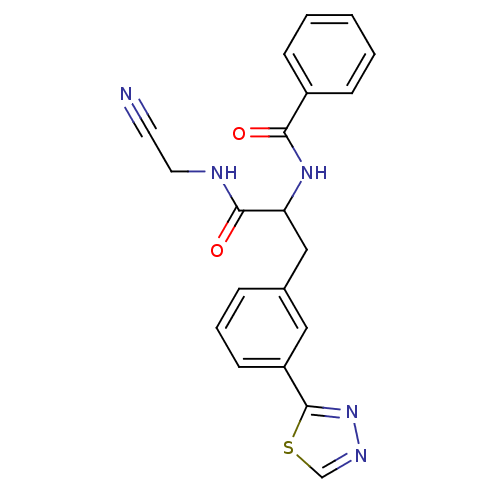
(CHEMBL559880)Show SMILES O=C(NCC#N)C(Cc1cccc(c1)-c1nncs1)NC(=O)c1ccccc1 Show InChI InChI=1S/C20H17N5O2S/c21-9-10-22-19(27)17(24-18(26)15-6-2-1-3-7-15)12-14-5-4-8-16(11-14)20-25-23-13-28-20/h1-8,11,13,17H,10,12H2,(H,22,27)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50263026
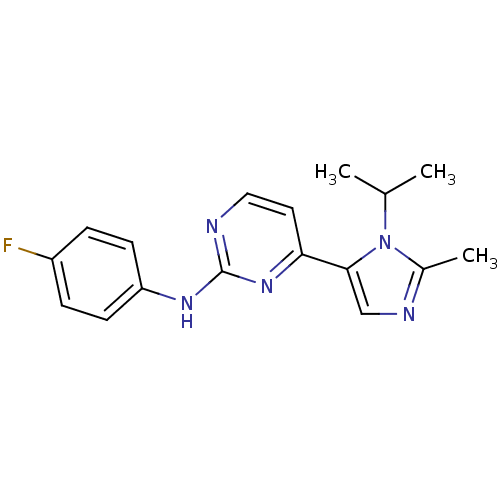
(CHEMBL478407 | N-(4-fluorophenyl)-4-(1-isopropyl-2...)Show InChI InChI=1S/C17H18FN5/c1-11(2)23-12(3)20-10-16(23)15-8-9-19-17(22-15)21-14-6-4-13(18)5-7-14/h4-11H,1-3H3,(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
Bioorg Med Chem Lett 18: 4442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.027
BindingDB Entry DOI: 10.7270/Q24749PG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50263068

(CHEMBL477785 | N-(4-(4-(2-(dimethylamino)ethylsulf...)Show SMILES CC(C)n1c(C)ncc1-c1ccnc(Nc2ccc(cc2)N2CCN(CC2)S(=O)(=O)CCN(C)C)n1 Show InChI InChI=1S/C25H36N8O2S/c1-19(2)33-20(3)27-18-24(33)23-10-11-26-25(29-23)28-21-6-8-22(9-7-21)31-12-14-32(15-13-31)36(34,35)17-16-30(4)5/h6-11,18-19H,12-17H2,1-5H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
Bioorg Med Chem Lett 18: 4442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.027
BindingDB Entry DOI: 10.7270/Q24749PG |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414640

(CHEMBL562844)Show SMILES Cc1nnc(s1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C23H27N7O2S/c1-14-27-28-22(33-14)16-8-6-7-15(11-16)12-17(20(31)25-10-9-24)26-21(32)18-13-19(23(2,3)4)29-30(18)5/h6-8,11,13,17H,10,12H2,1-5H3,(H,25,31)(H,26,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50041611
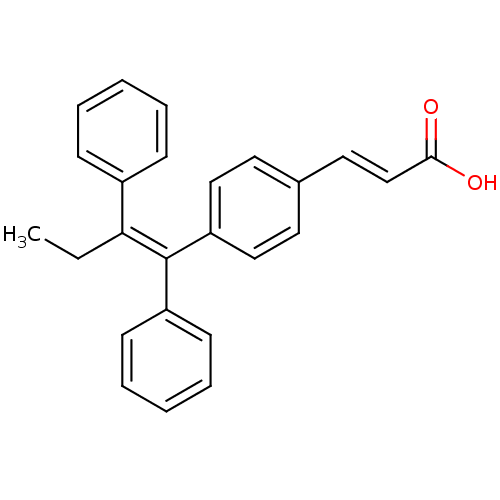
((2E)-3-{4-[(1E)-1,2-DIPHENYLBUT-1-ENYL]PHENYL}ACRY...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O2/c1-2-23(20-9-5-3-6-10-20)25(21-11-7-4-8-12-21)22-16-13-19(14-17-22)15-18-24(26)27/h3-18H,2H2,1H3,(H,26,27)/b18-15+,25-23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125077

(CHEMBL3623001)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-16(2)15-27-17(3)14-21-20-6-4-5-7-22(20)26-24(21)25(27)19-11-8-18(9-12-19)10-13-23(28)29/h4-13,16-17,25-26H,14-15H2,1-3H3,(H,28,29)/b13-10+/t17-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414641

(CHEMBL554065)Show SMILES COCc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C24H29N7O4/c1-24(2,3)19-13-18(31(4)30-19)22(33)27-17(21(32)26-10-9-25)12-15-7-6-8-16(11-15)23-29-28-20(35-23)14-34-5/h6-8,11,13,17H,10,12,14H2,1-5H3,(H,26,32)(H,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414639
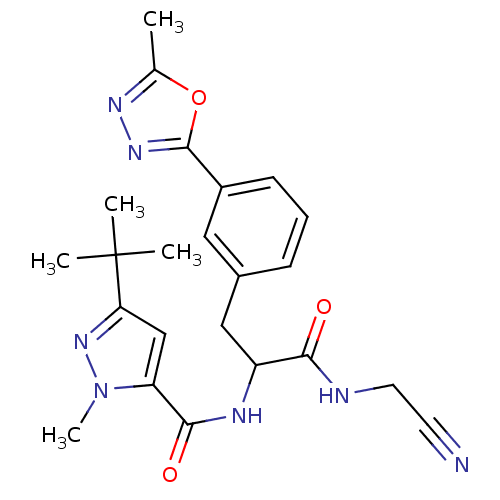
(CHEMBL550872)Show SMILES Cc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C23H27N7O3/c1-14-27-28-22(33-14)16-8-6-7-15(11-16)12-17(20(31)25-10-9-24)26-21(32)18-13-19(23(2,3)4)29-30(18)5/h6-8,11,13,17H,10,12H2,1-5H3,(H,25,31)(H,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414638
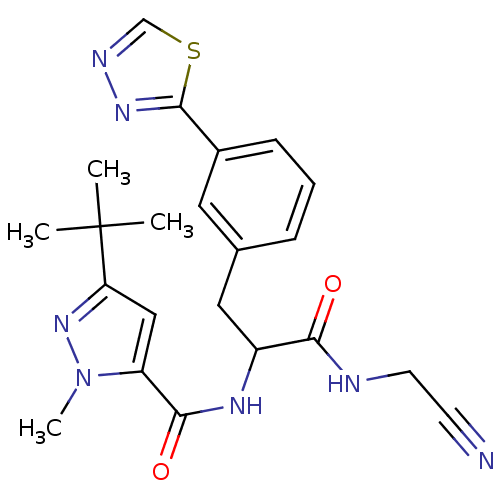
(CHEMBL549378)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nncs1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C22H25N7O2S/c1-22(2,3)18-12-17(29(4)28-18)20(31)26-16(19(30)24-9-8-23)11-14-6-5-7-15(10-14)21-27-25-13-32-21/h5-7,10,12-13,16H,9,11H2,1-4H3,(H,24,30)(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50263112

(1-(4-(4-(5-fluoro-4-(1-isopropyl-2-methyl-1H-imida...)Show SMILES CC(C)n1c(C)ncc1-c1nc(Nc2ccc(cc2)N2CCN(CC2)C(=O)CO)ncc1F Show InChI InChI=1S/C23H28FN7O2/c1-15(2)31-16(3)25-13-20(31)22-19(24)12-26-23(28-22)27-17-4-6-18(7-5-17)29-8-10-30(11-9-29)21(33)14-32/h4-7,12-13,15,32H,8-11,14H2,1-3H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
Bioorg Med Chem Lett 18: 4442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.027
BindingDB Entry DOI: 10.7270/Q24749PG |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414639

(CHEMBL550872)Show SMILES Cc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C23H27N7O3/c1-14-27-28-22(33-14)16-8-6-7-15(11-16)12-17(20(31)25-10-9-24)26-21(32)18-13-19(23(2,3)4)29-30(18)5/h6-8,11,13,17H,10,12H2,1-5H3,(H,25,31)(H,26,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414638

(CHEMBL549378)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nncs1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C22H25N7O2S/c1-22(2,3)18-12-17(29(4)28-18)20(31)26-16(19(30)24-9-8-23)11-14-6-5-7-15(10-14)21-27-25-13-32-21/h5-7,10,12-13,16H,9,11H2,1-4H3,(H,24,30)(H,26,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414642

(CHEMBL549791)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CO)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C23H27N7O4/c1-23(2,3)18-12-17(30(4)29-18)21(33)26-16(20(32)25-9-8-24)11-14-6-5-7-15(10-14)22-28-27-19(13-31)34-22/h5-7,10,12,16,31H,9,11,13H2,1-4H3,(H,25,32)(H,26,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125077

(CHEMBL3623001)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-16(2)15-27-17(3)14-21-20-6-4-5-7-22(20)26-24(21)25(27)19-11-8-18(9-12-19)10-13-23(28)29/h4-13,16-17,25-26H,14-15H2,1-3H3,(H,28,29)/b13-10+/t17-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948

(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50414633
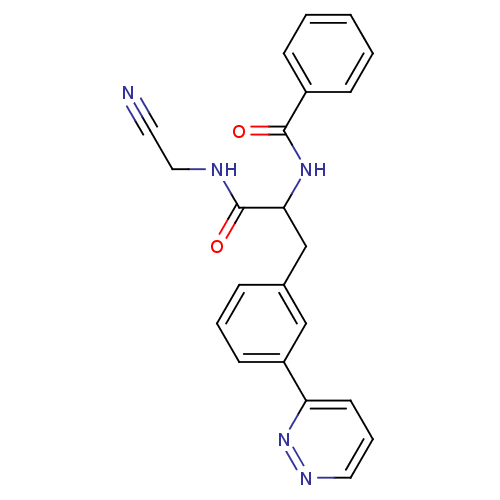
(CHEMBL556436)Show SMILES O=C(NCC#N)C(Cc1cccc(c1)-c1cccnn1)NC(=O)c1ccccc1 Show InChI InChI=1S/C22H19N5O2/c23-11-13-24-22(29)20(26-21(28)17-7-2-1-3-8-17)15-16-6-4-9-18(14-16)19-10-5-12-25-27-19/h1-10,12,14,20H,13,15H2,(H,24,29)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414642

(CHEMBL549791)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CO)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C23H27N7O4/c1-23(2,3)18-12-17(30(4)29-18)21(33)26-16(20(32)25-9-8-24)11-14-6-5-7-15(10-14)22-28-27-19(13-31)34-22/h5-7,10,12,16,31H,9,11,13H2,1-4H3,(H,25,32)(H,26,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50262981
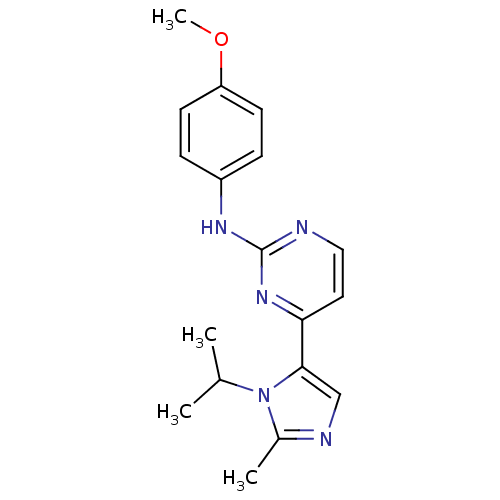
(4-(1-isopropyl-2-methyl-1H-imidazol-5-yl)-N-(4-met...)Show InChI InChI=1S/C18H21N5O/c1-12(2)23-13(3)20-11-17(23)16-9-10-19-18(22-16)21-14-5-7-15(24-4)8-6-14/h5-12H,1-4H3,(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
Bioorg Med Chem Lett 18: 4442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.027
BindingDB Entry DOI: 10.7270/Q24749PG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50263146

(5-fluoro-4-(1-isopropyl-2-methyl-1H-imidazol-5-yl)...)Show SMILES CC(C)n1c(C)ncc1-c1nc(Nc2ccc(cc2)N2CCOCC2)ncc1F Show InChI InChI=1S/C21H25FN6O/c1-14(2)28-15(3)23-13-19(28)20-18(22)12-24-21(26-20)25-16-4-6-17(7-5-16)27-8-10-29-11-9-27/h4-7,12-14H,8-11H2,1-3H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
Bioorg Med Chem Lett 18: 4442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.027
BindingDB Entry DOI: 10.7270/Q24749PG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50263113

(4-(1-isopropyl-2-methyl-1H-imidazol-5-yl)-N-(4-mor...)Show SMILES CC(C)n1c(C)ncc1-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 Show InChI InChI=1S/C21H26N6O/c1-15(2)27-16(3)23-14-20(27)19-8-9-22-21(25-19)24-17-4-6-18(7-5-17)26-10-12-28-13-11-26/h4-9,14-15H,10-13H2,1-3H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
Bioorg Med Chem Lett 18: 4442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.027
BindingDB Entry DOI: 10.7270/Q24749PG |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50026697
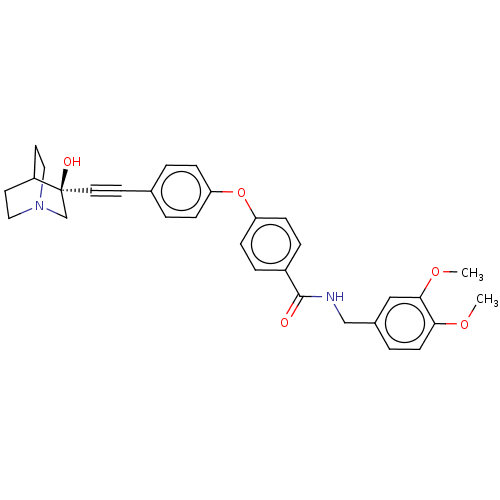
(CHEMBL1940287)Show SMILES COc1ccc(CNC(=O)c2ccc(Oc3ccc(cc3)C#C[C@]3(O)CN4CCC3CC4)cc2)cc1OC |r,wU:23.24,(-11.57,-13.27,;-10.23,-14.05,;-8.9,-13.28,;-7.55,-14.06,;-6.22,-13.29,;-6.22,-11.75,;-4.88,-10.98,;-3.54,-11.76,;-2.21,-10.99,;-2.2,-9.45,;-.87,-11.77,;.47,-11,;1.8,-11.78,;1.78,-13.32,;3.11,-14.1,;4.45,-13.33,;4.46,-11.79,;5.8,-11.03,;7.13,-11.82,;7.12,-13.35,;5.79,-14.11,;8.47,-11.05,;9.8,-10.29,;11.11,-9.51,;11.1,-11.03,;11.09,-7.98,;12.4,-7.21,;14.52,-7.63,;14.56,-9.81,;12.43,-10.25,;13.73,-9.47,;13.71,-7.97,;.45,-14.09,;-.88,-13.32,;-7.55,-10.97,;-8.89,-11.74,;-10.22,-10.96,;-11.56,-11.72,)| Show InChI InChI=1S/C31H32N2O5/c1-36-28-12-5-23(19-29(28)37-2)20-32-30(34)24-6-10-27(11-7-24)38-26-8-3-22(4-9-26)13-16-31(35)21-33-17-14-25(31)15-18-33/h3-12,19,25,35H,14-15,17-18,20-21H2,1-2H3,(H,32,34)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MMP13 using Mca-Pro-cyclohexyl-Ala-Gly-Nva-His-Ala- Dap(Dnp)-NH2 as substrate by fluorometric analysis |
Bioorg Med Chem Lett 22: 271-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.034
BindingDB Entry DOI: 10.7270/Q20R9PTN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at progesterone receptor in human MCF7 cells in presence of 0.25 uM tamoxifen |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50084948

(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM31992
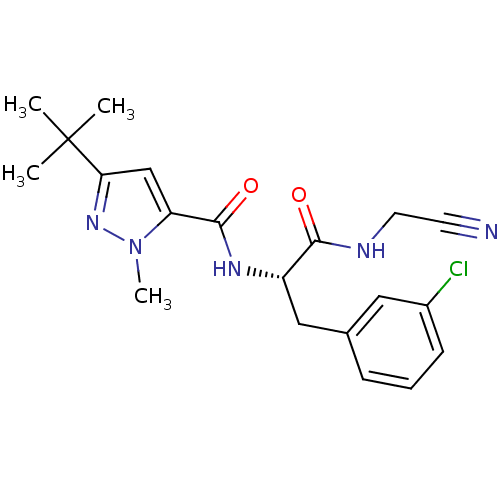
(Dipeptidyl nitrile inhibitor, 25)Show SMILES Cn1nc(cc1C(=O)N[C@@H](Cc1cccc(Cl)c1)C(=O)NCC#N)C(C)(C)C |r| Show InChI InChI=1S/C20H24ClN5O2/c1-20(2,3)17-12-16(26(4)25-17)19(28)24-15(18(27)23-9-8-22)11-13-6-5-7-14(21)10-13/h5-7,10,12,15H,9,11H2,1-4H3,(H,23,27)(H,24,28)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM31993

(Dipeptidyl nitrile inhibitor, 26)Show SMILES Cc1cccc(C[C@H](NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 |r| Show InChI InChI=1S/C21H27N5O2/c1-14-7-6-8-15(11-14)12-16(19(27)23-10-9-22)24-20(28)17-13-18(21(2,3)4)25-26(17)5/h6-8,11,13,16H,10,12H2,1-5H3,(H,23,27)(H,24,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31992

(Dipeptidyl nitrile inhibitor, 25)Show SMILES Cn1nc(cc1C(=O)N[C@@H](Cc1cccc(Cl)c1)C(=O)NCC#N)C(C)(C)C |r| Show InChI InChI=1S/C20H24ClN5O2/c1-20(2,3)17-12-16(26(4)25-17)19(28)24-15(18(27)23-9-8-22)11-13-6-5-7-14(21)10-13/h5-7,10,12,15H,9,11H2,1-4H3,(H,23,27)(H,24,28)/t15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125081
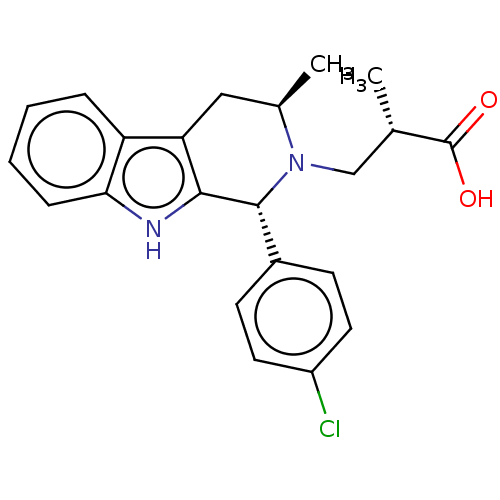
(CHEMBL3622997)Show SMILES C[C@@H](CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(Cl)cc1)C(O)=O |r| Show InChI InChI=1S/C22H23ClN2O2/c1-13(22(26)27)12-25-14(2)11-18-17-5-3-4-6-19(17)24-20(18)21(25)15-7-9-16(23)10-8-15/h3-10,13-14,21,24H,11-12H2,1-2H3,(H,26,27)/t13-,14+,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at progesterone receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data