 Found 865 hits with Last Name = 'kaufman' and Initial = 'd'
Found 865 hits with Last Name = 'kaufman' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50152609
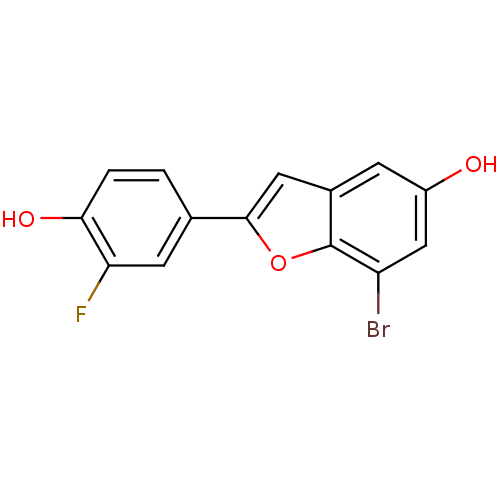
(7-Bromo-2-(3-fluoro-4-hydroxy-phenyl)-benzofuran-5...)Show InChI InChI=1S/C14H8BrFO3/c15-10-6-9(17)3-8-5-13(19-14(8)10)7-1-2-12(18)11(16)4-7/h1-6,17-18H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Estrogen receptor beta |
Bioorg Med Chem Lett 14: 4925-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.029
BindingDB Entry DOI: 10.7270/Q2TM79K8 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50096333
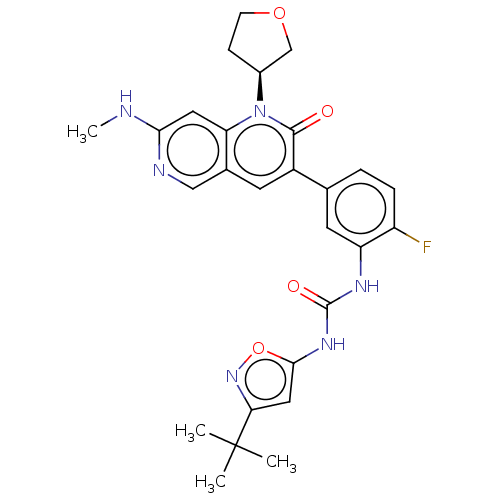
(CHEMBL3577114)Show SMILES CNc1cc2n([C@H]3CCOC3)c(=O)c(cc2cn1)-c1ccc(F)c(NC(=O)Nc2cc(no2)C(C)(C)C)c1 |r| Show InChI InChI=1S/C21H13F2N3O/c22-14-8-10-18-16(13-14)21(27)26(19-7-2-1-6-17(19)23)20(25-18)11-9-15-5-3-4-12-24-15/h1-13H/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) |
J Med Chem 58: 4165-79 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00067
BindingDB Entry DOI: 10.7270/Q2ZS2Z85 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50152616
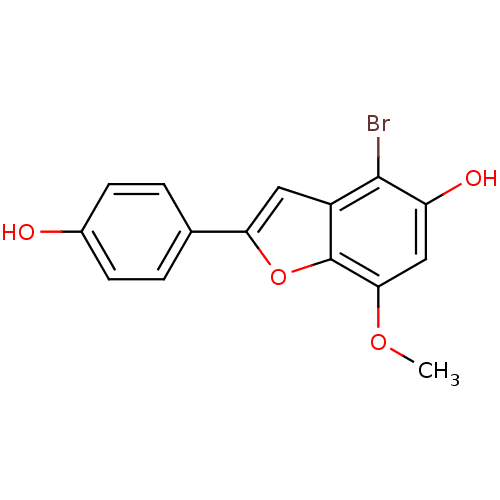
(4-Bromo-2-(4-hydroxy-phenyl)-7-methoxy-benzofuran-...)Show InChI InChI=1S/C15H11BrO4/c1-19-13-7-11(18)14(16)10-6-12(20-15(10)13)8-2-4-9(17)5-3-8/h2-7,17-18H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Estrogen receptor beta |
Bioorg Med Chem Lett 14: 4925-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.029
BindingDB Entry DOI: 10.7270/Q2TM79K8 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306072

(2-isobutyl-1-(3-(3-(methylsulfonyl)phenoxy)phenyl)...)Show SMILES CC(C)Cc1nc2c(cccc2n1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C25H23F3N2O3S/c1-16(2)13-23-29-24-21(25(26,27)28)11-6-12-22(24)30(23)17-7-4-8-18(14-17)33-19-9-5-10-20(15-19)34(3,31)32/h4-12,14-16H,13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305075
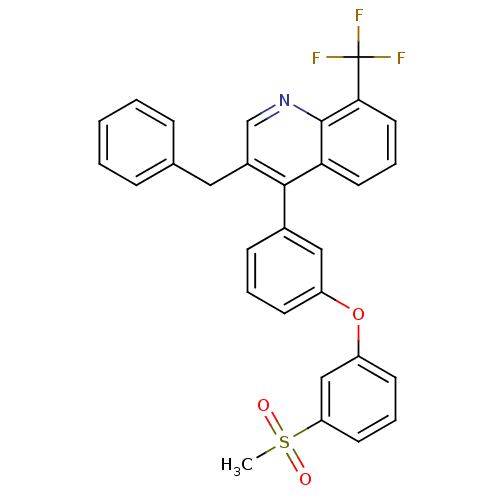
(3-benzyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C30H22F3NO3S/c1-38(35,36)25-13-6-12-24(18-25)37-23-11-5-10-21(17-23)28-22(16-20-8-3-2-4-9-20)19-34-29-26(28)14-7-15-27(29)30(31,32)33/h2-15,17-19H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305077
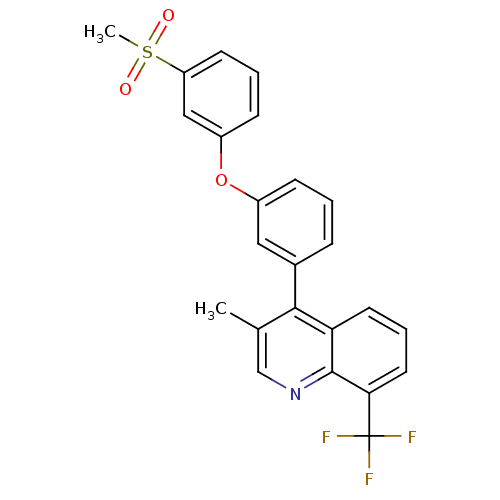
(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50096333

(CHEMBL3577114)Show SMILES CNc1cc2n([C@H]3CCOC3)c(=O)c(cc2cn1)-c1ccc(F)c(NC(=O)Nc2cc(no2)C(C)(C)C)c1 |r| Show InChI InChI=1S/C21H13F2N3O/c22-14-8-10-18-16(13-14)21(27)26(19-7-2-1-6-17(19)23)20(25-18)11-9-15-5-3-4-12-24-15/h1-13H/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of recombinant BRAF V600E mutant (unknown origin) assessed as ADP formation measured for 5 hrs by pyruvate kinase/lactate dehydrogenase co... |
J Med Chem 58: 4165-79 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00067
BindingDB Entry DOI: 10.7270/Q2ZS2Z85 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306070
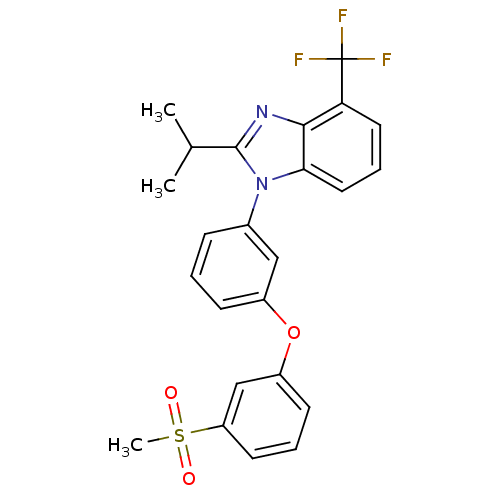
(2-isopropyl-1-(3-(3-(methylsulfonyl)phenoxy)phenyl...)Show SMILES CC(C)c1nc2c(cccc2n1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H21F3N2O3S/c1-15(2)23-28-22-20(24(25,26)27)11-6-12-21(22)29(23)16-7-4-8-17(13-16)32-18-9-5-10-19(14-18)33(3,30)31/h4-15H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50152628

(2-(3-Fluoro-4-hydroxy-phenyl)-5-hydroxy-benzofuran...)Show InChI InChI=1S/C15H8FNO3/c16-12-5-8(1-2-13(12)19)14-6-9-3-11(18)4-10(7-17)15(9)20-14/h1-6,18-19H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Estrogen receptor beta |
Bioorg Med Chem Lett 14: 4925-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.029
BindingDB Entry DOI: 10.7270/Q2TM79K8 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305072
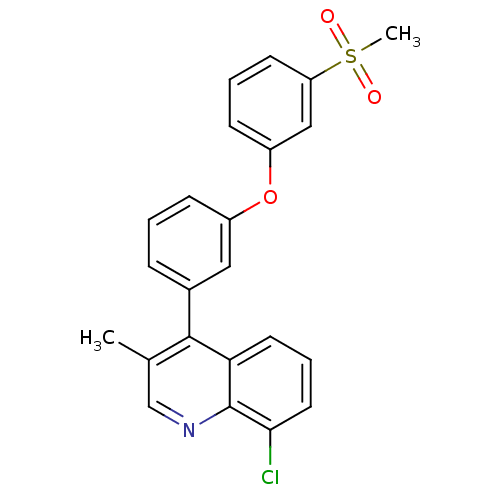
(8-chloro-3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)...)Show SMILES Cc1cnc2c(Cl)cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1 Show InChI InChI=1S/C23H18ClNO3S/c1-15-14-25-23-20(10-5-11-21(23)24)22(15)16-6-3-7-17(12-16)28-18-8-4-9-19(13-18)29(2,26)27/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306073
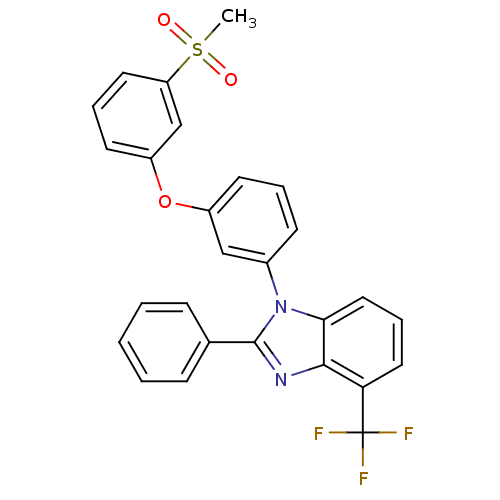
(1-(3-(3-(methylsulfonyl)phenoxy)phenyl)-2-phenyl-4...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-n2c(nc3c(cccc23)C(F)(F)F)-c2ccccc2)c1 Show InChI InChI=1S/C27H19F3N2O3S/c1-36(33,34)22-13-6-12-21(17-22)35-20-11-5-10-19(16-20)32-24-15-7-14-23(27(28,29)30)25(24)31-26(32)18-8-3-2-4-9-18/h2-17H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305073
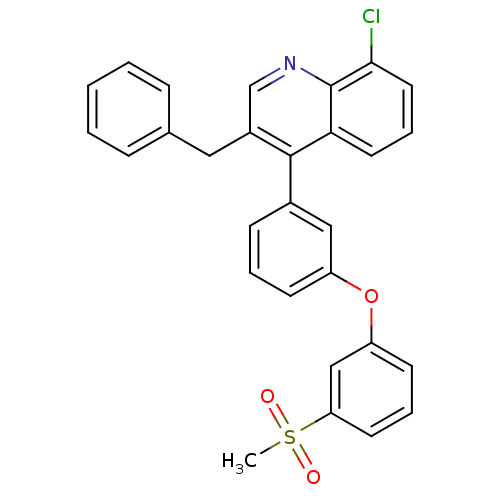
(3-benzyl-8-chloro-4-(3-(3-(methylsulfonyl)phenoxy)...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(Cl)cccc23)c1 Show InChI InChI=1S/C29H22ClNO3S/c1-35(32,33)25-13-6-12-24(18-25)34-23-11-5-10-21(17-23)28-22(16-20-8-3-2-4-9-20)19-31-29-26(28)14-7-15-27(29)30/h2-15,17-19H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50222709
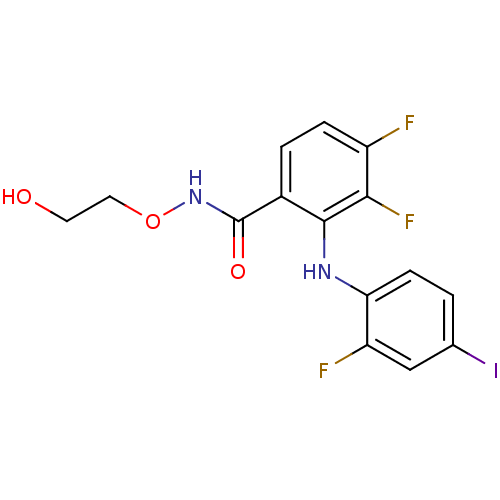
(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-N-(2-h...)Show InChI InChI=1S/C15H12F3IN2O3/c16-10-3-2-9(15(23)21-24-6-5-22)14(13(10)18)20-12-4-1-8(19)7-11(12)17/h1-4,7,20,22H,5-6H2,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of MEK assessed as inhibition of ERK phosphorylation by Raf-MEK-ERK cascade assay |
J Med Chem 50: 5090-102 (2007)
Article DOI: 10.1021/jm0704548
BindingDB Entry DOI: 10.7270/Q2474DMT |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50096323
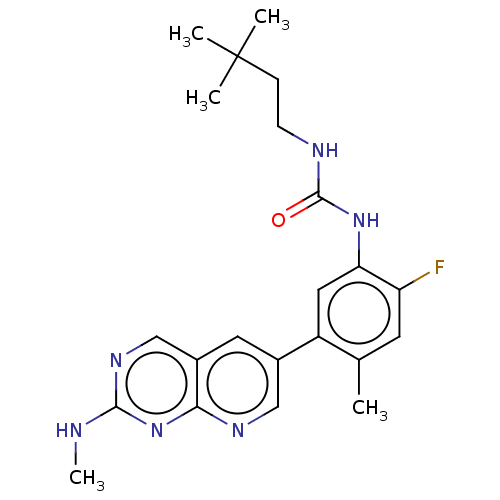
(CHEMBL3577123)Show SMILES CNc1ncc2cc(cnc2n1)-c1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C Show InChI InChI=1S/C22H17N3O2/c1-27-20-12-5-4-11-19(20)25-21(14-13-16-8-6-7-15-23-16)24-18-10-3-2-9-17(18)22(25)26/h2-15H,1H3/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of wild type CRAF (unknown origin) assessed as ADP formation measured for 5 hrs by pyruvate kinase/lactate dehydrogenase coupled assay in ... |
J Med Chem 58: 4165-79 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00067
BindingDB Entry DOI: 10.7270/Q2ZS2Z85 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50096332
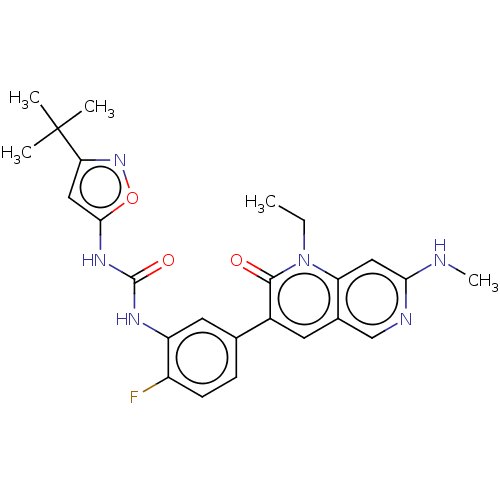
(CHEMBL3577115)Show SMILES CCn1c2cc(NC)ncc2cc(-c2ccc(F)c(NC(=O)Nc3cc(no3)C(C)(C)C)c2)c1=O Show InChI InChI=1S/C22H15ClFN3O2/c23-18-6-1-2-7-20(18)27-21(11-9-15-4-3-5-16(13-28)25-15)26-19-10-8-14(24)12-17(19)22(27)29/h1-12,28H,13H2/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of recombinant BRAF V600E mutant (unknown origin) assessed as ADP formation measured for 5 hrs by pyruvate kinase/lactate dehydrogenase co... |
J Med Chem 58: 4165-79 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00067
BindingDB Entry DOI: 10.7270/Q2ZS2Z85 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306074

(2-(4-fluorobenzyl)-1-(3-(3-(methylsulfonyl)phenoxy...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-n2c(Cc3ccc(F)cc3)nc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C28H20F4N2O3S/c1-38(35,36)23-8-3-7-22(17-23)37-21-6-2-5-20(16-21)34-25-10-4-9-24(28(30,31)32)27(25)33-26(34)15-18-11-13-19(29)14-12-18/h2-14,16-17H,15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306068
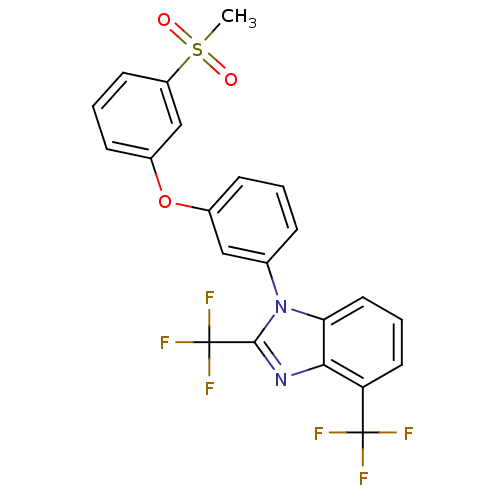
(1-(3-(3-(methylsulfonyl)phenoxy)phenyl)-2,4-bis(tr...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-n2c(nc3c(cccc23)C(F)(F)F)C(F)(F)F)c1 Show InChI InChI=1S/C22H14F6N2O3S/c1-34(31,32)16-8-3-7-15(12-16)33-14-6-2-5-13(11-14)30-18-10-4-9-17(21(23,24)25)19(18)29-20(30)22(26,27)28/h2-12H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305064
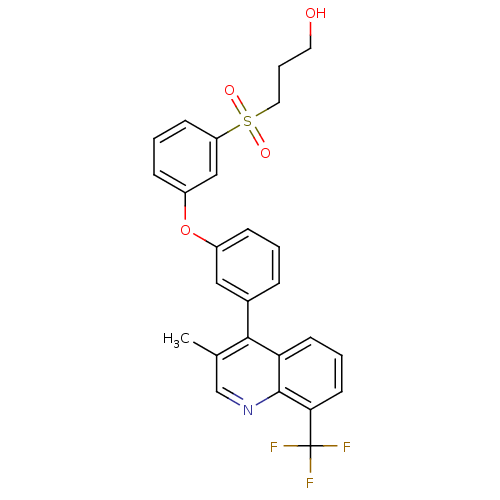
(3-(3-(3-(3-methyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(=O)(=O)CCCO)c1)C(F)(F)F Show InChI InChI=1S/C26H22F3NO4S/c1-17-16-30-25-22(10-4-11-23(25)26(27,28)29)24(17)18-6-2-7-19(14-18)34-20-8-3-9-21(15-20)35(32,33)13-5-12-31/h2-4,6-11,14-16,31H,5,12-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20024
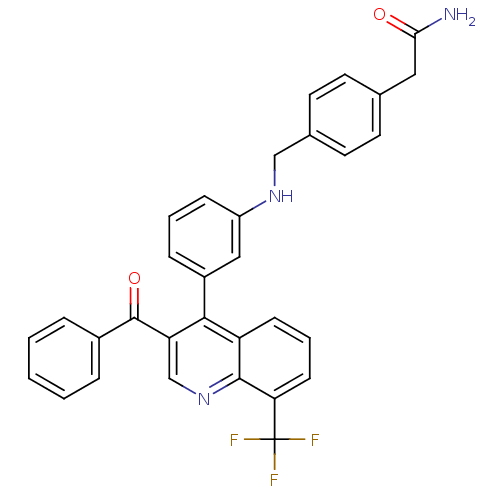
(2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...)Show SMILES NC(=O)Cc1ccc(CNc2cccc(c2)-c2c(cnc3c(cccc23)C(F)(F)F)C(=O)c2ccccc2)cc1 Show InChI InChI=1S/C32H24F3N3O2/c33-32(34,35)27-11-5-10-25-29(26(19-38-30(25)27)31(40)22-6-2-1-3-7-22)23-8-4-9-24(17-23)37-18-21-14-12-20(13-15-21)16-28(36)39/h1-15,17,19,37H,16,18H2,(H2,36,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | 316 | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
Bioorg Med Chem 15: 3321-33 (2007)
Article DOI: 10.1016/j.bmc.2007.03.013
BindingDB Entry DOI: 10.7270/Q2VH5M37 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20015

(2-[4-({[3-(3-benzyl-8-chloroquinolin-4-yl)phenyl]a...)Show SMILES OC(=O)Cc1ccc(CNc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(Cl)cccc23)cc1 Show InChI InChI=1S/C31H25ClN2O2/c32-28-11-5-10-27-30(25(20-34-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)33-19-23-14-12-22(13-15-23)17-29(35)36/h1-15,18,20,33H,16-17,19H2,(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | 23 | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
Bioorg Med Chem 15: 3321-33 (2007)
Article DOI: 10.1016/j.bmc.2007.03.013
BindingDB Entry DOI: 10.7270/Q2VH5M37 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306075
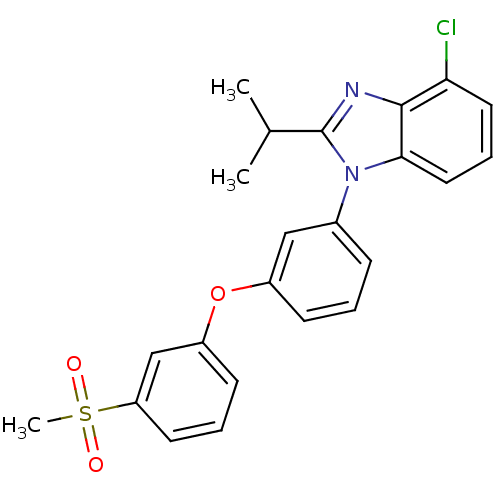
(4-chloro-2-isopropyl-1-(3-(3-(methylsulfonyl)pheno...)Show SMILES CC(C)c1nc2c(Cl)cccc2n1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1 Show InChI InChI=1S/C23H21ClN2O3S/c1-15(2)23-25-22-20(24)11-6-12-21(22)26(23)16-7-4-8-17(13-16)29-18-9-5-10-19(14-18)30(3,27)28/h4-15H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50152629
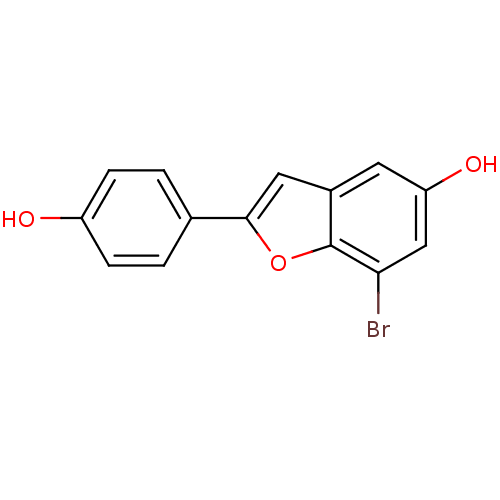
(7-Bromo-2-(4-hydroxy-phenyl)-benzofuran-5-ol | CHE...)Show InChI InChI=1S/C14H9BrO3/c15-12-7-11(17)5-9-6-13(18-14(9)12)8-1-3-10(16)4-2-8/h1-7,16-17H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Estrogen receptor alpha |
Bioorg Med Chem Lett 14: 4925-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.029
BindingDB Entry DOI: 10.7270/Q2TM79K8 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50096325
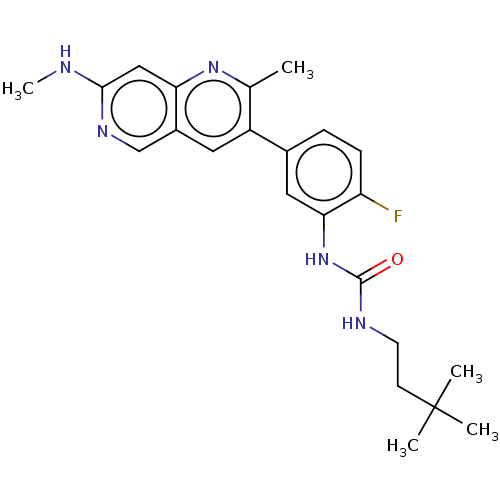
(CHEMBL3577120)Show SMILES CNc1cc2nc(C)c(cc2cn1)-c1ccc(F)c(NC(=O)NCCC(C)(C)C)c1 Show InChI InChI=1S/C22H16ClN3O/c1-15-6-2-3-8-20(15)26-21(12-10-17-7-4-5-13-24-17)25-19-14-16(23)9-11-18(19)22(26)27/h2-14H,1H3/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of wild type CRAF (unknown origin) assessed as ADP formation measured for 5 hrs by pyruvate kinase/lactate dehydrogenase coupled assay in ... |
J Med Chem 58: 4165-79 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00067
BindingDB Entry DOI: 10.7270/Q2ZS2Z85 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305070
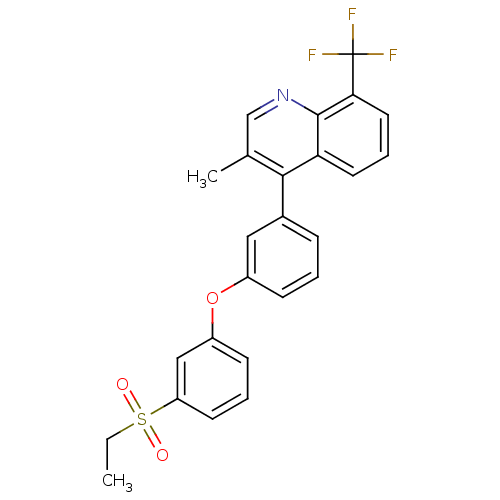
(4-(3-(3-(ethylsulfonyl)phenoxy)phenyl)-3-methyl-8-...)Show SMILES CCS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(C)cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C25H20F3NO3S/c1-3-33(30,31)20-10-5-9-19(14-20)32-18-8-4-7-17(13-18)23-16(2)15-29-24-21(23)11-6-12-22(24)25(26,27)28/h4-15H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589660

(CHEMBL5193071)Show SMILES CC(C)Nc1ncc(-c2ccc(Oc3ccnc(c3)-c3cnn(C)c3)c(C)n2)c(=O)[nH]1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
J Med Chem 48: 680-93 (2005)
Article DOI: 10.1016/j.bmcl.2022.128928
BindingDB Entry DOI: 10.7270/Q2FN1B5Z |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50152626

(7-Chloro-2-(4-hydroxy-phenyl)-benzofuran-5-ol | CH...)Show InChI InChI=1S/C14H9ClO3/c15-12-7-11(17)5-9-6-13(18-14(9)12)8-1-3-10(16)4-2-8/h1-7,16-17H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Estrogen receptor beta |
Bioorg Med Chem Lett 14: 4925-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.029
BindingDB Entry DOI: 10.7270/Q2TM79K8 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306069

(2-ethyl-1-(3-(3-(methylsulfonyl)phenoxy)phenyl)-4-...)Show SMILES CCc1nc2c(cccc2n1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C23H19F3N2O3S/c1-3-21-27-22-19(23(24,25)26)11-6-12-20(22)28(21)15-7-4-8-16(13-15)31-17-9-5-10-18(14-17)32(2,29)30/h4-14H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50177716
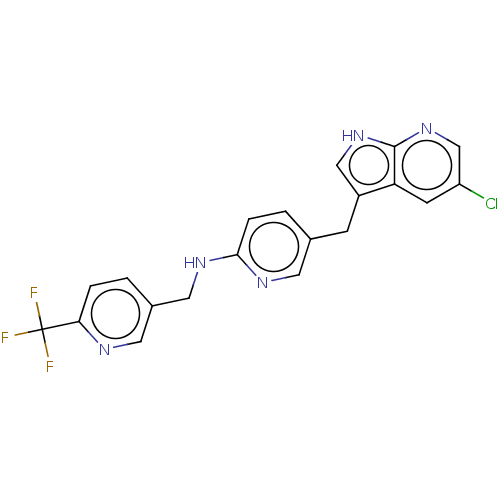
(CHEMBL3813873 | US11679110, Compound Pexidartinib ...)Show SMILES FC(F)(F)c1ccc(CNc2ccc(Cc3c[nH]c4ncc(Cl)cc34)cn2)cn1 Show InChI InChI=1S/C20H15ClF3N5/c21-15-6-16-14(10-28-19(16)29-11-15)5-12-2-4-18(26-7-12)27-9-13-1-3-17(25-8-13)20(22,23)24/h1-4,6-8,10-11H,5,9H2,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
J Med Chem 48: 680-93 (2005)
Article DOI: 10.1016/j.bmcl.2022.128928
BindingDB Entry DOI: 10.7270/Q2FN1B5Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50177716

(CHEMBL3813873 | US11679110, Compound Pexidartinib ...)Show SMILES FC(F)(F)c1ccc(CNc2ccc(Cc3c[nH]c4ncc(Cl)cc34)cn2)cn1 Show InChI InChI=1S/C20H15ClF3N5/c21-15-6-16-14(10-28-19(16)29-11-15)5-12-2-4-18(26-7-12)27-9-13-1-3-17(25-8-13)20(22,23)24/h1-4,6-8,10-11H,5,9H2,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128929
BindingDB Entry DOI: 10.7270/Q29W0KGP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305068
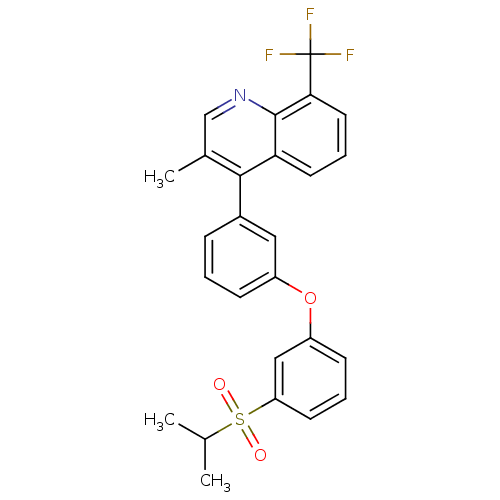
(4-(3-(3-(isopropylsulfonyl)phenoxy)phenyl)-3-methy...)Show SMILES CC(C)S(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(C)cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C26H22F3NO3S/c1-16(2)34(31,32)21-10-5-9-20(14-21)33-19-8-4-7-18(13-19)24-17(3)15-30-25-22(24)11-6-12-23(25)26(27,28)29/h4-16H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50096332

(CHEMBL3577115)Show SMILES CCn1c2cc(NC)ncc2cc(-c2ccc(F)c(NC(=O)Nc3cc(no3)C(C)(C)C)c2)c1=O Show InChI InChI=1S/C22H15ClFN3O2/c23-18-6-1-2-7-20(18)27-21(11-9-15-4-3-5-16(13-28)25-15)26-19-10-8-14(24)12-17(19)22(27)29/h1-12,28H,13H2/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) |
J Med Chem 58: 4165-79 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00067
BindingDB Entry DOI: 10.7270/Q2ZS2Z85 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM35107
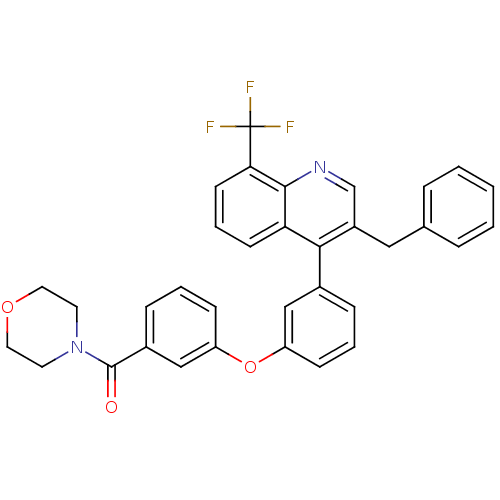
(biarylether amide quinoline, 4g)Show SMILES FC(F)(F)c1cccc2c(c(Cc3ccccc3)cnc12)-c1cccc(Oc2cccc(c2)C(=O)N2CCOCC2)c1 Show InChI InChI=1S/C34H27F3N2O3/c35-34(36,37)30-14-6-13-29-31(26(22-38-32(29)30)19-23-7-2-1-3-8-23)24-9-4-11-27(20-24)42-28-12-5-10-25(21-28)33(40)39-15-17-41-18-16-39/h1-14,20-22H,15-19H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | 138 | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
Bioorg Med Chem 17: 1663-70 (2009)
Article DOI: 10.1016/j.bmc.2008.12.048
BindingDB Entry DOI: 10.7270/Q2QR4VGV |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20001
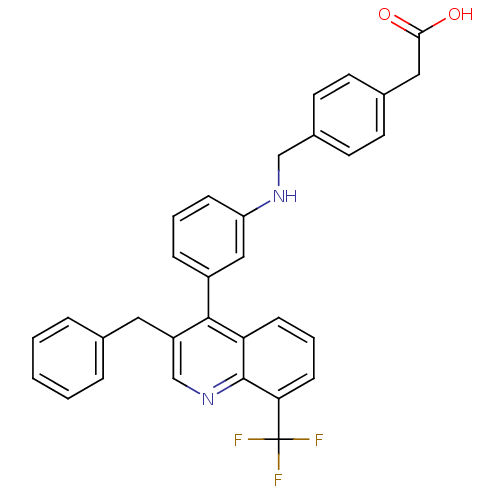
(2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...)Show SMILES OC(=O)Cc1ccc(CNc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H25F3N2O2/c33-32(34,35)28-11-5-10-27-30(25(20-37-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)36-19-23-14-12-22(13-15-23)17-29(38)39/h1-15,18,20,36H,16-17,19H2,(H,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | 33 | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
Bioorg Med Chem 15: 3321-33 (2007)
Article DOI: 10.1016/j.bmc.2007.03.013
BindingDB Entry DOI: 10.7270/Q2VH5M37 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305060
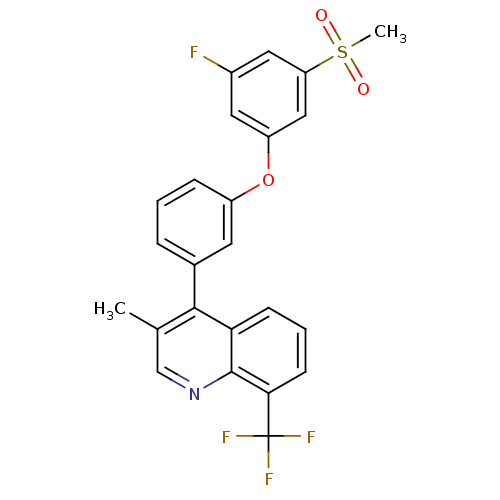
(4-(3-(3-fluoro-5-(methylsulfonyl)phenoxy)phenyl)-3...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cc(F)cc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H17F4NO3S/c1-14-13-29-23-20(7-4-8-21(23)24(26,27)28)22(14)15-5-3-6-17(9-15)32-18-10-16(25)11-19(12-18)33(2,30)31/h3-13H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50152629

(7-Bromo-2-(4-hydroxy-phenyl)-benzofuran-5-ol | CHE...)Show InChI InChI=1S/C14H9BrO3/c15-12-7-11(17)5-9-6-13(18-14(9)12)8-1-3-10(16)4-2-8/h1-7,16-17H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Estrogen receptor beta |
Bioorg Med Chem Lett 14: 4925-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.029
BindingDB Entry DOI: 10.7270/Q2TM79K8 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50305075

(3-benzyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C30H22F3NO3S/c1-38(35,36)25-13-6-12-24(18-25)37-23-11-5-10-21(17-23)28-22(16-20-8-3-2-4-9-20)19-34-29-26(28)14-7-15-27(29)30(31,32)33/h2-15,17-19H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRbeta-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor [538-972]
(Homo sapiens (Human)) | BDBM181020
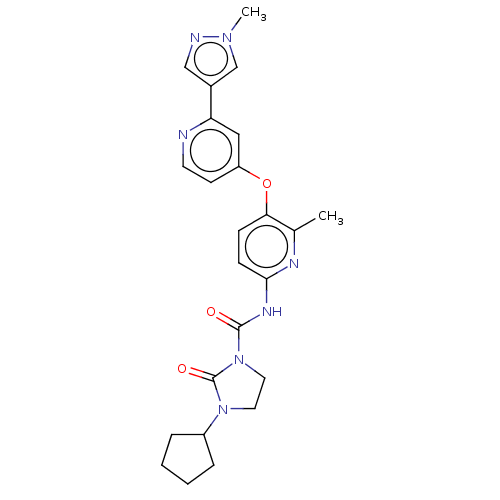
(US9133183, Example 29)Show SMILES Cc1nc(NC(=O)N2CCN(C3CCCC3)C2=O)ccc1Oc1ccnc(c1)-c1cnn(C)c1 Show InChI InChI=1S/C24H27N7O3/c1-16-21(34-19-9-10-25-20(13-19)17-14-26-29(2)15-17)7-8-22(27-16)28-23(32)31-12-11-30(24(31)33)18-5-3-4-6-18/h7-10,13-15,18H,3-6,11-12H2,1-2H3,(H,27,28,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Deciphera Pharmaceuticals, LLC
US Patent
| Assay Description
Activity of unphosphorylated c-FMS kinase (uFMS, Seq. ID no. 1) was determined by following the production of ADP from the FMS kinase reaction with A... |
US Patent US9133183 (2015)
BindingDB Entry DOI: 10.7270/Q2DN43TD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50096323

(CHEMBL3577123)Show SMILES CNc1ncc2cc(cnc2n1)-c1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C Show InChI InChI=1S/C22H17N3O2/c1-27-20-12-5-4-11-19(20)25-21(14-13-16-8-6-7-15-23-16)24-18-10-3-2-9-17(18)22(25)26/h2-15H,1H3/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of recombinant BRAF V600E mutant (unknown origin) assessed as ADP formation measured for 5 hrs by pyruvate kinase/lactate dehydrogenase co... |
J Med Chem 58: 4165-79 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00067
BindingDB Entry DOI: 10.7270/Q2ZS2Z85 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50152617

(CHEMBL360385 | [4-Bromo-5-hydroxy-2-(4-hydroxy-phe...)Show InChI InChI=1S/C16H10BrNO3/c17-15-12-8-14(9-1-3-11(19)4-2-9)21-16(12)10(5-6-18)7-13(15)20/h1-4,7-8,19-20H,5H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Estrogen receptor beta |
Bioorg Med Chem Lett 14: 4925-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.029
BindingDB Entry DOI: 10.7270/Q2TM79K8 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50222709

(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-N-(2-h...)Show InChI InChI=1S/C15H12F3IN2O3/c16-10-3-2-9(15(23)21-24-6-5-22)14(13(10)18)20-12-4-1-8(19)7-11(12)17/h1-4,7,20,22H,5-6H2,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of MEK in mouse colon 26 carcinoma cells assessed as inhibition of ERK phosphorylation by ELISA |
J Med Chem 50: 5090-102 (2007)
Article DOI: 10.1021/jm0704548
BindingDB Entry DOI: 10.7270/Q2474DMT |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50589670
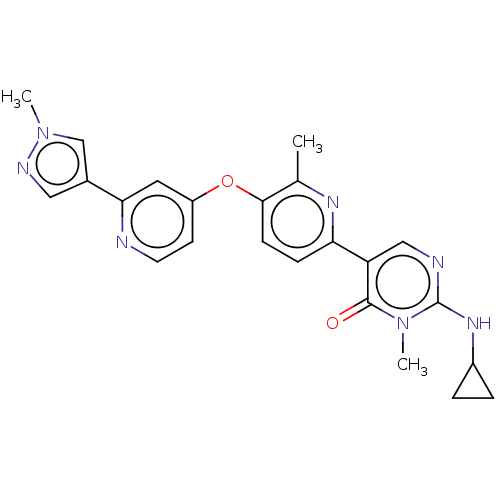
(CHEMBL5183210)Show SMILES Cc1nc(ccc1Oc1ccnc(c1)-c1cnn(C)c1)-c1cnc(NC2CC2)n(C)c1=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
J Med Chem 48: 680-93 (2005)
Article DOI: 10.1016/j.bmcl.2022.128928
BindingDB Entry DOI: 10.7270/Q2FN1B5Z |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20000

(2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES OC(=O)Cc1ccc(COc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H24F3NO3/c33-32(34,35)28-11-5-10-27-30(25(19-36-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)39-20-23-14-12-22(13-15-23)17-29(37)38/h1-15,18-19H,16-17,20H2,(H,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | 93 | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
Bioorg Med Chem 17: 1663-70 (2009)
Article DOI: 10.1016/j.bmc.2008.12.048
BindingDB Entry DOI: 10.7270/Q2QR4VGV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM35094
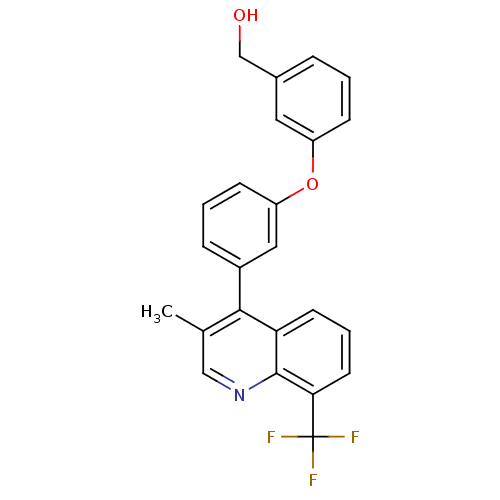
(biarylether alcohol quinoline, 5f)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(CO)c2)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO2/c1-15-13-28-23-20(9-4-10-21(23)24(25,26)27)22(15)17-6-3-8-19(12-17)30-18-7-2-5-16(11-18)14-29/h2-13,29H,14H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | 233 | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... |
Bioorg Med Chem 17: 8086-92 (2009)
Article DOI: 10.1016/j.bmc.2009.10.001
BindingDB Entry DOI: 10.7270/Q2VH5M54 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20000

(2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES OC(=O)Cc1ccc(COc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H24F3NO3/c33-32(34,35)28-11-5-10-27-30(25(19-36-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)39-20-23-14-12-22(13-15-23)17-29(37)38/h1-15,18-19H,16-17,20H2,(H,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | 71 | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... |
Bioorg Med Chem 17: 8086-92 (2009)
Article DOI: 10.1016/j.bmc.2009.10.001
BindingDB Entry DOI: 10.7270/Q2VH5M54 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50152624
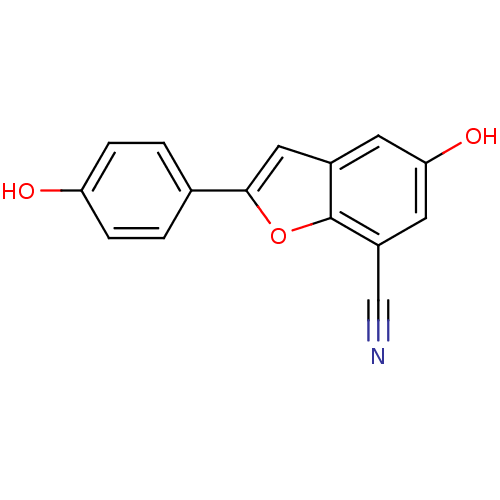
(5-HYDROXY-2-(4-HYDROXYPHENYL)-1-BENZOFURAN-7-CARBO...)Show InChI InChI=1S/C15H9NO3/c16-8-11-6-13(18)5-10-7-14(19-15(10)11)9-1-3-12(17)4-2-9/h1-7,17-18H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Estrogen receptor beta |
Bioorg Med Chem Lett 14: 4925-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.029
BindingDB Entry DOI: 10.7270/Q2TM79K8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRbeta LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306077
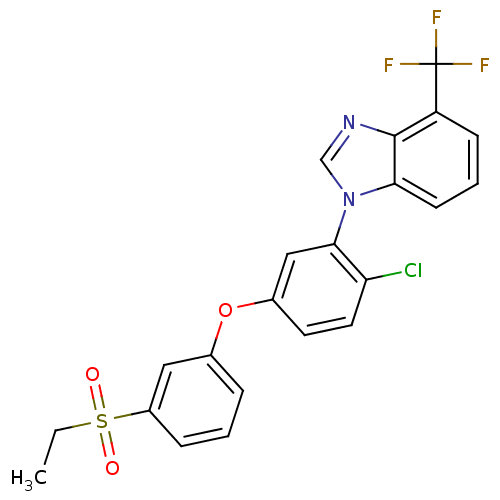
(1-(2-chloro-5-(3-(ethylsulfonyl)phenoxy)phenyl)-4-...)Show SMILES CCS(=O)(=O)c1cccc(Oc2ccc(Cl)c(c2)-n2cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C22H16ClF3N2O3S/c1-2-32(29,30)16-6-3-5-14(11-16)31-15-9-10-18(23)20(12-15)28-13-27-21-17(22(24,25)26)7-4-8-19(21)28/h3-13H,2H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305067
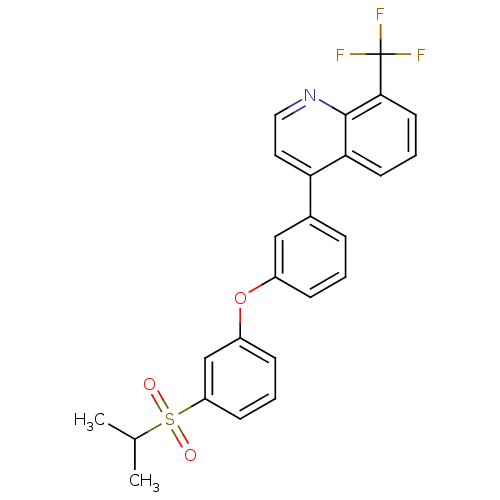
(4-(3-(3-(isopropylsulfonyl)phenoxy)phenyl)-8-(trif...)Show SMILES CC(C)S(=O)(=O)c1cccc(Oc2cccc(c2)-c2ccnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C25H20F3NO3S/c1-16(2)33(30,31)20-9-4-8-19(15-20)32-18-7-3-6-17(14-18)21-12-13-29-24-22(21)10-5-11-23(24)25(26,27)28/h3-16H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRbeta-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data