

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042365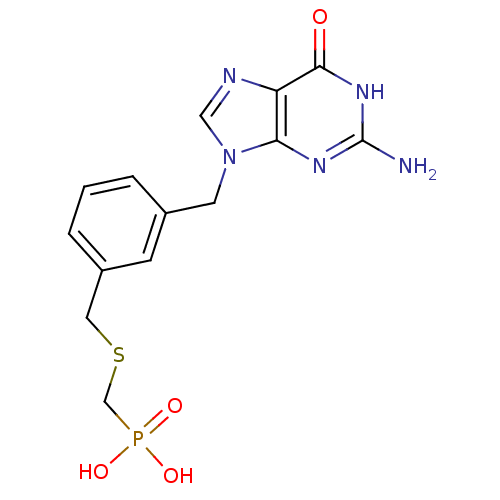 (CHEMBL117417 | [3-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033660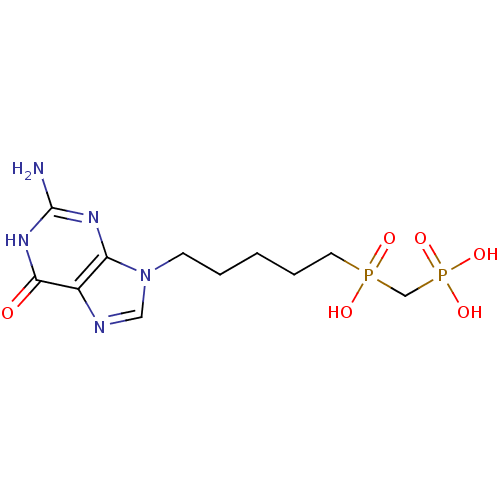 (CHEMBL269745 | {[5-(2-Amino-6-oxo-1,6-dihydro-puri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033663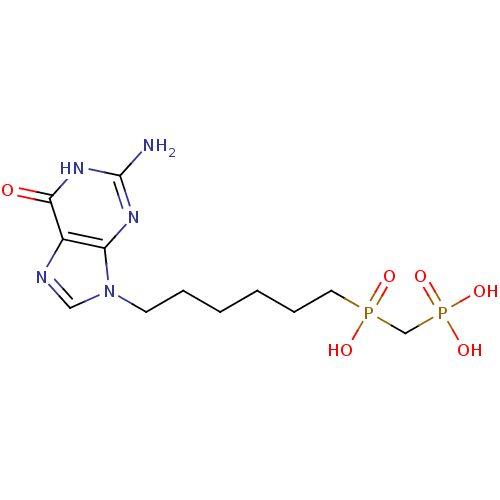 (CHEMBL7319 | {[6-(2-Amino-6-oxo-1,6-dihydro-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033666 (2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033666 (2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042370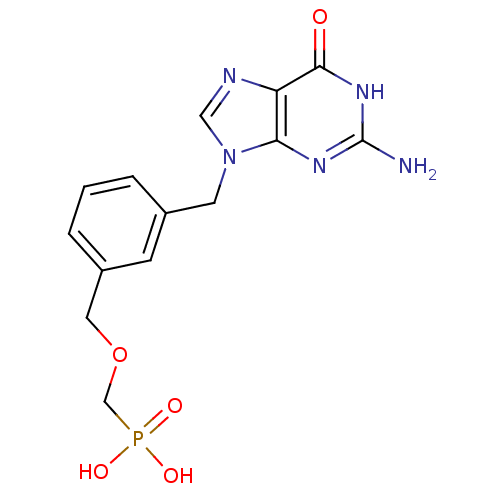 (CHEMBL326595 | [3-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042364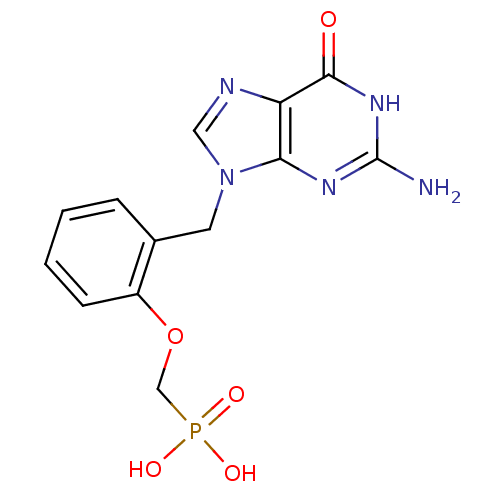 (CHEMBL327010 | [2-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033661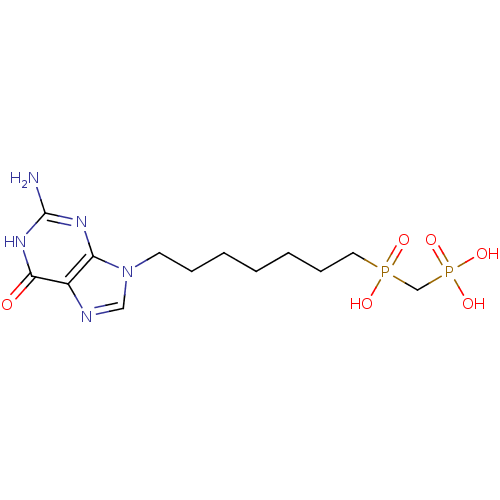 (CHEMBL7247 | {[7-(2-Amino-6-oxo-1,6-dihydro-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033666 (2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042372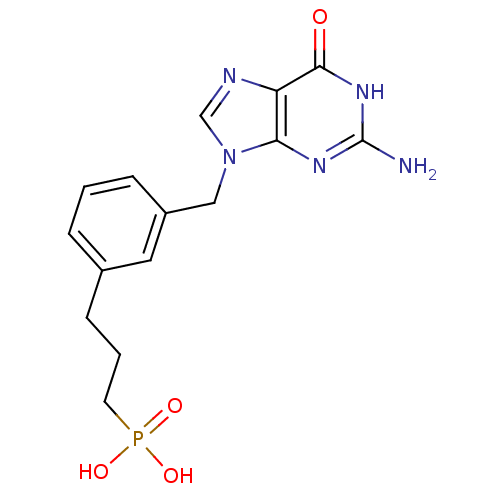 (CHEMBL117127 | {3-[3-(2-Amino-6-oxo-1,6-dihydro-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042363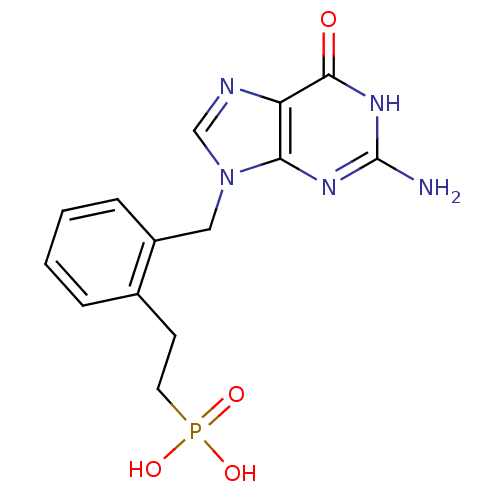 (CHEMBL333946 | {2-[2-(2-Amino-6-oxo-1,6-dihydro-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042367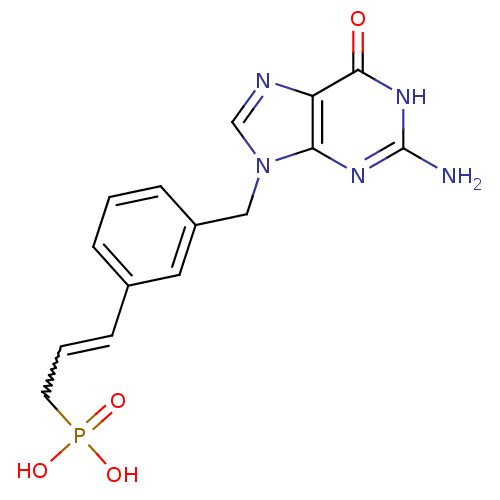 (CHEMBL325716 | {3-[3-(2-Amino-6-oxo-1,6-dihydro-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042369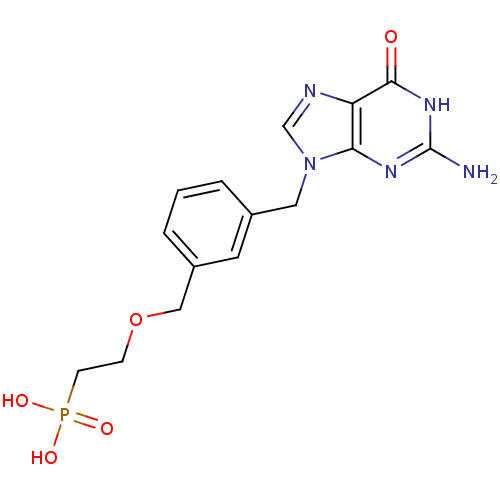 (CHEMBL119243 | {2-[3-(2-Amino-6-oxo-1,6-dihydro-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033664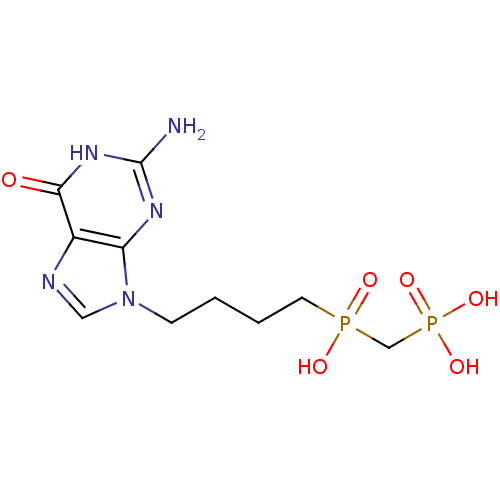 (CHEMBL7068 | {[4-(2-Amino-6-oxo-1,6-dihydro-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042362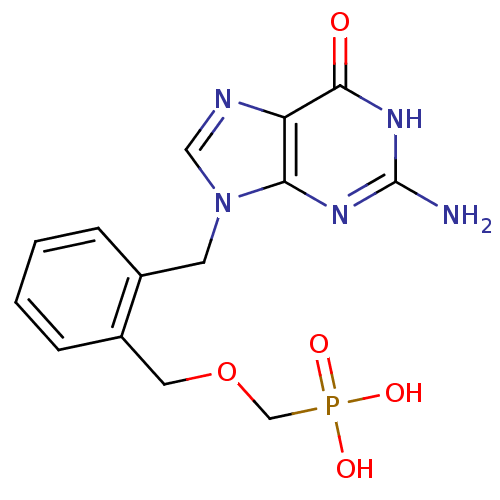 (CHEMBL331133 | [2-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033663 (CHEMBL7319 | {[6-(2-Amino-6-oxo-1,6-dihydro-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042366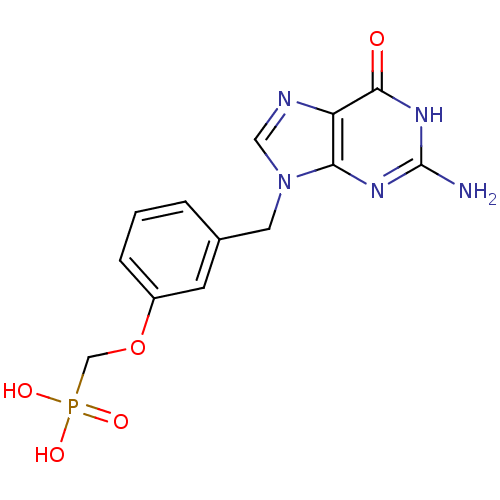 (CHEMBL116580 | [3-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033660 (CHEMBL269745 | {[5-(2-Amino-6-oxo-1,6-dihydro-puri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042361 (CHEMBL324839 | {3-[2-(2-Amino-6-oxo-1,6-dihydro-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033661 (CHEMBL7247 | {[7-(2-Amino-6-oxo-1,6-dihydro-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033665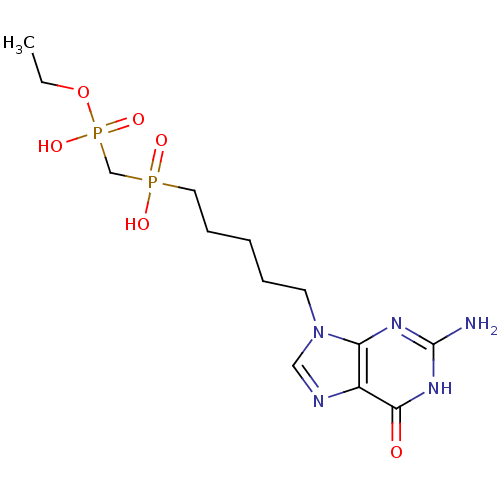 (CHEMBL268378 | {[5-(2-Amino-6-oxo-1,6-dihydro-puri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033664 (CHEMBL7068 | {[4-(2-Amino-6-oxo-1,6-dihydro-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033662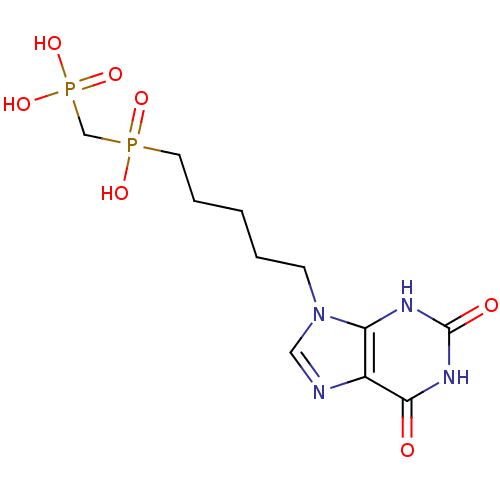 (CHEMBL7194 | {Hydroxy-[5-(2-hydroxy-6-oxo-1,6-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine decarboxylase (Rattus norvegicus) | BDBM50010139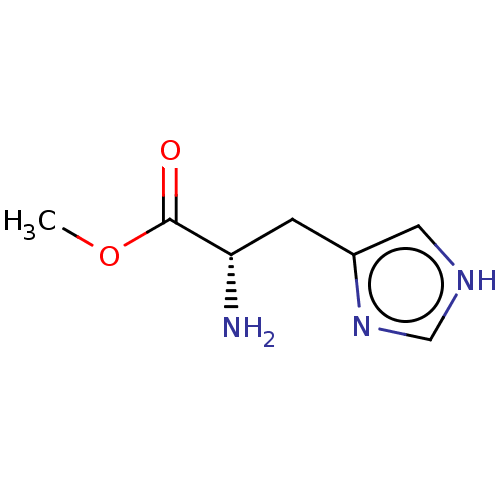 (CHEMBL54664 | L-Histidine Methyl Ester) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of histidine decarboxylase in Sprague-Dawley rat stomach assessed as decrease in 14CO2 production using L-histidine-carboxyl-14C as substr... | J Med Chem 20: 506-9 (1977) BindingDB Entry DOI: 10.7270/Q2FN17QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042360 (CHEMBL117588 | [4-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042368 (CHEMBL446671 | [4-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033665 (CHEMBL268378 | {[5-(2-Amino-6-oxo-1,6-dihydro-puri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033662 (CHEMBL7194 | {Hydroxy-[5-(2-hydroxy-6-oxo-1,6-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042371 (CHEMBL327003 | [4-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Tested in vitro for inhibition of [3H]spiperone binding to dopamine receptor D2 | J Med Chem 36: 3417-23 (1993) BindingDB Entry DOI: 10.7270/Q2S75GZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007534 (3-Bromo-N-((S)-1-ethyl-pyrrolidin-2-ylmethyl)-2-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Tested in vitro for inhibition of [3H]spiperone binding to dopamine receptor D2 | J Med Chem 36: 3417-23 (1993) BindingDB Entry DOI: 10.7270/Q2S75GZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030972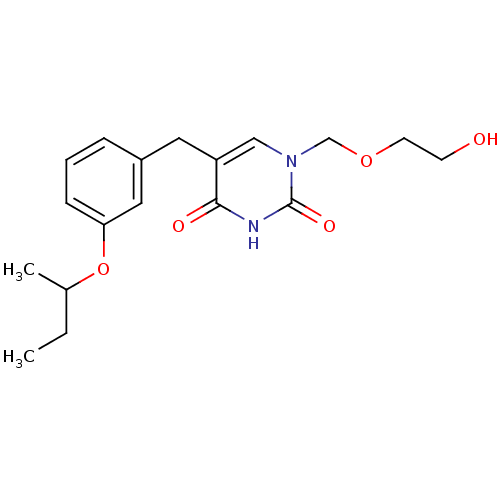 (5-(3-sec-Butoxy-benzyl)-1-(2-hydroxy-ethoxymethyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031005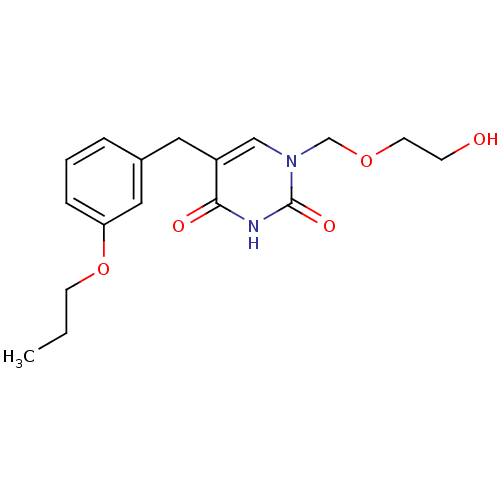 (1-(2-Hydroxy-ethoxymethyl)-5-(3-propoxy-benzyl)-1H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030974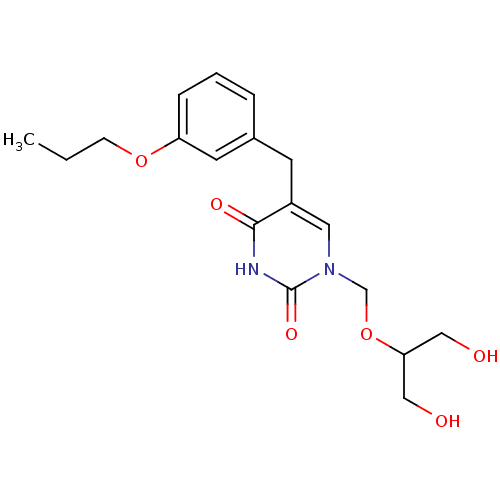 (1-(2-Hydroxy-1-hydroxymethyl-ethoxymethyl)-5-(3-pr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031009 (1-(2-Hydroxy-ethoxymethyl)-5-(3-isobutoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030990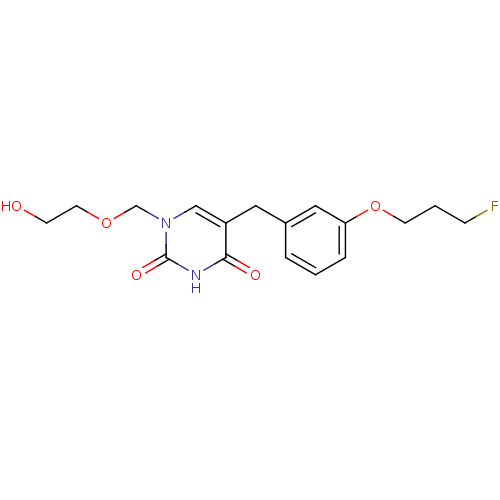 (5-[3-(3-Fluoro-propoxy)-benzyl]-1-(2-hydroxy-ethox...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030980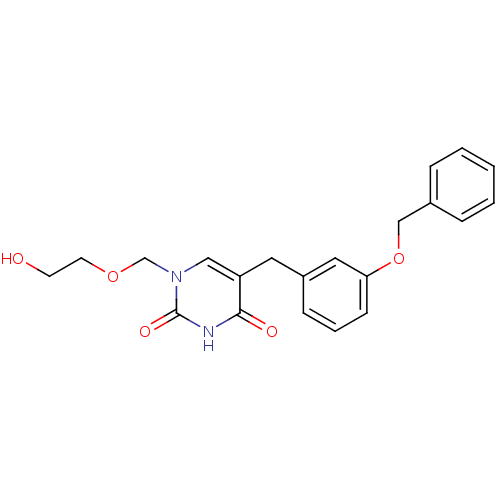 (1-((2-HYDROXYETHOXY)METHYL)-5-(3-(BENZYLOXY)BENZYL...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031006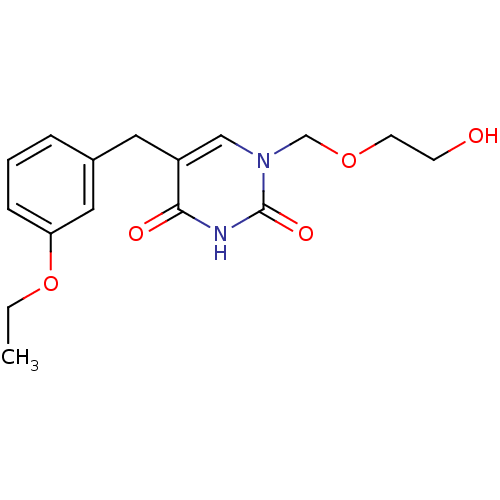 (5-(3-Ethoxy-benzyl)-1-(2-hydroxy-ethoxymethyl)-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031007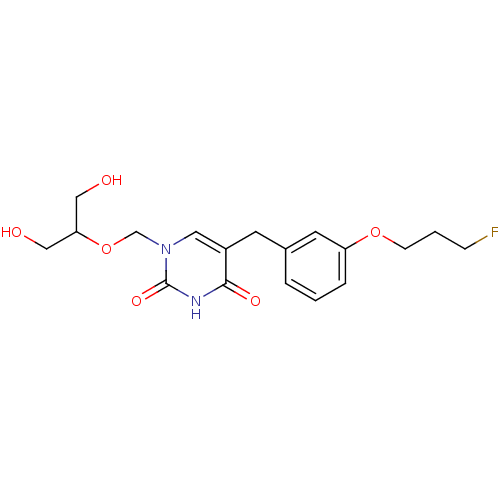 (5-[3-(3-Fluoro-propoxy)-benzyl]-1-(2-hydroxy-1-hyd...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030999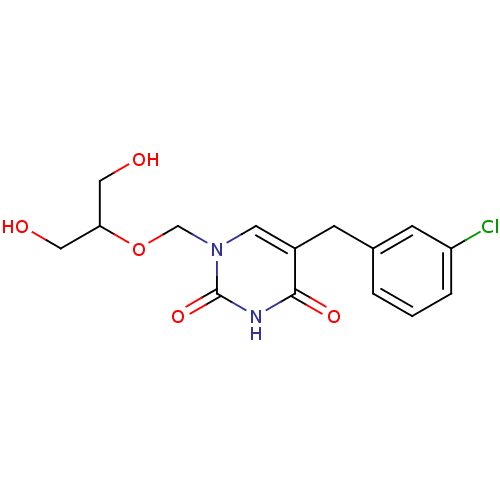 (5-(3-Chloro-benzyl)-1-(2-hydroxy-1-hydroxymethyl-e...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030983 (5-(3-Allyloxy-benzyl)-1-(2-hydroxy-ethoxymethyl)-1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50031004 (1-(2-Hydroxy-ethoxymethyl)-5-(3-isopropoxy-benzyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030995 (5-(3-Butoxy-benzyl)-1-(2-hydroxy-ethoxymethyl)-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50122955 (2-(4,6-Difluoro-indan-1-ylidene)-acetamide | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Monoamine oxidase B (MAO-B) | J Med Chem 46: 399-408 (2003) Article DOI: 10.1021/jm020067s BindingDB Entry DOI: 10.7270/Q2VH5N7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM11638 (CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Tested in vitro for inhibition of [3H]spiperone binding to dopamine receptor D2 | J Med Chem 36: 3417-23 (1993) BindingDB Entry DOI: 10.7270/Q2S75GZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030984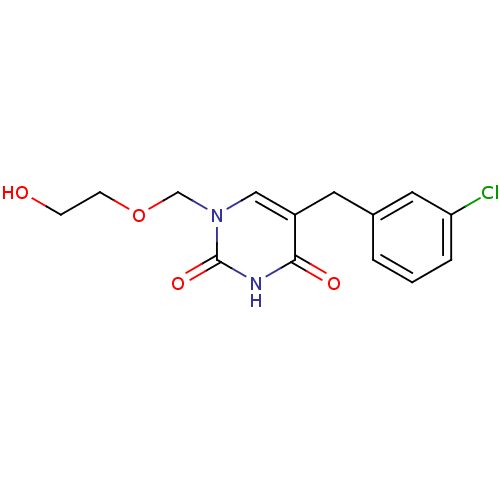 (5-(3-Chloro-benzyl)-1-(2-hydroxy-ethoxymethyl)-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030981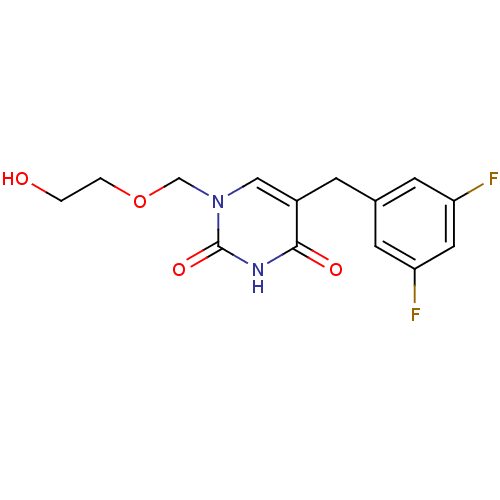 (5-(3,5-Difluoro-benzyl)-1-(2-hydroxy-ethoxymethyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50026387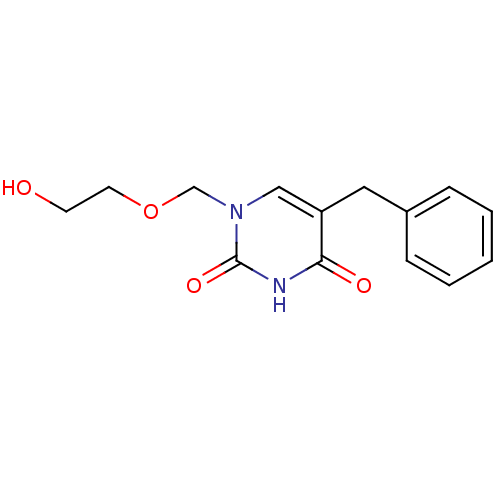 (1-((2-HYDROXYETHOXY)METHYL)-5-BENZYLPYRIMIDINE-2,4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine phosphorylase 1 (Mus musculus) | BDBM50030976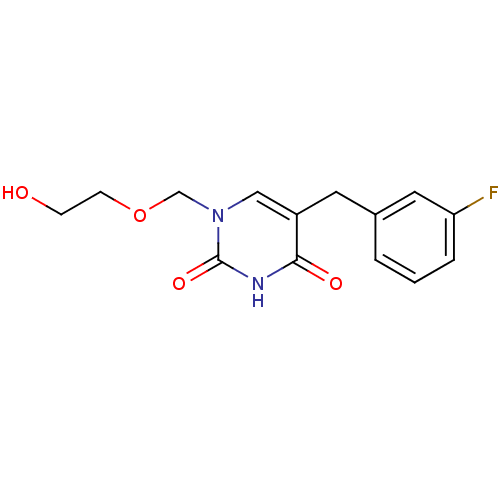 (5-(3-Fluoro-benzyl)-1-(2-hydroxy-ethoxymethyl)-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of uridine phosphorylase (UrdPase) from murine liver. | J Med Chem 38: 3850-6 (1995) BindingDB Entry DOI: 10.7270/Q2B27T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50005520 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against Glycinamide ribonucleotide transformylase(GAR-TFase) against hog liver | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 98 total ) | Next | Last >> |