

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM11023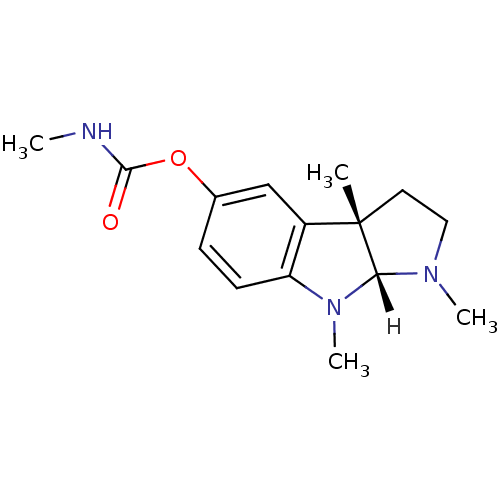 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM11023 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.7 | n/a |
GC University | Assay Description The AChE inhibition assay was performed according to the Ellman's method. Total volume of 100 �L reaction mixture contained 60 �L 50 mM Na2HPO4 b... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM11023 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | 7.7 | n/a |
GC University | Assay Description The AChE inhibition assay was performed according to the Ellman's method. Total volume of 100 �L reaction mixture contained 60 �L 50 mM Na2HPO4 b... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM11023 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The butyrylcholinesterase (BChE) inhibition activity was determined according to the Ellman's method with minor modifications. Total volume of th... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175305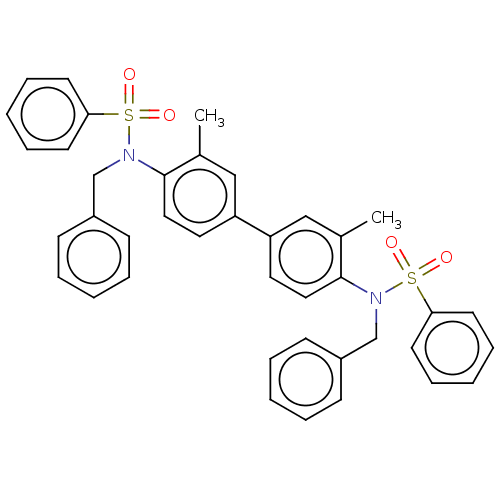 (N,N'-(3,3'-dimethylbiphenyl-4,4'-diyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175304 (N,N'-(3,3'-dimethylbiphenyl-4,4'-diyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175300 (N,N'-(3,3'-dimethylbiphenyl-4,4'-diyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175296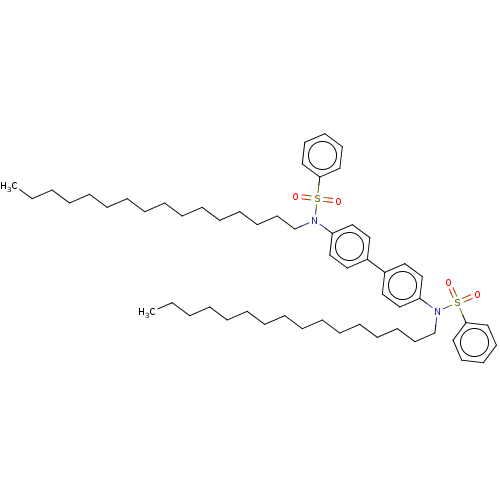 (N,N’-(biphenyl-4,4’-diyl)bis(N-hexadecyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.63E+3 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM175296 (N,N’-(biphenyl-4,4’-diyl)bis(N-hexadecyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.74E+3 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The butyrylcholinesterase (BChE) inhibition activity was determined according to the Ellman's method with minor modifications. Total volume of th... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175292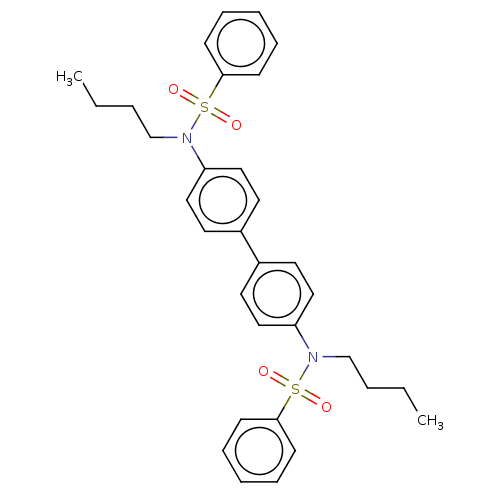 (N,N'-(biphenyl-4,4'-diyl)bis(N-butylbenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.41E+3 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM173580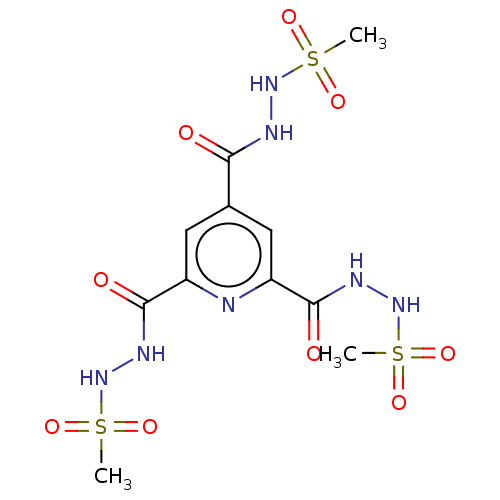 (N',N'',N'''-(pyridine-2,4,...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
GC University | Assay Description The AChE inhibition assay was performed according to the Ellman's method. Total volume of 100 �L reaction mixture contained 60 �L 50 mM Na2HPO4 b... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM175300 (N,N'-(3,3'-dimethylbiphenyl-4,4'-diyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The butyrylcholinesterase (BChE) inhibition activity was determined according to the Ellman's method with minor modifications. Total volume of th... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM175304 (N,N'-(3,3'-dimethylbiphenyl-4,4'-diyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.47E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The butyrylcholinesterase (BChE) inhibition activity was determined according to the Ellman's method with minor modifications. Total volume of th... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM173576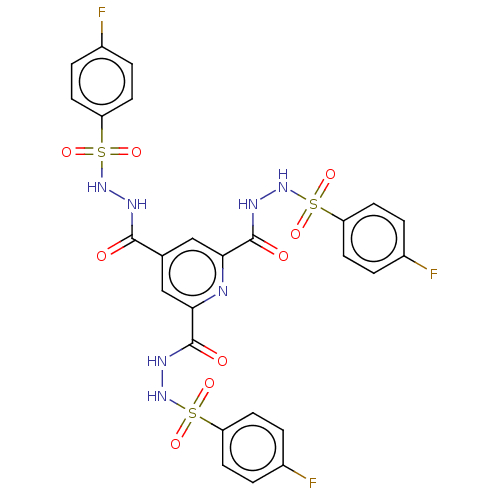 (N',N'',N'''-(pyridine-2,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.56E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GC University | Assay Description The α-glucosidase inhibition activity was performed with slight modifications according to method. Total volume of 100 �L reaction mixture conta... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM173575 (N',N'',N'''-(pyridine-2,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GC University | Assay Description The α-glucosidase inhibition activity was performed with slight modifications according to method. Total volume of 100 �L reaction mixture conta... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM173577 (N',N'',N'''-(pyridine-2,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GC University | Assay Description The α-glucosidase inhibition activity was performed with slight modifications according to method. Total volume of 100 �L reaction mixture conta... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM173581 (N',N'',N'''-(pyridine-2,4,...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.91E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
GC University | Assay Description The AChE inhibition assay was performed according to the Ellman's method. Total volume of 100 �L reaction mixture contained 60 �L 50 mM Na2HPO4 b... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM175305 (N,N'-(3,3'-dimethylbiphenyl-4,4'-diyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.13E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The butyrylcholinesterase (BChE) inhibition activity was determined according to the Ellman's method with minor modifications. Total volume of th... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM173574 (N',N'',N'''-(pyridine-2,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.22E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GC University | Assay Description The α-glucosidase inhibition activity was performed with slight modifications according to method. Total volume of 100 �L reaction mixture conta... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM175297 (N,N'-(biphenyl-4,4'-diyl)bis(N-benzylbenze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.58E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The butyrylcholinesterase (BChE) inhibition activity was determined according to the Ellman's method with minor modifications. Total volume of th... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50333465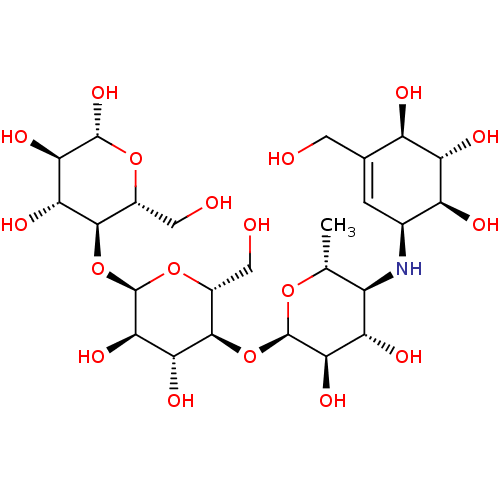 ((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | 6.8 | 37 |
COMSATS Institute of Information Technology, Lahore 54000, Pakistan | Assay Description Reaction mixture (100 µL) contained 70 µL 50 mM Na2HPO4 buffer, pH 6.8, 10 µL test compound (0.5 mM), followed by the addition of 10 &... | Bioorg Chem 54: 96-104 (2014) Article DOI: 10.1016/j.bioorg.2014.05.003 BindingDB Entry DOI: 10.7270/Q2NG4P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM23406 ((3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5-{[(2R,3R,4S,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GC University | Assay Description The α-glucosidase inhibition activity was performed with slight modifications according to method. Total volume of 100 �L reaction mixture conta... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175288 (N,N'-(biphenyl-4,4'-diyl)dibenzenesulfonam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.23E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175303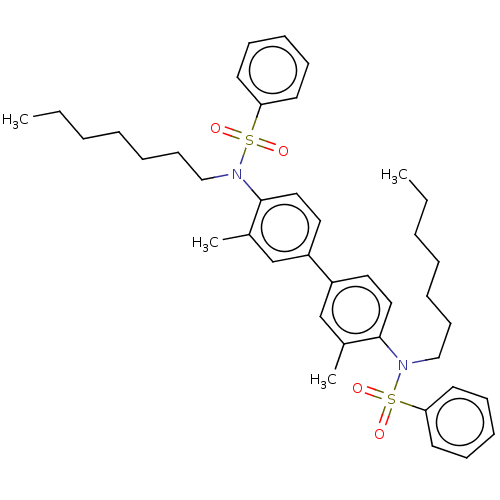 (N,N'-(3,3'-dimethylbiphenyl-4,4'-diyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.32E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM173574 (N',N'',N'''-(pyridine-2,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.38E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
GC University | Assay Description The AChE inhibition assay was performed according to the Ellman's method. Total volume of 100 �L reaction mixture contained 60 �L 50 mM Na2HPO4 b... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM173580 (N',N'',N'''-(pyridine-2,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.48E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
GC University | Assay Description The AChE inhibition assay was performed according to the Ellman's method. Total volume of 100 �L reaction mixture contained 60 �L 50 mM Na2HPO4 b... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM175303 (N,N'-(3,3'-dimethylbiphenyl-4,4'-diyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.87E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The butyrylcholinesterase (BChE) inhibition activity was determined according to the Ellman's method with minor modifications. Total volume of th... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175302 (N,N'-(3,3'-dimethylbiphenyl-4,4'-diyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.96E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM173574 (N',N'',N'''-(pyridine-2,4,...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.02E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
GC University | Assay Description The AChE inhibition assay was performed according to the Ellman's method. Total volume of 100 �L reaction mixture contained 60 �L 50 mM Na2HPO4 b... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175301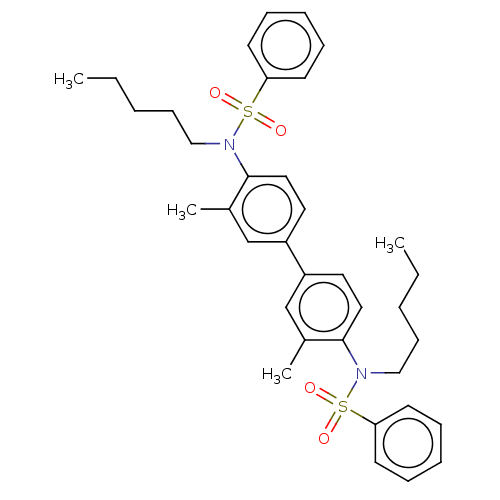 (N,N'-(3,3'-dimethylbiphenyl-4,4'-diyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.12E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175295 (N,N'-(biphenyl-4,4'-diyl)bis(N-heptylbenze...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.86E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175297 (N,N'-(biphenyl-4,4'-diyl)bis(N-benzylbenze...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.16E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175291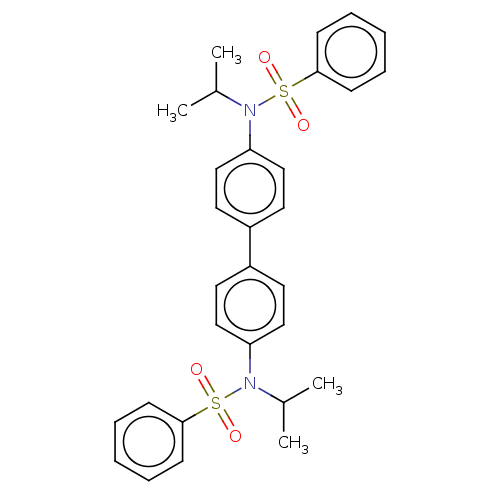 (N,N'-(biphenyl-4,4'-diyl)bis(N-isopropylbe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.32E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175294 (N,N'-(biphenyl-4,4'-diyl)bis(N-hexylbenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.86E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175293 (N,N'-(biphenyl-4,4'-diyl)bis(N-pentylbenze...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.96E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175298 (N,N'-(3,3'-dimethylbiphenyl-4,4'-diyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.63E+4 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175299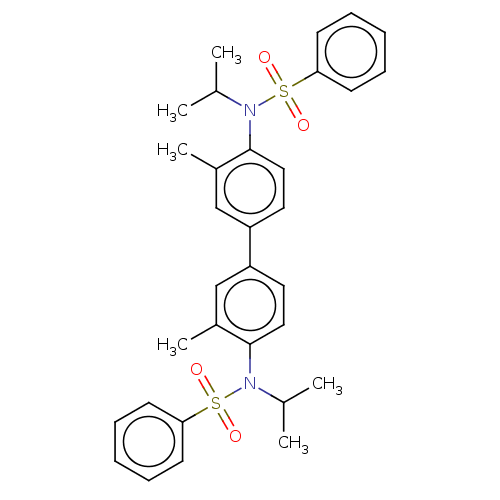 (N,N'-(3,3'-dimethylbiphenyl-4,4'-diyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175290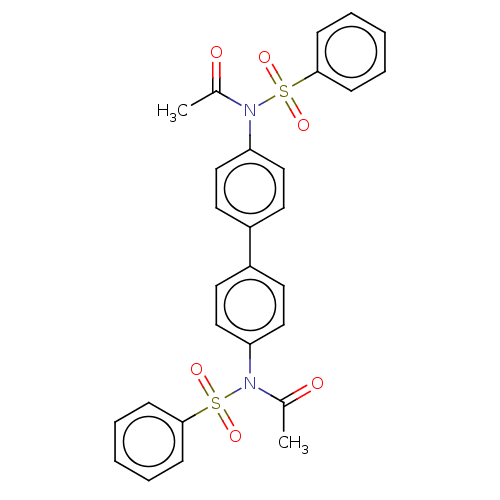 (N,N'-(biphenyl-4,4'-diyl)bis(N-(phenylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM173581 (N',N'',N'''-(pyridine-2,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+5 | n/a | n/a | n/a | n/a | 7.7 | n/a |
GC University | Assay Description The AChE inhibition assay was performed according to the Ellman's method. Total volume of 100 �L reaction mixture contained 60 �L 50 mM Na2HPO4 b... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM112646 (Ethyl 6-methyl-4-(4-methylphenyl)-2-oxo-1,2,3,4- t...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+5 | n/a | n/a | n/a | n/a | 6.8 | 37 |
COMSATS Institute of Information Technology, Lahore 54000, Pakistan | Assay Description Reaction mixture (100 µL) contained 70 µL 50 mM Na2HPO4 buffer, pH 6.8, 10 µL test compound (0.5 mM), followed by the addition of 10 &... | Bioorg Chem 54: 96-104 (2014) Article DOI: 10.1016/j.bioorg.2014.05.003 BindingDB Entry DOI: 10.7270/Q2NG4P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM112652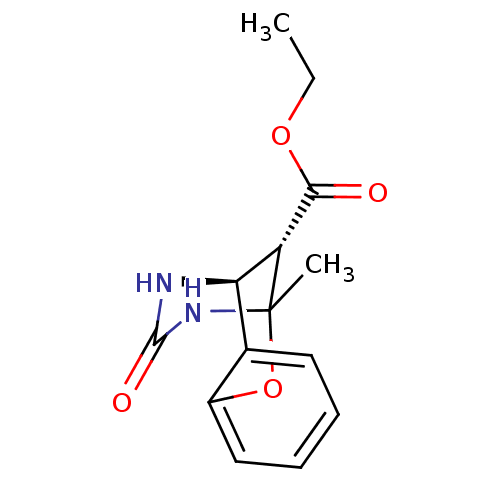 ((�)2,6-Methano-4-oxo-3,4,5,6-tetrahydro-2H- [1,3,5...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+5 | n/a | n/a | n/a | n/a | 6.8 | 37 |
COMSATS Institute of Information Technology, Lahore 54000, Pakistan | Assay Description Reaction mixture (100 µL) contained 70 µL 50 mM Na2HPO4 buffer, pH 6.8, 10 µL test compound (0.5 mM), followed by the addition of 10 &... | Bioorg Chem 54: 96-104 (2014) Article DOI: 10.1016/j.bioorg.2014.05.003 BindingDB Entry DOI: 10.7270/Q2NG4P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM175289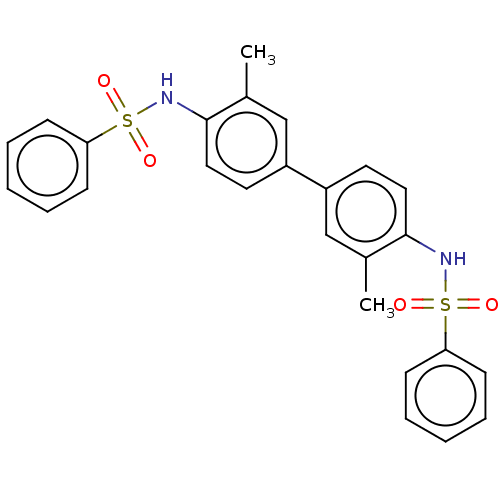 (N,N'-(3,3'-dimethylbiphenyl-4,4'-diyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+5 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The AChE inhibition activity was determined according to the Ellman's method with slight modifications. Total volume of the reaction mixture was ... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM175292 (N,N'-(biphenyl-4,4'-diyl)bis(N-butylbenzen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The butyrylcholinesterase (BChE) inhibition activity was determined according to the Ellman's method with minor modifications. Total volume of th... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM112651 (Ethyl 6-methyl-4-(4-methylphenyl)-2-thioxo-1,2,3,4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | 6.8 | 37 |
COMSATS Institute of Information Technology, Lahore 54000, Pakistan | Assay Description Reaction mixture (100 µL) contained 70 µL 50 mM Na2HPO4 buffer, pH 6.8, 10 µL test compound (0.5 mM), followed by the addition of 10 &... | Bioorg Chem 54: 96-104 (2014) Article DOI: 10.1016/j.bioorg.2014.05.003 BindingDB Entry DOI: 10.7270/Q2NG4P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM173578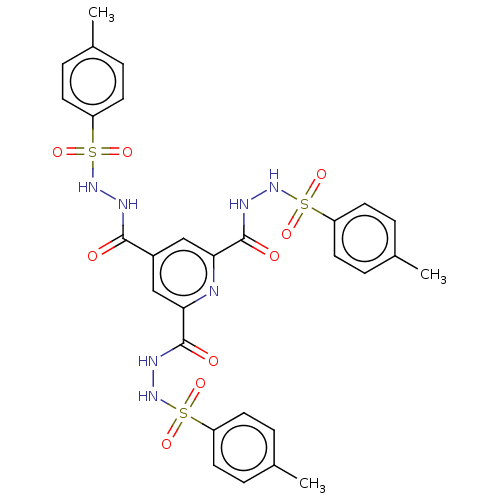 (N',N'',N'''-(pyridine-2,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | 6.8 | n/a |
GC University | Assay Description The α-glucosidase inhibition activity was performed with slight modifications according to method. Total volume of 100 �L reaction mixture conta... | Bioorg Chem 63: 64-71 (2015) Article DOI: 10.1016/j.bioorg.2015.09.008 BindingDB Entry DOI: 10.7270/Q2RJ4H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM112642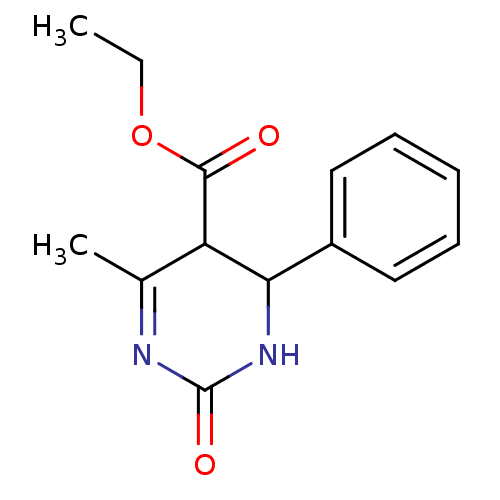 (Ethyl 6-methyl-2-oxo-4-phenyl-1,2,3,4-tetrahydropy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.43E+5 | n/a | n/a | n/a | n/a | 6.8 | 37 |
COMSATS Institute of Information Technology, Lahore 54000, Pakistan | Assay Description Reaction mixture (100 µL) contained 70 µL 50 mM Na2HPO4 buffer, pH 6.8, 10 µL test compound (0.5 mM), followed by the addition of 10 &... | Bioorg Chem 54: 96-104 (2014) Article DOI: 10.1016/j.bioorg.2014.05.003 BindingDB Entry DOI: 10.7270/Q2NG4P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM175290 (N,N'-(biphenyl-4,4'-diyl)bis(N-(phenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+5 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The butyrylcholinesterase (BChE) inhibition activity was determined according to the Ellman's method with minor modifications. Total volume of th... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM175288 (N,N'-(biphenyl-4,4'-diyl)dibenzenesulfonam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.03E+5 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Government College University | Assay Description The butyrylcholinesterase (BChE) inhibition activity was determined according to the Ellman's method with minor modifications. Total volume of th... | Bioorg Chem 64: 13-20 (2016) Article DOI: 10.1016/j.bioorg.2015.11.002 BindingDB Entry DOI: 10.7270/Q20V8BJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM112643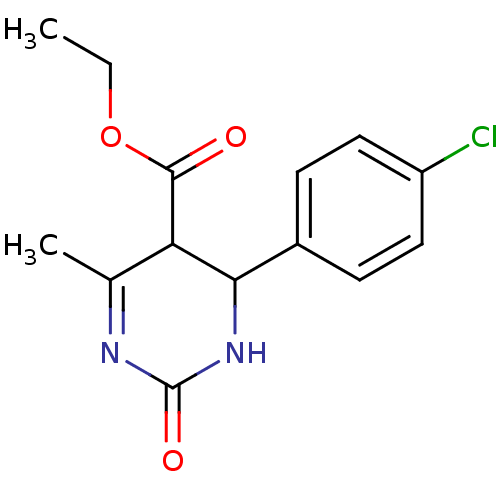 (Ethyl 4-(4-chlorophenyl)-6-methyl-2-oxo-1,2,3,4- t...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.13E+5 | n/a | n/a | n/a | n/a | 6.8 | 37 |
COMSATS Institute of Information Technology, Lahore 54000, Pakistan | Assay Description Reaction mixture (100 µL) contained 70 µL 50 mM Na2HPO4 buffer, pH 6.8, 10 µL test compound (0.5 mM), followed by the addition of 10 &... | Bioorg Chem 54: 96-104 (2014) Article DOI: 10.1016/j.bioorg.2014.05.003 BindingDB Entry DOI: 10.7270/Q2NG4P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM112645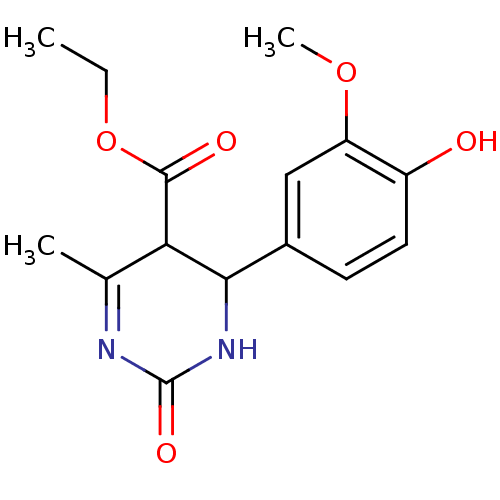 (Ethyl 4-(4-hydroxy-3-methoxyphenyl)-6-methyl-2-oxo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.05E+5 | n/a | n/a | n/a | n/a | 6.8 | 37 |
COMSATS Institute of Information Technology, Lahore 54000, Pakistan | Assay Description Reaction mixture (100 µL) contained 70 µL 50 mM Na2HPO4 buffer, pH 6.8, 10 µL test compound (0.5 mM), followed by the addition of 10 &... | Bioorg Chem 54: 96-104 (2014) Article DOI: 10.1016/j.bioorg.2014.05.003 BindingDB Entry DOI: 10.7270/Q2NG4P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 57 total ) | Next | Last >> |