

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442489 (CHEMBL2440417) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of wild-type human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli using geraniol as substrate by dou... | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321717 (7-hydroxy-2-(4-methoxyphenylimino)-N-(pyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of dehydrogenase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of geraniol deh... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321718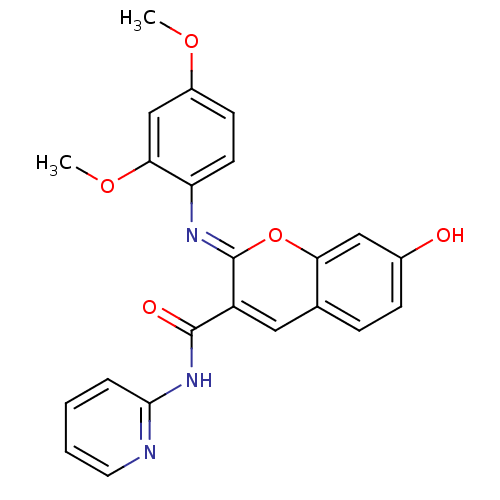 (2-(2,4-dimethoxyphenylimino)-7-hydroxy-N-(pyridin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of dehydrogenase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of geraniol deh... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321715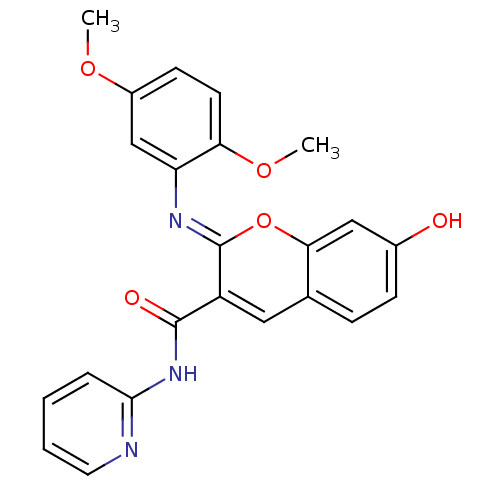 (2-(2,5-dimethoxyphenylimino)-7-hydroxy-N-(pyridin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of dehydrogenase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of geraniol deh... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321716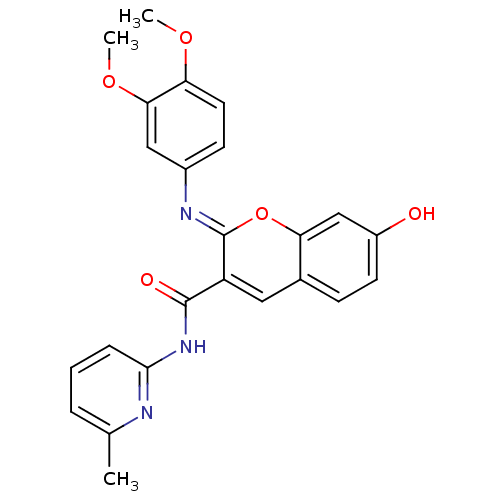 (2-(3,4-dimethoxyphenylimino)-7-hydroxy-N-(6-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of dehydrogenase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of geraniol deh... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50135288 ((1S,2R,3S,4R)-4-(6-Amino-purin-9-yl)-cyclopentane-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50135288 ((1S,2R,3S,4R)-4-(6-Amino-purin-9-yl)-cyclopentane-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against P. falciparum S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50135289 ((1S,2R,3S,4R)-4-(6-Amino-2-fluoro-purin-9-yl)-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50135289 ((1S,2R,3S,4R)-4-(6-Amino-2-fluoro-purin-9-yl)-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50081472 ((2S,3S)-4-(6-Amino-purin-9-yl)-2,3-dihydroxy-butyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of human SAHH | Bioorg Med Chem 16: 6575-9 (2008) Article DOI: 10.1016/j.bmc.2008.05.020 BindingDB Entry DOI: 10.7270/Q2SN08SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50081472 ((2S,3S)-4-(6-Amino-purin-9-yl)-2,3-dihydroxy-butyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Affinity and reactivity of an affinity-labeling reagent, was assessed from the inhibitory constant value | Bioorg Med Chem Lett 9: 2737-40 (1999) BindingDB Entry DOI: 10.7270/Q28C9VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50244094 (9-[(10R,20S,30S,40R)-30,40-Epoxy-20-hydroxy-cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of human SAHH | Bioorg Med Chem 16: 6575-9 (2008) Article DOI: 10.1016/j.bmc.2008.05.020 BindingDB Entry DOI: 10.7270/Q2SN08SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50244095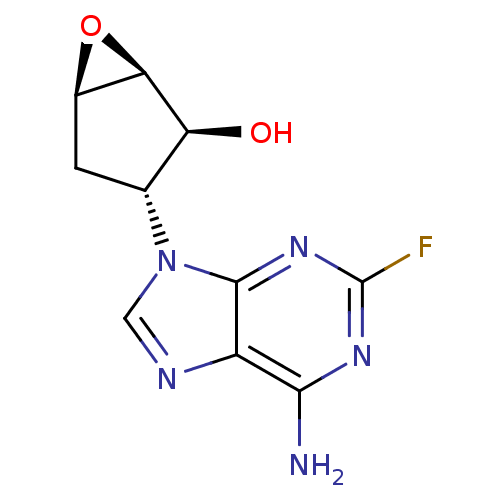 (9-[(10R,20S,30R,50R)-30,40-Epoxy-20-hydroxy-cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of human SAHH | Bioorg Med Chem 16: 6575-9 (2008) Article DOI: 10.1016/j.bmc.2008.05.020 BindingDB Entry DOI: 10.7270/Q2SN08SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085553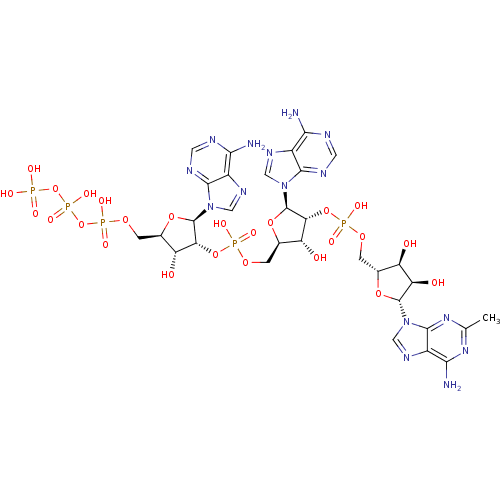 (CHEMBL214603 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Compound was tested for its binding ability, by displacement of p(A2'p)3A3'[32p]p5'Cp from recombinant human ribonuclease L | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085558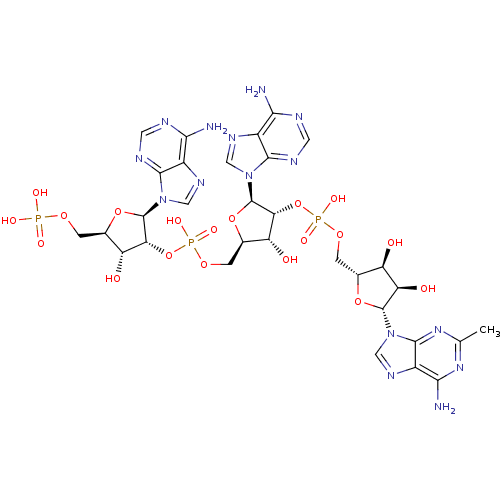 (CHEMBL402676 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of poly (U) 3'[32P]p5'C3'p | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442489 (CHEMBL2440417) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321717 (7-hydroxy-2-(4-methoxyphenylimino)-N-(pyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of reductase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of NADPH linked pyr... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321717 (7-hydroxy-2-(4-methoxyphenylimino)-N-(pyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50442506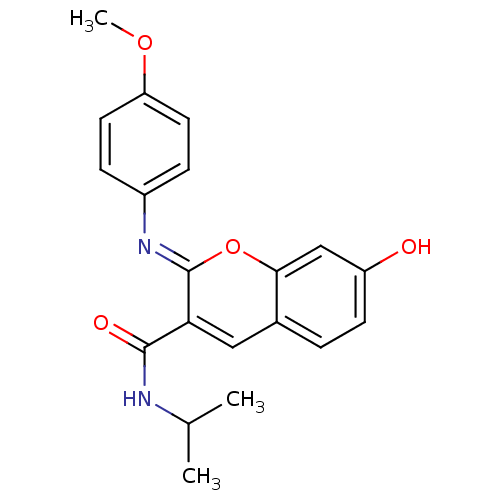 (CHEMBL2440411) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B1 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM50038843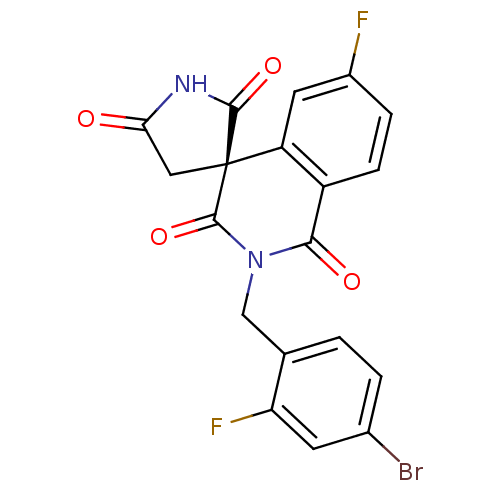 ((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human aldehyde reductase expressed in Escherichia coli BL21(DE3) mediated D-glucuronate reduction | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442501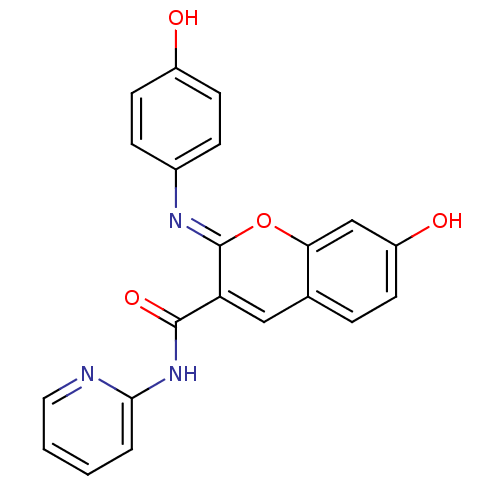 (CHEMBL2440422) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085554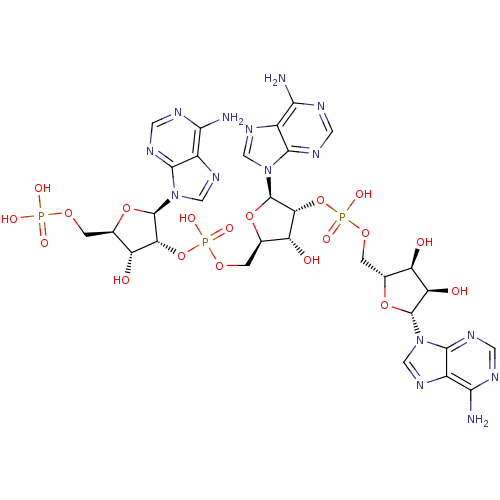 (5'-O-MONOPHOSPHORYLADENYLYL(2'->5')ADENYLYL(2'->5'...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of [32P]-pC11U2C7 | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442504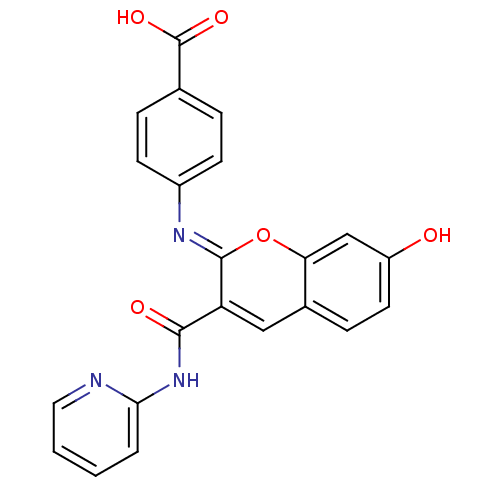 (CHEMBL2440423) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442499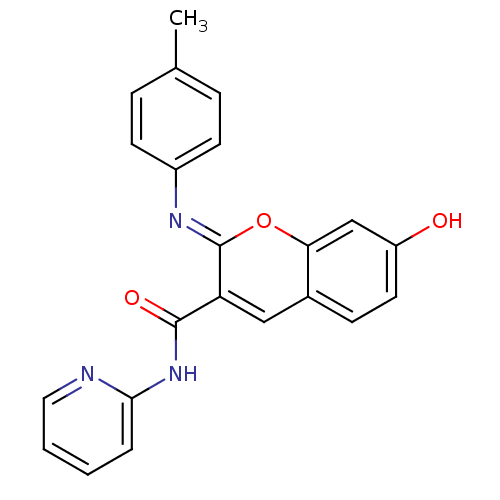 (CHEMBL2440424) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442495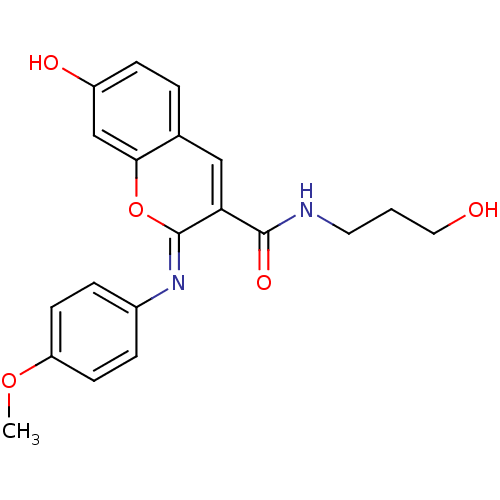 (CHEMBL2440416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442506 (CHEMBL2440411) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16314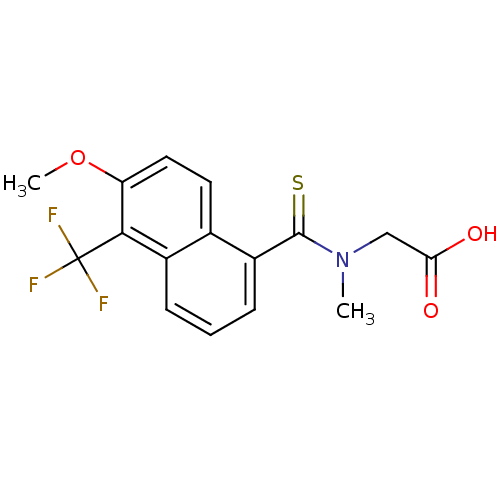 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human aldose reductase expressed in Escherichia coli BL21(DE3) mediated NADPH linked pyridine-3-aldehyde reducti... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442498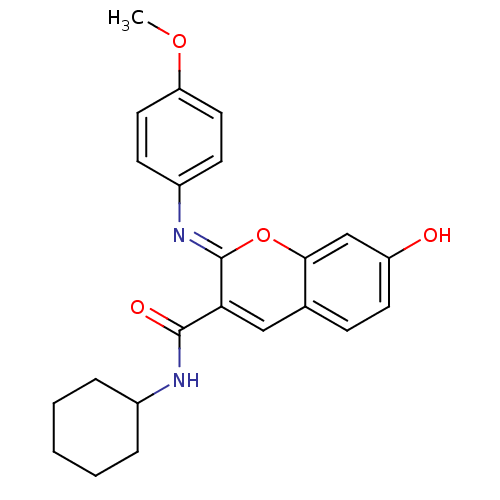 (CHEMBL2440414) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442502 (CHEMBL2440415) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50321717 (7-hydroxy-2-(4-methoxyphenylimino)-N-(pyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B1 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50321717 (7-hydroxy-2-(4-methoxyphenylimino)-N-(pyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human aldose reductase expressed in Escherichia coli BL21(DE3) mediated NADPH linked pyridine-3-aldehyde reducti... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442494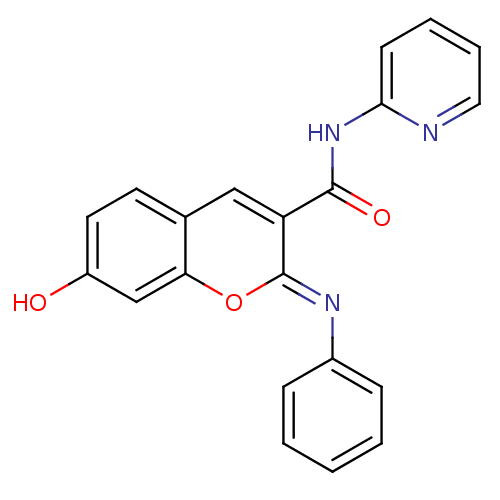 (CHEMBL2440428) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50442505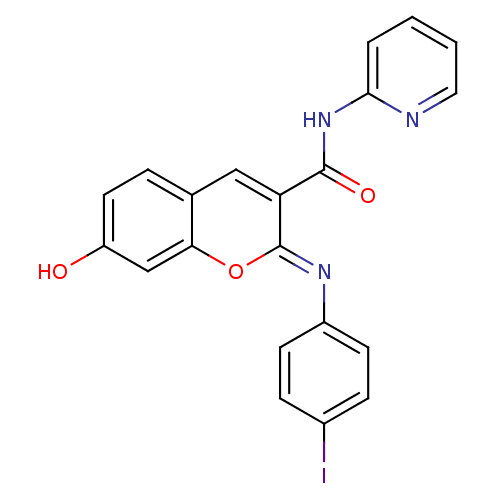 (CHEMBL2440426) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B1 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442503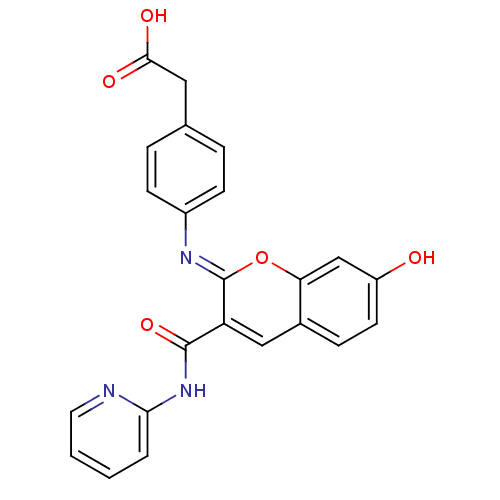 (CHEMBL2440427) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442505 (CHEMBL2440426) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442500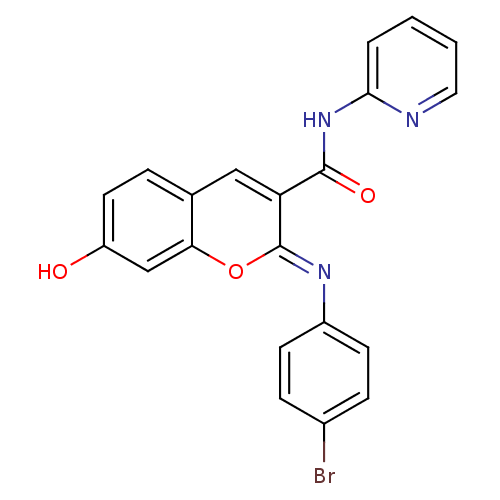 (CHEMBL2440425) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321718 (2-(2,4-dimethoxyphenylimino)-7-hydroxy-N-(pyridin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of reductase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of NADPH linked pyr... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442496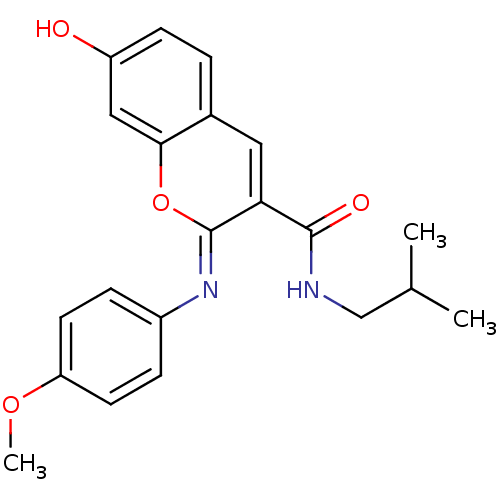 (CHEMBL2440413) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442497 (CHEMBL2440412) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50442504 (CHEMBL2440423) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B1 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50024764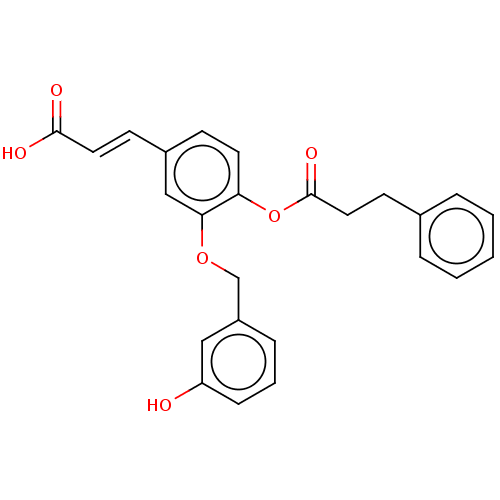 (CHEMBL3337721) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay | Bioorg Med Chem 22: 5220-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.007 BindingDB Entry DOI: 10.7270/Q28P623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50442503 (CHEMBL2440427) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B1 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50442502 (CHEMBL2440415) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B1 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50049730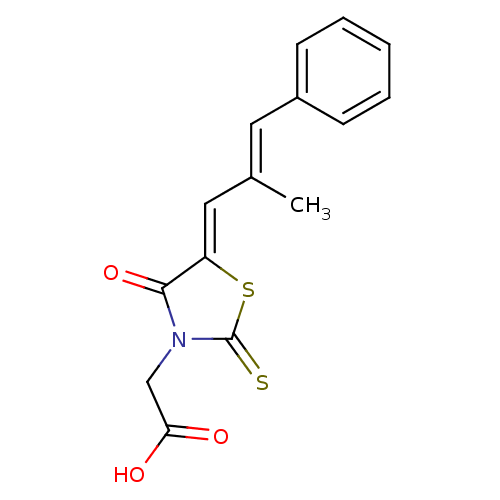 (2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human aldose reductase expressed in Escherichia coli BL21(DE3) mediated NADPH linked pyridine-3-aldehyde reducti... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50321718 (2-(2,4-dimethoxyphenylimino)-7-hydroxy-N-(pyridin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human aldose reductase expressed in Escherichia coli BL21(DE3) mediated NADPH linked pyridine-3-aldehyde reducti... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321715 (2-(2,5-dimethoxyphenylimino)-7-hydroxy-N-(pyridin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of reductase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of NADPH linked pyr... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50442501 (CHEMBL2440422) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B1 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50442489 (CHEMBL2440417) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B1 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085556 (CHEMBL405496 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Compound was tested for its binding ability, by displacement of p(A2'p)3A3'[32p]p5'Cp from recombinant human ribonuclease L | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50038843 ((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human aldose reductase expressed in Escherichia coli BL21(DE3) mediated NADPH linked pyridine-3-aldehyde reducti... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 180 total ) | Next | Last >> |