

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50150028 ((5R,6R)-1-Benzoyl-5-benzyl-6-hydroxy-2,4-bis-(4-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease | Bioorg Med Chem Lett 14: 4075-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.036 BindingDB Entry DOI: 10.7270/Q25B01ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM192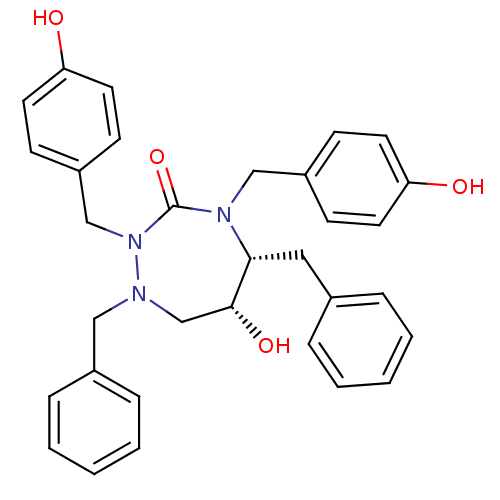 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxyp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease | Bioorg Med Chem Lett 14: 4075-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.036 BindingDB Entry DOI: 10.7270/Q25B01ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50150033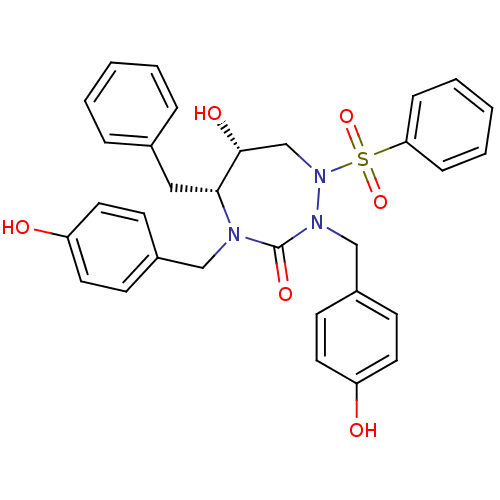 ((5R,6R)-1-Benzenesulfonyl-5-benzyl-6-hydroxy-2,4-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease | Bioorg Med Chem Lett 14: 4075-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.036 BindingDB Entry DOI: 10.7270/Q25B01ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin D (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin D (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50174973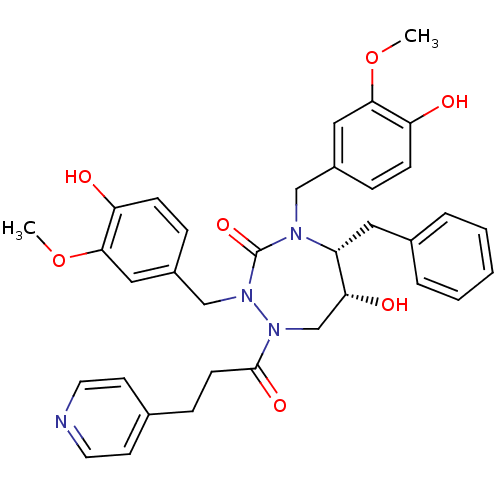 ((5R,6R)-2,4-bis(4-hydroxy-3-methoxybenzyl)-5-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HIV1 protease | Bioorg Med Chem Lett 15: 5499-503 (2005) Article DOI: 10.1016/j.bmcl.2005.08.093 BindingDB Entry DOI: 10.7270/Q2ZP45PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50174993 ((5R,6R)-2,4-bis(4-hydroxy-3-methoxybenzyl)-5-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HIV1 protease | Bioorg Med Chem Lett 15: 5499-503 (2005) Article DOI: 10.1016/j.bmcl.2005.08.093 BindingDB Entry DOI: 10.7270/Q2ZP45PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50174986 ((5R,6R)-2,4-bis(4-hydroxy-3-methoxybenzyl)-5-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HIV1 protease | Bioorg Med Chem Lett 15: 5499-503 (2005) Article DOI: 10.1016/j.bmcl.2005.08.093 BindingDB Entry DOI: 10.7270/Q2ZP45PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12210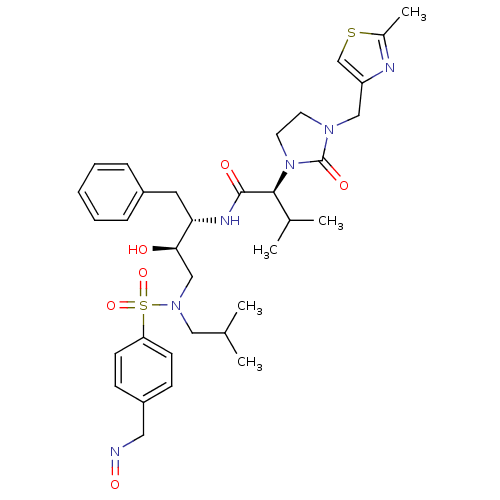 ((2S)-N-[(2S,3R)-3-hydroxy-4-({4-[(1E)-(hydroxyimin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50174969 ((5R,6R)-2,4-bis(4-hydroxy-3-methoxybenzyl)-5-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HIV1 protease | Bioorg Med Chem Lett 15: 5499-503 (2005) Article DOI: 10.1016/j.bmcl.2005.08.093 BindingDB Entry DOI: 10.7270/Q2ZP45PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50174985 ((5R,6R)-2,4-bis(4-hydroxy-3-methoxybenzyl)-5-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HIV1 protease | Bioorg Med Chem Lett 15: 5499-503 (2005) Article DOI: 10.1016/j.bmcl.2005.08.093 BindingDB Entry DOI: 10.7270/Q2ZP45PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12213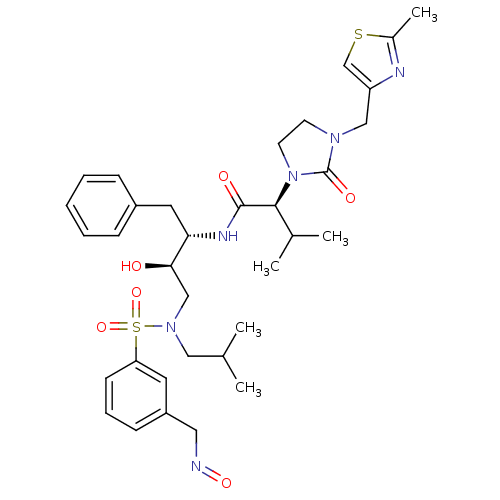 ((2S)-N-[(2S,3R)-3-hydroxy-4-({3-[(1E)-(hydroxyimin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12209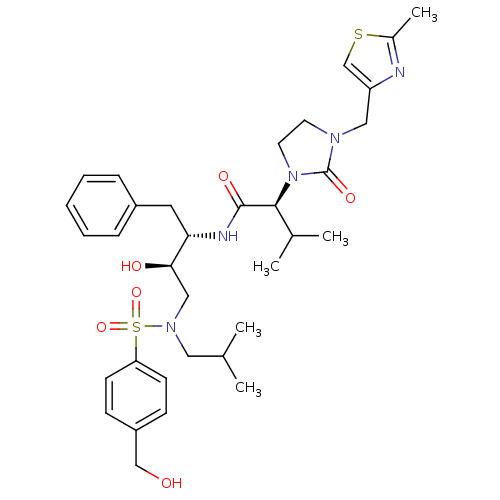 ((2S)-N-[(2S,3R)-3-hydroxy-4-{[4-(hydroxymethyl)ben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50250183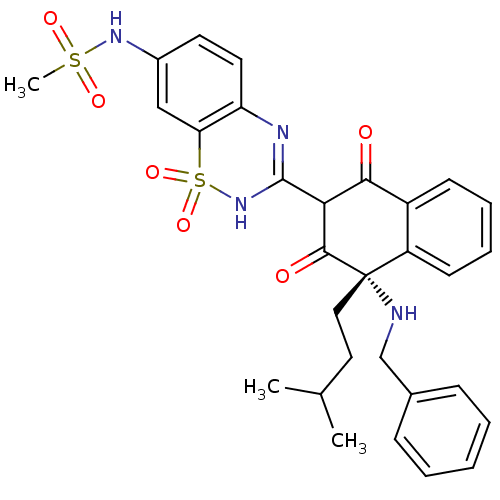 (CHEMBL503188 | N-{3-[(4S)-4-Benzylamino-1-hydroxy-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV1b Con1 NS5B assessed as [3H]UTP incorporation into RNA by scintillation counting | J Med Chem 52: 3174-83 (2009) Article DOI: 10.1021/jm801485z BindingDB Entry DOI: 10.7270/Q2ZW1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50249586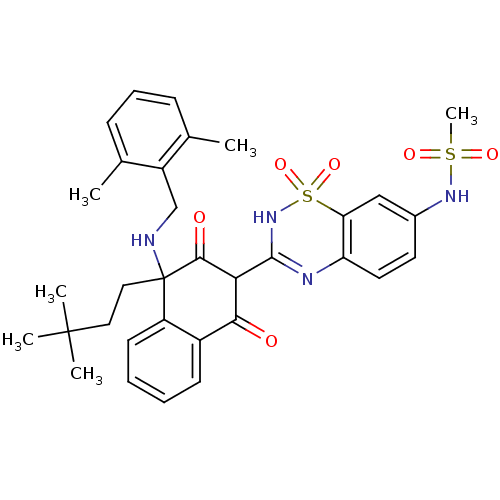 (CHEMBL504729 | N-{3-[4-[(2,6-Dimethylbenzyl)amino]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV1b Con1 NS5B assessed as [3H]UTP incorporation into RNA by scintillation counting | J Med Chem 52: 3174-83 (2009) Article DOI: 10.1021/jm801485z BindingDB Entry DOI: 10.7270/Q2ZW1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12198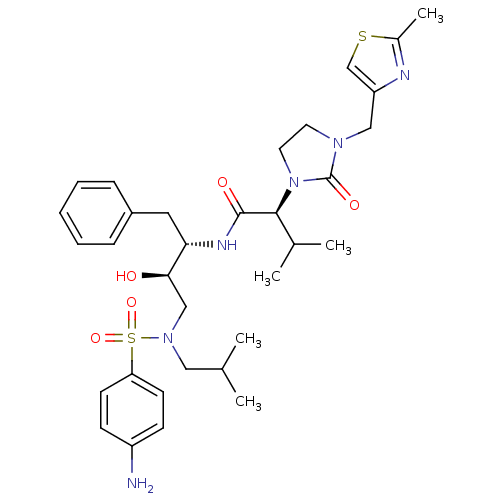 ((2S)-N-[(2S,3R)-4-[(4-aminobenzene)(2-methylpropyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12203 ((2S)-N-[(2S,3R)-4-[(4-acetylbenzene)(2-methylpropy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12212 ((2S)-N-[(2S,3R)-3-hydroxy-4-{[3-(hydroxymethyl)ben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310767 (Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310765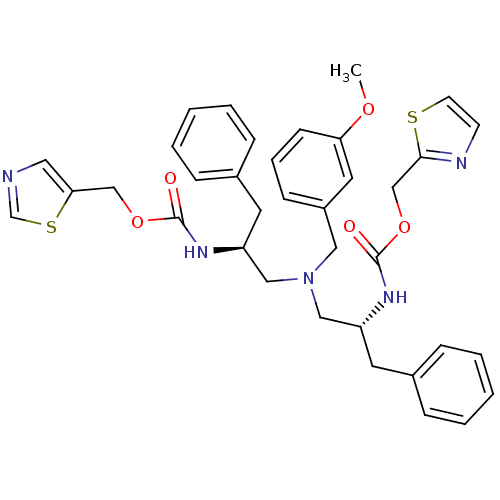 (CHEMBL1077937 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310761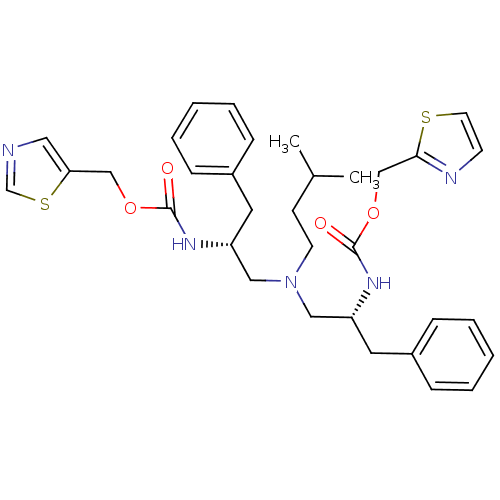 (Bis-N-[(R)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12208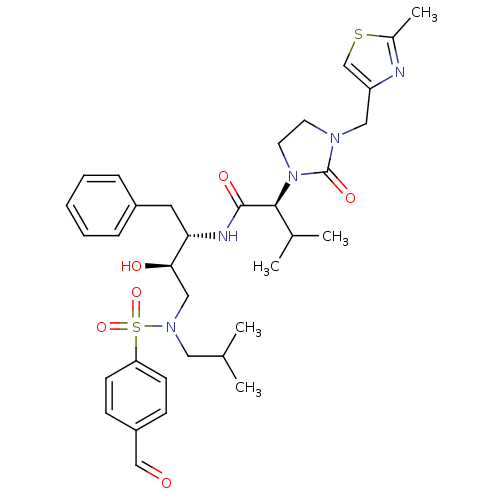 ((2S)-N-[(2S,3R)-4-[(4-formylbenzene)(2-methylpropy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310760 (Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310764 (Bis-N-[(R)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310751 (CHEMBL1079228 | Tris-N-[2-(thiazol-5-ylmethoxycarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310768 (Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310776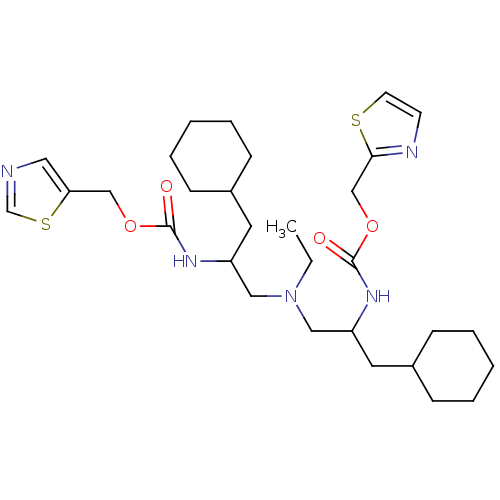 (Bis-N-[2-(thiazol-5-ylmethoxycarbonylamino)-3-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310766 (Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310759 (Bis-N-[(R)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310769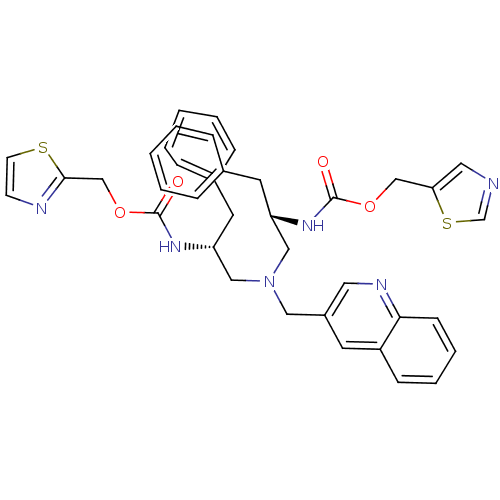 (CHEMBL1077941 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310763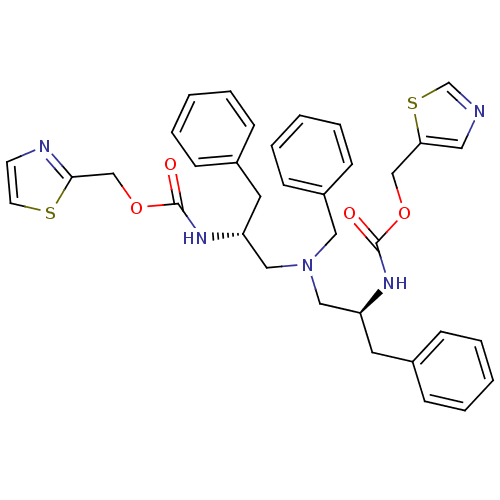 (CHEMBL1077935 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310775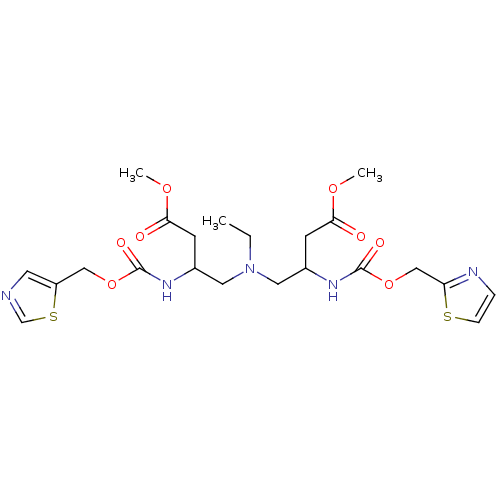 (Bis-N-[2-(thiazol-5-ylmethoxycarbonylamino)-3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310758 (CHEMBL1077930 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310749 (CHEMBL1079896 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310778 (CHEMBL1078122 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310750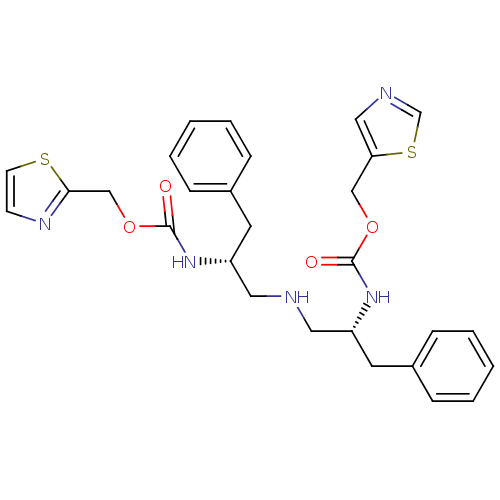 (Bis-N-[(R)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310753 (CHEMBL1079230 | N-[(S)-2-thiazol-5-ylmethoxycarbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12207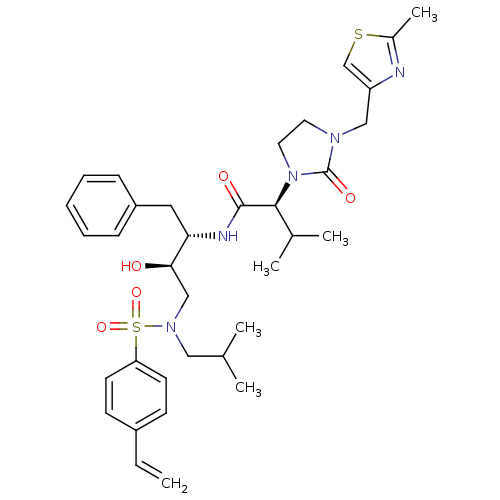 ((2S)-N-[(2S,3R)-4-[(4-ethenylbenzene)(2-methylprop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310757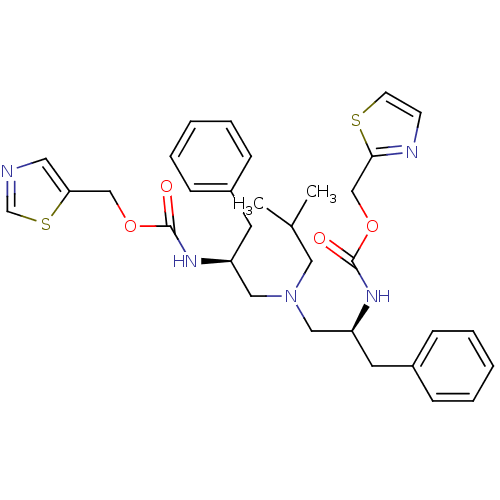 (Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310752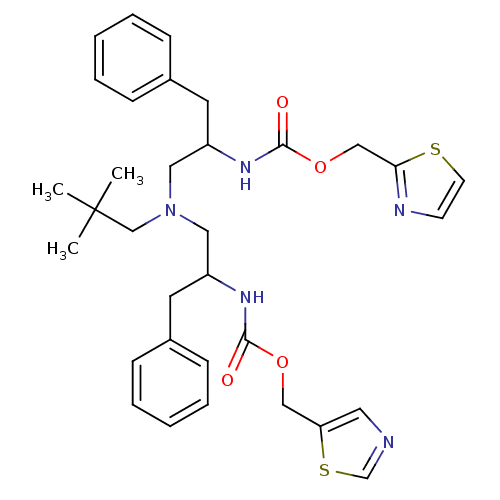 (Bis-N-[2-(thiazol-5-ylmethoxycarbonylamino)-3-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310755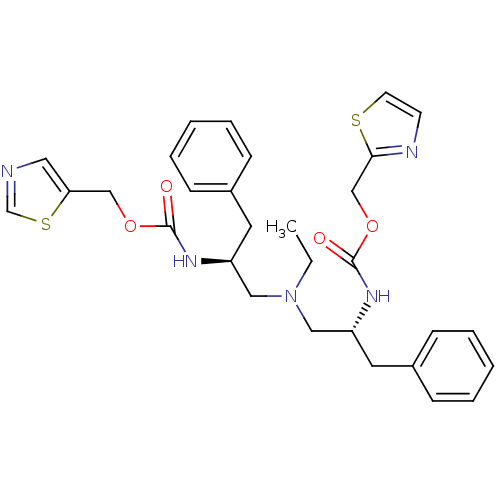 (CHEMBL1081147 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50310756 (Bis-N-[(R)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline | Bioorg Med Chem Lett 19: 5444-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.118 BindingDB Entry DOI: 10.7270/Q21R6QN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 126 total ) | Next | Last >> |