 Found 391 hits with Last Name = 'knick' and Initial = 'v'
Found 391 hits with Last Name = 'knick' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Candida albicans) | BDBM50049912

(7-(1,1-Dimethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]...)Show InChI InChI=1S/C16H21N5/c1-5-16(3,4)21-9(2)8-10-12(21)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Candida albicans) | BDBM50049905
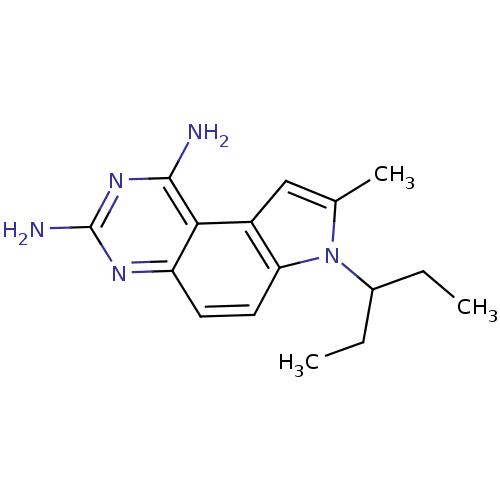
(7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C16H21N5/c1-4-10(5-2)21-9(3)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-8,10H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049907

(7-(1-Ethyl-propyl)-8-isopropyl-7H-pyrrolo[3,2-f]qu...)Show InChI InChI=1S/C18H25N5/c1-5-11(6-2)23-14-8-7-13-16(17(19)22-18(20)21-13)12(14)9-15(23)10(3)4/h7-11H,5-6H2,1-4H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049901
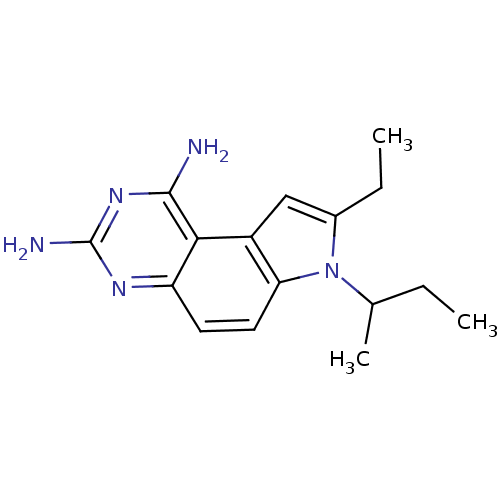
(7-sec-Butyl-8-ethyl-7H-pyrrolo[3,2-f]quinazoline-1...)Show InChI InChI=1S/C16H21N5/c1-4-9(3)21-10(5-2)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-9H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049906
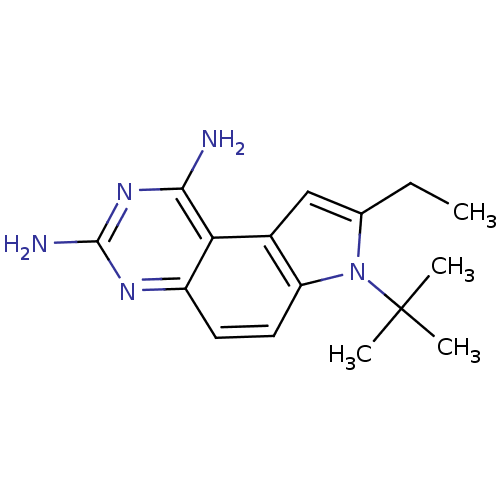
(7-tert-Butyl-8-ethyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C16H21N5/c1-5-9-8-10-12(21(9)16(2,3)4)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049911

(8-tert-Butyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diam...)Show InChI InChI=1S/C14H17N5/c1-14(2,3)10-6-7-8(17-10)4-5-9-11(7)12(15)19-13(16)18-9/h4-6,17H,1-3H3,(H4,15,16,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049898

(8-Isopropyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C13H15N5/c1-6(2)10-5-7-8(16-10)3-4-9-11(7)12(14)18-13(15)17-9/h3-6,16H,1-2H3,(H4,14,15,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049909

(8-tert-Butyl-7-isopropyl-7H-pyrrolo[3,2-f]quinazol...)Show InChI InChI=1S/C17H23N5/c1-9(2)22-12-7-6-11-14(15(18)21-16(19)20-11)10(12)8-13(22)17(3,4)5/h6-9H,1-5H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049897

(8-Ethyl-7-(1-ethyl-propyl)-7H-pyrrolo[3,2-f]quinaz...)Show InChI InChI=1S/C17H23N5/c1-4-10(5-2)22-11(6-3)9-12-14(22)8-7-13-15(12)16(18)21-17(19)20-13/h7-10H,4-6H2,1-3H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM18043
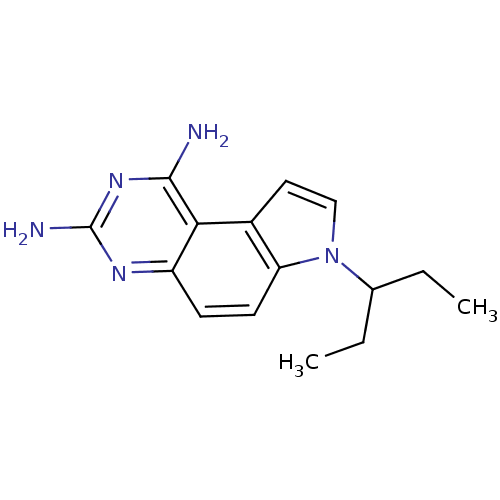
(1,3-DIAMINO-7-(1-ETHYEPROPYE)-7H-PYRRALO-[3,2-F]QU...)Show InChI InChI=1S/C15H19N5/c1-3-9(4-2)20-8-7-10-12(20)6-5-11-13(10)14(16)19-15(17)18-11/h5-9H,3-4H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049899
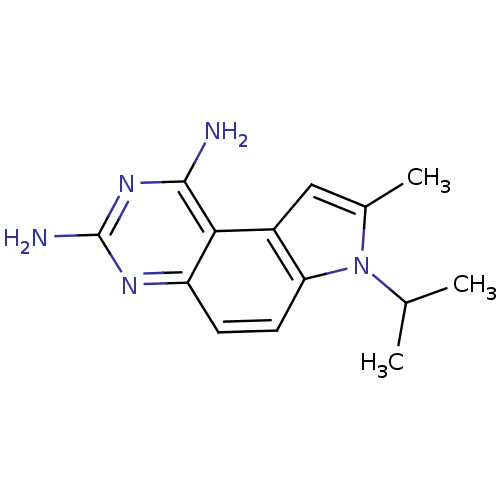
(7-Isopropyl-8-methyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C14H17N5/c1-7(2)19-8(3)6-9-11(19)5-4-10-12(9)13(15)18-14(16)17-10/h4-7H,1-3H3,(H4,15,16,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049914

(7-(3,4,5-Trimethoxy-benzyl)-7H-pyrrolo[3,2-f]quina...)Show SMILES COc1cc(Cn2ccc3c2ccc2nc(N)nc(N)c32)cc(OC)c1OC Show InChI InChI=1S/C20H21N5O3/c1-26-15-8-11(9-16(27-2)18(15)28-3)10-25-7-6-12-14(25)5-4-13-17(12)19(21)24-20(22)23-13/h4-9H,10H2,1-3H3,(H4,21,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049905

(7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C16H21N5/c1-4-10(5-2)21-9(3)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-8,10H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049913
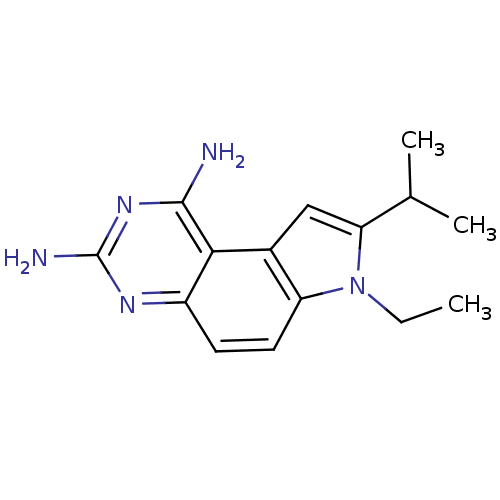
(7-Ethyl-8-isopropyl-7H-pyrrolo[3,2-f]quinazoline-1...)Show InChI InChI=1S/C15H19N5/c1-4-20-11-6-5-10-13(14(16)19-15(17)18-10)9(11)7-12(20)8(2)3/h5-8H,4H2,1-3H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049902

(7,8-Diethyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C14H17N5/c1-3-8-7-9-11(19(8)4-2)6-5-10-12(9)13(15)18-14(16)17-10/h5-7H,3-4H2,1-2H3,(H4,15,16,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049896
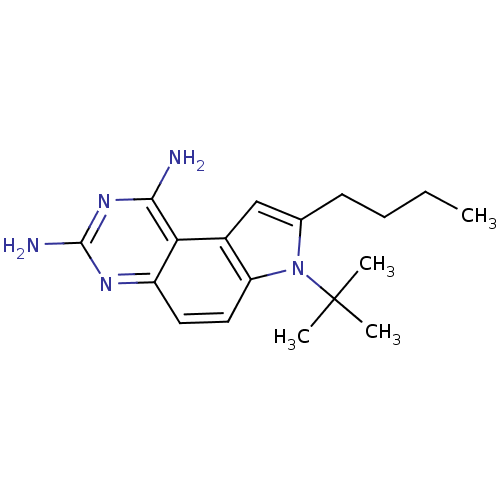
(8-Butyl-7-tert-butyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C18H25N5/c1-5-6-7-11-10-12-14(23(11)18(2,3)4)9-8-13-15(12)16(19)22-17(20)21-13/h8-10H,5-7H2,1-4H3,(H4,19,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049903
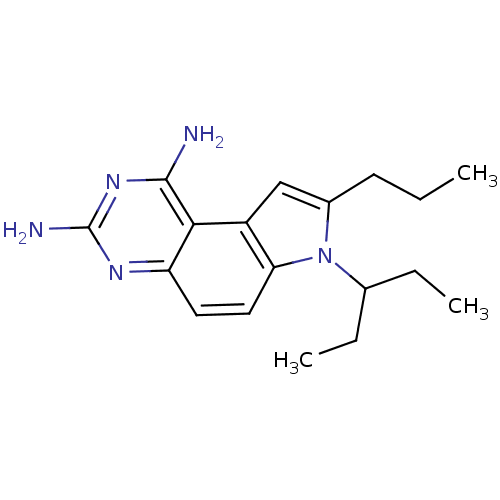
(7-(1-Ethyl-propyl)-8-propyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C18H25N5/c1-4-7-12-10-13-15(23(12)11(5-2)6-3)9-8-14-16(13)17(19)22-18(20)21-14/h8-11H,4-7H2,1-3H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049912

(7-(1,1-Dimethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]...)Show InChI InChI=1S/C16H21N5/c1-5-16(3,4)21-9(2)8-10-12(21)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049910

(7-Ethyl-8-propyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C15H19N5/c1-3-5-9-8-10-12(20(9)4-2)7-6-11-13(10)14(16)19-15(17)18-11/h6-8H,3-5H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049915
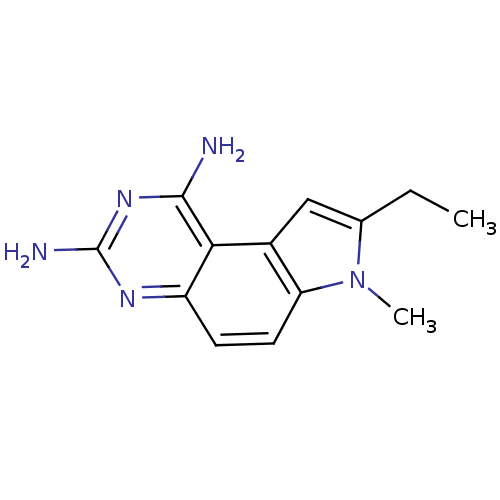
(8-Ethyl-7-methyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C13H15N5/c1-3-7-6-8-10(18(7)2)5-4-9-11(8)12(14)17-13(15)16-9/h4-6H,3H2,1-2H3,(H4,14,15,16,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049908
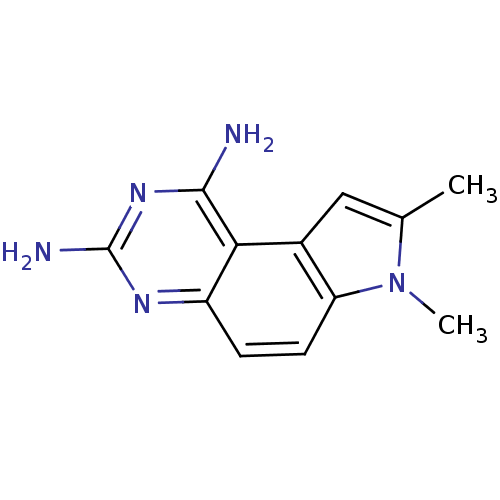
(7,8-Dimethyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diam...)Show InChI InChI=1S/C12H13N5/c1-6-5-7-9(17(6)2)4-3-8-10(7)11(13)16-12(14)15-8/h3-5H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18043

(1,3-DIAMINO-7-(1-ETHYEPROPYE)-7H-PYRRALO-[3,2-F]QU...)Show InChI InChI=1S/C15H19N5/c1-3-9(4-2)20-8-7-10-12(20)6-5-11-13(10)14(16)19-15(17)18-11/h5-9H,3-4H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049914

(7-(3,4,5-Trimethoxy-benzyl)-7H-pyrrolo[3,2-f]quina...)Show SMILES COc1cc(Cn2ccc3c2ccc2nc(N)nc(N)c32)cc(OC)c1OC Show InChI InChI=1S/C20H21N5O3/c1-26-15-8-11(9-16(27-2)18(15)28-3)10-25-7-6-12-14(25)5-4-13-17(12)19(21)24-20(22)23-13/h4-9H,10H2,1-3H3,(H4,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049899

(7-Isopropyl-8-methyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C14H17N5/c1-7(2)19-8(3)6-9-11(19)5-4-10-12(9)13(15)18-14(16)17-10/h4-7H,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049898

(8-Isopropyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C13H15N5/c1-6(2)10-5-7-8(16-10)3-4-9-11(7)12(14)18-13(15)17-9/h3-6,16H,1-2H3,(H4,14,15,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049902

(7,8-Diethyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C14H17N5/c1-3-8-7-9-11(19(8)4-2)6-5-10-12(9)13(15)18-14(16)17-10/h5-7H,3-4H2,1-2H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049904
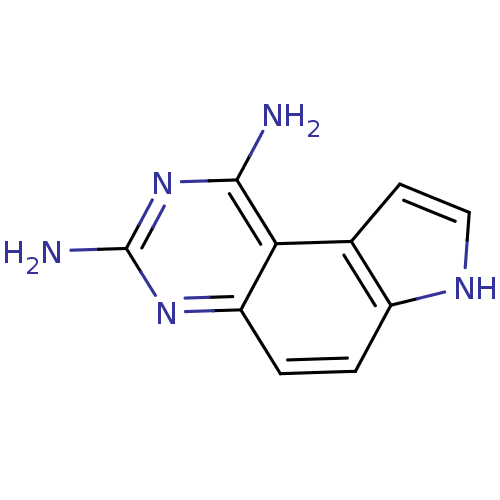
(7H-Pyrrolo[3,2-f]quinazoline-1,3-diamine | CHEMBL3...)Show InChI InChI=1S/C10H9N5/c11-9-8-5-3-4-13-6(5)1-2-7(8)14-10(12)15-9/h1-4,13H,(H4,11,12,14,15) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049910

(7-Ethyl-8-propyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C15H19N5/c1-3-5-9-8-10-12(20(9)4-2)7-6-11-13(10)14(16)19-15(17)18-11/h6-8H,3-5H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049900
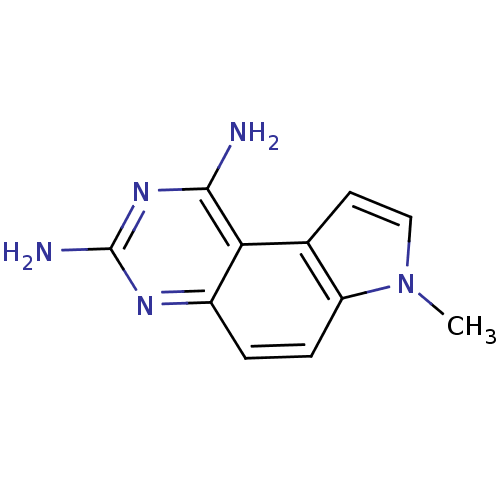
(7-Methyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine ...)Show InChI InChI=1S/C11H11N5/c1-16-5-4-6-8(16)3-2-7-9(6)10(12)15-11(13)14-7/h2-5H,1H3,(H4,12,13,14,15) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049915

(8-Ethyl-7-methyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C13H15N5/c1-3-7-6-8-10(18(7)2)5-4-9-11(8)12(14)17-13(15)16-9/h4-6H,3H2,1-2H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049896

(8-Butyl-7-tert-butyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C18H25N5/c1-5-6-7-11-10-12-14(23(11)18(2,3)4)9-8-13-15(12)16(19)22-17(20)21-13/h8-10H,5-7H2,1-4H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049908

(7,8-Dimethyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diam...)Show InChI InChI=1S/C12H13N5/c1-6-5-7-9(17(6)2)4-3-8-10(7)11(13)16-12(14)15-8/h3-5H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049900

(7-Methyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine ...)Show InChI InChI=1S/C11H11N5/c1-16-5-4-6-8(16)3-2-7-9(6)10(12)15-11(13)14-7/h2-5H,1H3,(H4,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Tubulin alpha-1A chain
(Sus scrofa (Pig)) | BDBM50280140
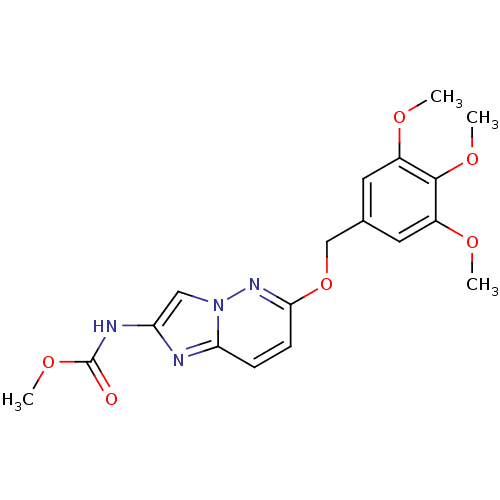
(CHEMBL418565 | [6-(3,4,5-Trimethoxy-benzyloxy)-imi...)Show SMILES COC(=O)Nc1cn2nc(OCc3cc(OC)c(OC)c(OC)c3)ccc2n1 Show InChI InChI=1S/C18H20N4O6/c1-24-12-7-11(8-13(25-2)17(12)26-3)10-28-16-6-5-15-19-14(9-22(15)21-16)20-18(23)27-4/h5-9H,10H2,1-4H3,(H,20,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for competitive inhibition with radioligand [3H]colchicine at colchicine site of mammalian tubulin |
Bioorg Med Chem Lett 2: 1257-1262 (1992)
Article DOI: 10.1016/S0960-894X(00)80225-5
BindingDB Entry DOI: 10.7270/Q25X29DB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049904

(7H-Pyrrolo[3,2-f]quinazoline-1,3-diamine | CHEMBL3...)Show InChI InChI=1S/C10H9N5/c11-9-8-5-3-4-13-6(5)1-2-7(8)14-10(12)15-9/h1-4,13H,(H4,11,12,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50293153
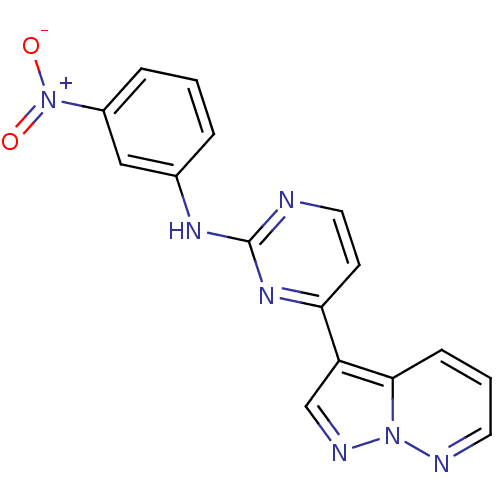
(CHEMBL526110 | N-(3-nitrophenyl)-4-(pyrazolo[1,5-b...)Show SMILES [O-][N+](=O)c1cccc(Nc2nccc(n2)-c2cnn3ncccc23)c1 Show InChI InChI=1S/C16H11N7O2/c24-23(25)12-4-1-3-11(9-12)20-16-17-8-6-14(21-16)13-10-19-22-15(13)5-2-7-18-22/h1-10H,(H,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2 by HTRF assay |
Bioorg Med Chem Lett 18: 5758-62 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.069
BindingDB Entry DOI: 10.7270/Q2J67GZN |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50293153

(CHEMBL526110 | N-(3-nitrophenyl)-4-(pyrazolo[1,5-b...)Show SMILES [O-][N+](=O)c1cccc(Nc2nccc(n2)-c2cnn3ncccc23)c1 Show InChI InChI=1S/C16H11N7O2/c24-23(25)12-4-1-3-11(9-12)20-16-17-8-6-14(21-16)13-10-19-22-15(13)5-2-7-18-22/h1-10H,(H,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 by radioactive glutathione plate-binding assay |
Bioorg Med Chem Lett 18: 5758-62 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.069
BindingDB Entry DOI: 10.7270/Q2J67GZN |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM26467
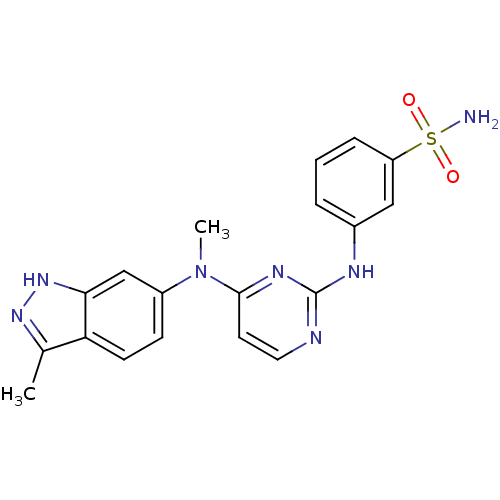
(3-({4-[methyl(3-methyl-1H-indazol-6-yl)amino]pyrim...)Show SMILES CN(c1ccc2c(C)n[nH]c2c1)c1ccnc(Nc2cccc(c2)S(N)(=O)=O)n1 Show InChI InChI=1S/C19H19N7O2S/c1-12-16-7-6-14(11-17(16)25-24-12)26(2)18-8-9-21-19(23-18)22-13-4-3-5-15(10-13)29(20,27)28/h3-11H,1-2H3,(H,24,25)(H2,20,27,28)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
The assay uses purified baculovirus-expressed GST-VEGFR2 interacting with biotinylated peptide substrates. HTRF is based on the proximity of europium... |
J Med Chem 51: 4632-40 (2008)
Article DOI: 10.1021/jm800566m
BindingDB Entry DOI: 10.7270/Q26971XR |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM26477
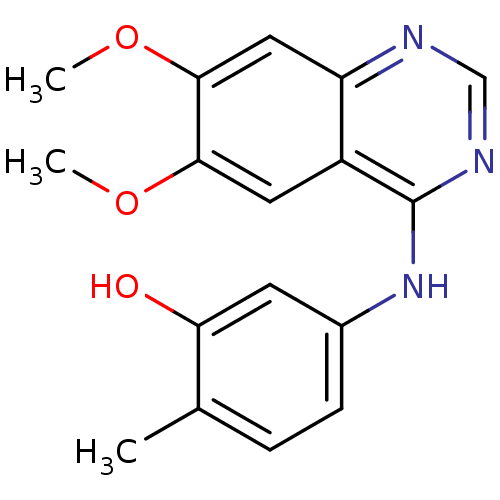
(5-[(6,7-dimethoxyquinazolin-4-yl)amino]-2-methylph...)Show InChI InChI=1S/C17H17N3O3/c1-10-4-5-11(6-14(10)21)20-17-12-7-15(22-2)16(23-3)8-13(12)18-9-19-17/h4-9,21H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
The assay uses purified baculovirus-expressed GST-VEGFR2 interacting with biotinylated peptide substrates. HTRF is based on the proximity of europium... |
J Med Chem 51: 4632-40 (2008)
Article DOI: 10.1021/jm800566m
BindingDB Entry DOI: 10.7270/Q26971XR |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306097

((2S)-1-(1H-indol-3-yl)-3-(5-(3-methyl-1H-indazol-5...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)cnc1-c1ccoc1C |r| Show InChI InChI=1S/C29H27N5O2/c1-17-25-12-19(7-8-28(25)34-33-17)26-13-22(15-32-29(26)23-9-10-35-18(23)2)36-16-21(30)11-20-14-31-27-6-4-3-5-24(20)27/h3-10,12-15,21,31H,11,16,30H2,1-2H3,(H,33,34)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length AKT1 |
Bioorg Med Chem Lett 20: 673-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.064
BindingDB Entry DOI: 10.7270/Q2D21XQ0 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306095
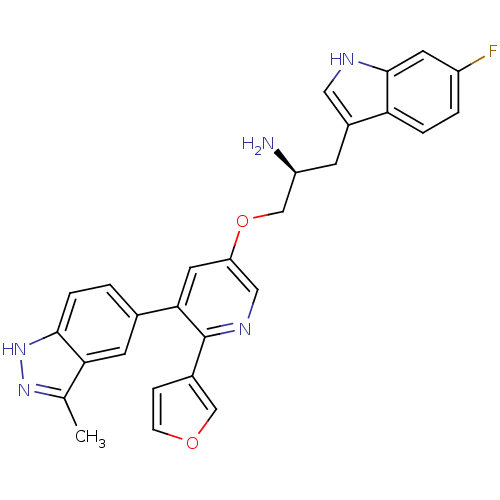
((S)-1-(6-fluoro-1H-indol-3-yl)-3-(6-(furan-3-yl)-5...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3cc(F)ccc23)cnc1-c1ccoc1 |r| Show InChI InChI=1S/C28H24FN5O2/c1-16-24-9-17(2-5-26(24)34-33-16)25-11-22(13-32-28(25)18-6-7-35-14-18)36-15-21(30)8-19-12-31-27-10-20(29)3-4-23(19)27/h2-7,9-14,21,31H,8,15,30H2,1H3,(H,33,34)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length AKT1 |
Bioorg Med Chem Lett 20: 673-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.064
BindingDB Entry DOI: 10.7270/Q2D21XQ0 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306094
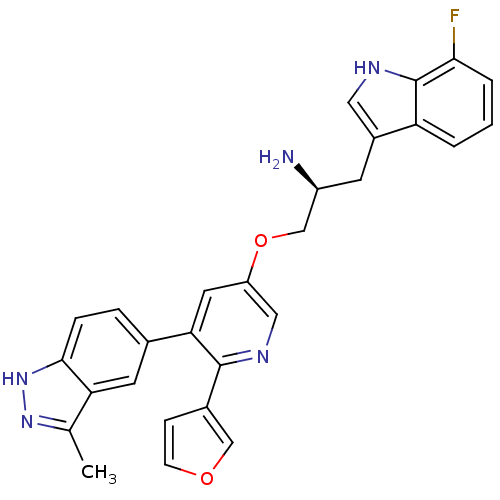
((S)-1-(7-fluoro-1H-indol-3-yl)-3-(6-(furan-3-yl)-5...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3c(F)cccc23)cnc1-c1ccoc1 |r| Show InChI InChI=1S/C28H24FN5O2/c1-16-23-10-17(5-6-26(23)34-33-16)24-11-21(13-32-27(24)18-7-8-35-14-18)36-15-20(30)9-19-12-31-28-22(19)3-2-4-25(28)29/h2-8,10-14,20,31H,9,15,30H2,1H3,(H,33,34)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length AKT1 |
Bioorg Med Chem Lett 20: 673-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.064
BindingDB Entry DOI: 10.7270/Q2D21XQ0 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM25004
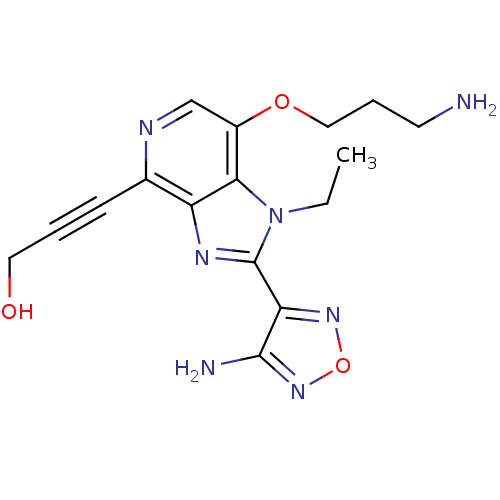
(3-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(3-aminoprop...)Show InChI InChI=1S/C16H19N7O3/c1-2-23-14-11(25-8-4-6-17)9-19-10(5-3-7-24)12(14)20-16(23)13-15(18)22-26-21-13/h9,24H,2,4,6-8,17H2,1H3,(H2,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306096
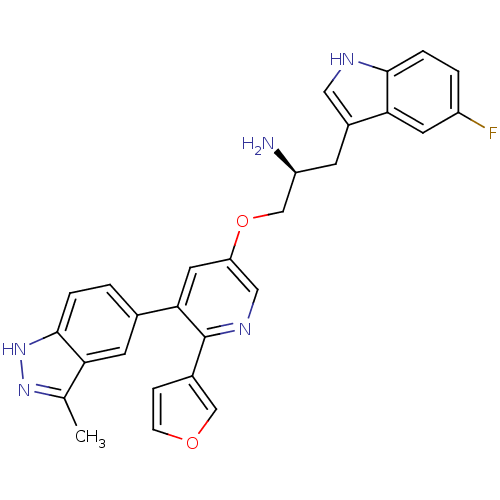
((S)-1-(5-fluoro-1H-indol-3-yl)-3-(6-(furan-3-yl)-5...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccc(F)cc23)cnc1-c1ccoc1 |r| Show InChI InChI=1S/C28H24FN5O2/c1-16-23-9-17(2-4-27(23)34-33-16)25-11-22(13-32-28(25)18-6-7-35-14-18)36-15-21(30)8-19-12-31-26-5-3-20(29)10-24(19)26/h2-7,9-14,21,31H,8,15,30H2,1H3,(H,33,34)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length AKT1 |
Bioorg Med Chem Lett 20: 673-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.064
BindingDB Entry DOI: 10.7270/Q2D21XQ0 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
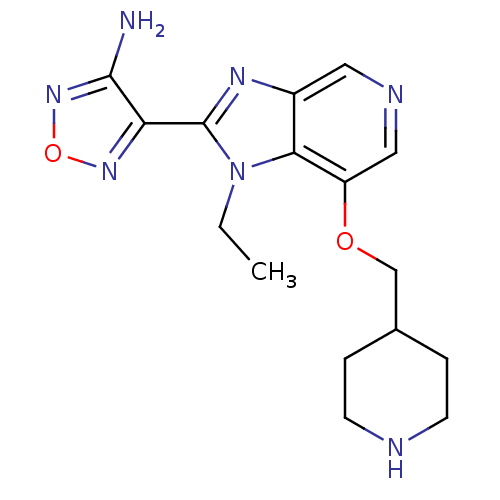
(Homo sapiens (Human)) | BDBM24994
(4-[1-ethyl-7-(piperidin-4-ylmethoxy)-1H-imidazo[4,...)Show InChI InChI=1S/C16H21N7O2/c1-2-23-14-11(20-16(23)13-15(17)22-25-21-13)7-19-8-12(14)24-9-10-3-5-18-6-4-10/h7-8,10,18H,2-6,9H2,1H3,(H2,17,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM5829

(1-[2-fluoro-5-(trifluoromethyl)phenyl]-3-{4-[methy...)Show SMILES CN(c1ccc(NC(=O)Nc2cc(ccc2F)C(F)(F)F)cc1)c1ccnc(Nc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C25H21F4N7O3S/c1-36(22-12-13-31-23(35-22)32-16-5-9-19(10-6-16)40(30,38)39)18-7-3-17(4-8-18)33-24(37)34-21-14-15(25(27,28)29)2-11-20(21)26/h2-14H,1H3,(H2,30,38,39)(H,31,32,35)(H2,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
The assay was using baculovirus-expressed recombinant protein kinase purified as the intracellular domain fused by GST tag, interacting with biotinyl... |
Bioorg Med Chem Lett 15: 3519-23 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.096
BindingDB Entry DOI: 10.7270/Q2KK990W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data