 Found 180 hits with Last Name = 'koovakkat' and Initial = 'sk'
Found 180 hits with Last Name = 'koovakkat' and Initial = 'sk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM17280

(1-[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-difluo...)Show SMILES CN1CCN=C1c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(N3CCCC(C3)C(O)=O)c2F)c1 |c:4| Show InChI InChI=1S/C28H28F2N6O5/c1-35-11-9-33-25(35)16-4-2-6-18(12-16)40-26-21(29)23(36-10-3-5-17(14-36)28(38)39)22(30)27(34-26)41-20-13-15(24(31)32)7-8-19(20)37/h2,4,6-8,12-13,17,37H,3,5,9-11,14H2,1H3,(H3,31,32)(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50088923
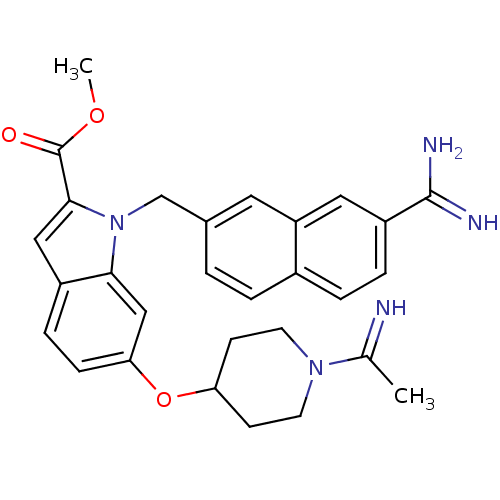
(1-(7-Carbamimidoyl-naphthalen-2-ylmethyl)-6-[1-(1-...)Show SMILES COC(=O)c1cc2ccc(OC3CCN(CC3)C(C)=N)cc2n1Cc1ccc2ccc(cc2c1)C(N)=N Show InChI InChI=1S/C29H31N5O3/c1-18(30)33-11-9-24(10-12-33)37-25-8-7-21-15-27(29(35)36-2)34(26(21)16-25)17-19-3-4-20-5-6-22(28(31)32)14-23(20)13-19/h3-8,13-16,24,30H,9-12,17H2,1-2H3,(H3,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17282

(7-({6-[(1-ethanimidoylpiperidin-4-yl)oxy]-2-methyl...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2nc(C)n(Cc3ccc4ccc(cc4c3)C(N)=N)c2c1 Show InChI InChI=1S/C27H30N6O/c1-17(28)32-11-9-23(10-12-32)34-24-7-8-25-26(15-24)33(18(2)31-25)16-19-3-4-20-5-6-21(27(29)30)14-22(20)13-19/h3-8,13-15,23,28H,9-12,16H2,1-2H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.270 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50088924

(1-(7-Carbamimidoyl-naphthalen-2-ylmethyl)-6-[1-(1-...)Show SMILES CCOC(=O)c1cc2ccc(OC3CCN(CC3)C(C)=N)cc2n1Cc1ccc2ccc(cc2c1)C(N)=N Show InChI InChI=1S/C30H33N5O3/c1-3-37-30(36)28-16-22-8-9-26(38-25-10-12-34(13-11-25)19(2)31)17-27(22)35(28)18-20-4-5-21-6-7-23(29(32)33)15-24(21)14-20/h4-9,14-17,25,31H,3,10-13,18H2,1-2H3,(H3,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17277
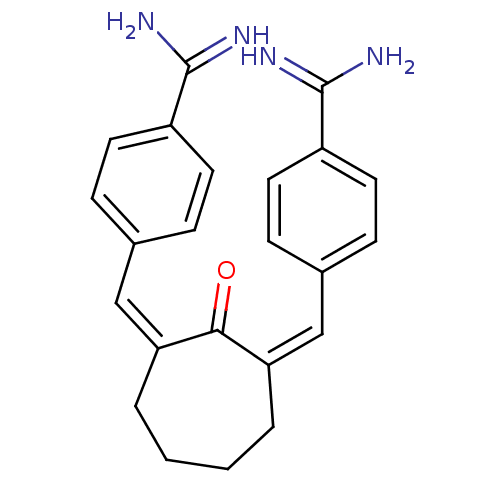
((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Coagulation factor X |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17277

((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.660 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066614

((E,E)-2,7-Bis(4-amidinobenzylidine)cycloheptan-1-o...)Show SMILES NC(=N)c1ccc(\C=C2/CCCC\C(=C/c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13+,20-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50088917
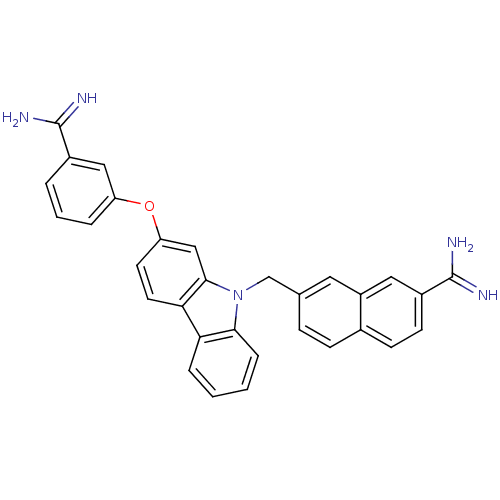
(7-[2-(3-Carbamimidoyl-phenoxy)-carbazol-9-ylmethyl...)Show SMILES NC(=N)c1cccc(Oc2ccc3c(c2)n(Cc2ccc4ccc(cc4c2)C(N)=N)c2ccccc32)c1 Show InChI InChI=1S/C31H25N5O/c32-30(33)21-4-3-5-24(16-21)37-25-12-13-27-26-6-1-2-7-28(26)36(29(27)17-25)18-19-8-9-20-10-11-22(31(34)35)15-23(20)14-19/h1-17H,18H2,(H3,32,33)(H3,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17281

(7-({2-[(1-ethanimidoylpiperidin-4-yl)oxy]-9H-carba...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2c(c1)n(Cc1ccc3ccc(cc3c1)C(N)=N)c1ccccc21 Show InChI InChI=1S/C31H31N5O/c1-20(32)35-14-12-25(13-15-35)37-26-10-11-28-27-4-2-3-5-29(27)36(30(28)18-26)19-21-6-7-22-8-9-23(31(33)34)17-24(22)16-21/h2-11,16-18,25,32H,12-15,19H2,1H3,(H3,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17281

(7-({2-[(1-ethanimidoylpiperidin-4-yl)oxy]-9H-carba...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2c(c1)n(Cc1ccc3ccc(cc3c1)C(N)=N)c1ccccc21 Show InChI InChI=1S/C31H31N5O/c1-20(32)35-14-12-25(13-15-35)37-26-10-11-28-27-4-2-3-5-29(27)36(30(28)18-26)19-21-6-7-22-8-9-23(31(33)34)17-24(22)16-21/h2-11,16-18,25,32H,12-15,19H2,1H3,(H3,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50088919

(1-(7-Carbamimidoyl-naphthalen-2-ylmethyl)-6-[1-(1-...)Show SMILES CN(C)C(=O)c1cc2ccc(OC3CCN(CC3)C(C)=N)cc2n1Cc1ccc2ccc(cc2c1)C(N)=N Show InChI InChI=1S/C30H34N6O2/c1-19(31)35-12-10-25(11-13-35)38-26-9-8-22-16-28(30(37)34(2)3)36(27(22)17-26)18-20-4-5-21-6-7-23(29(32)33)15-24(21)14-20/h4-9,14-17,25,31H,10-13,18H2,1-3H3,(H3,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50088920
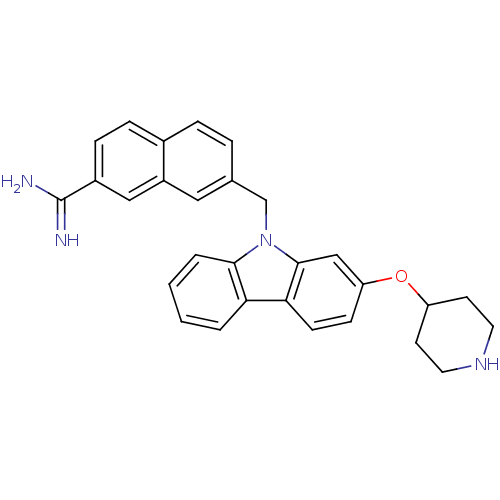
(7-[2-(Piperidin-4-yloxy)-carbazol-9-ylmethyl]-naph...)Show SMILES NC(=N)c1ccc2ccc(Cn3c4ccccc4c4ccc(OC5CCNCC5)cc34)cc2c1 Show InChI InChI=1S/C29H28N4O/c30-29(31)21-8-7-20-6-5-19(15-22(20)16-21)18-33-27-4-2-1-3-25(27)26-10-9-24(17-28(26)33)34-23-11-13-32-14-12-23/h1-10,15-17,23,32H,11-14,18H2,(H3,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50088932
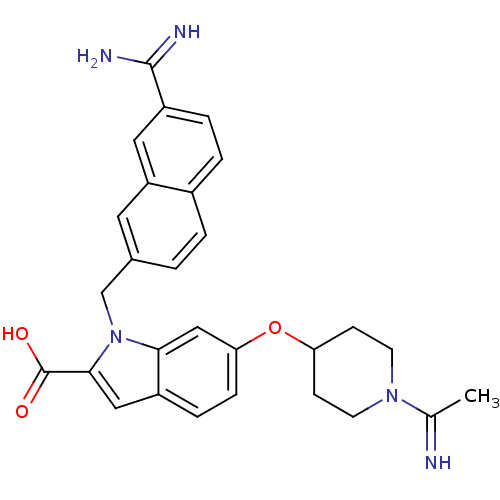
(1-(7-Carbamimidoyl-naphthalen-2-ylmethyl)-6-[1-(1-...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2cc(C(O)=O)n(Cc3ccc4ccc(cc4c3)C(N)=N)c2c1 Show InChI InChI=1S/C28H29N5O3/c1-17(29)32-10-8-23(9-11-32)36-24-7-6-20-14-26(28(34)35)33(25(20)15-24)16-18-2-3-19-4-5-21(27(30)31)13-22(19)12-18/h2-7,12-15,23,29H,8-11,16H2,1H3,(H3,30,31)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50088918
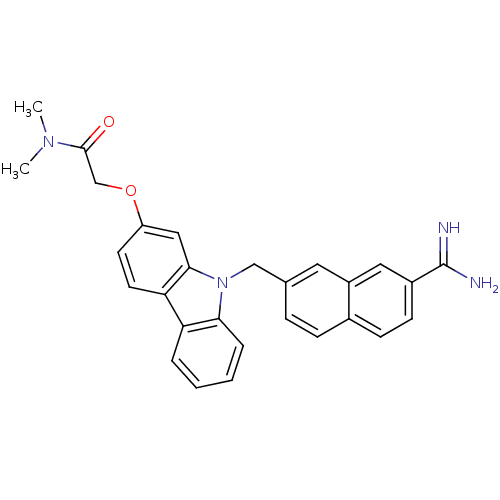
(2-[9-(7-Carbamimidoyl-naphthalen-2-ylmethyl)-9H-ca...)Show SMILES CN(C)C(=O)COc1ccc2c(c1)n(Cc1ccc3ccc(cc3c1)C(N)=N)c1ccccc21 Show InChI InChI=1S/C28H26N4O2/c1-31(2)27(33)17-34-22-11-12-24-23-5-3-4-6-25(23)32(26(24)15-22)16-18-7-8-19-9-10-20(28(29)30)14-21(19)13-18/h3-15H,16-17H2,1-2H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50088929

(7-[2-(4-Carbamimidoyl-phenoxy)-carbazol-9-ylmethyl...)Show SMILES NC(=N)c1ccc(Oc2ccc3c(c2)n(Cc2ccc4ccc(cc4c2)C(N)=N)c2ccccc32)cc1 Show InChI InChI=1S/C31H25N5O/c32-30(33)21-9-11-24(12-10-21)37-25-13-14-27-26-3-1-2-4-28(26)36(29(27)17-25)18-19-5-6-20-7-8-22(31(34)35)16-23(20)15-19/h1-17H,18H2,(H3,32,33)(H3,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50077190

(2N-[3-amino(imino)methylphenyl]-2N,4-dimethyl-6-[3...)Show SMILES CN(c1cccc(c1)C(N)=N)c1nc(Oc2cccc(c2)C(N)=N)c(F)c(C)c1F Show InChI InChI=1S/C21H20F2N6O/c1-11-16(22)20(29(2)14-7-3-5-12(9-14)18(24)25)28-21(17(11)23)30-15-8-4-6-13(10-15)19(26)27/h3-10H,1-2H3,(H3,24,25)(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17278
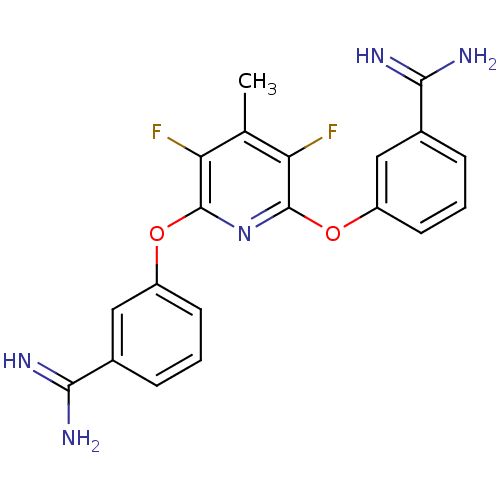
(3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...)Show SMILES Cc1c(F)c(Oc2cccc(c2)C(N)=N)nc(Oc2cccc(c2)C(N)=N)c1F Show InChI InChI=1S/C20H17F2N5O2/c1-10-15(21)19(28-13-6-2-4-11(8-13)17(23)24)27-20(16(10)22)29-14-7-3-5-12(9-14)18(25)26/h2-9H,1H3,(H3,23,24)(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17278

(3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...)Show SMILES Cc1c(F)c(Oc2cccc(c2)C(N)=N)nc(Oc2cccc(c2)C(N)=N)c1F Show InChI InChI=1S/C20H17F2N5O2/c1-10-15(21)19(28-13-6-2-4-11(8-13)17(23)24)27-20(16(10)22)29-14-7-3-5-12(9-14)18(25)26/h2-9H,1H3,(H3,23,24)(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 14 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM17282

(7-({6-[(1-ethanimidoylpiperidin-4-yl)oxy]-2-methyl...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2nc(C)n(Cc3ccc4ccc(cc4c3)C(N)=N)c2c1 Show InChI InChI=1S/C27H30N6O/c1-17(28)32-11-9-23(10-12-32)34-24-7-8-25-26(15-24)33(18(2)31-25)16-19-3-4-20-5-6-21(27(29)30)14-22(20)13-19/h3-8,13-15,23,28H,9-12,16H2,1-2H3,(H3,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 18 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50088921
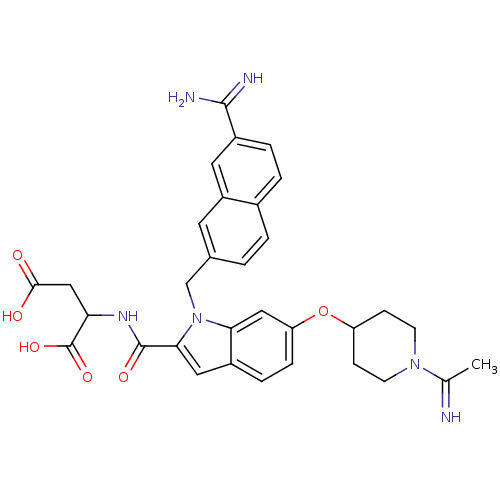
(2-({1-(7-Carbamimidoyl-naphthalen-2-ylmethyl)-6-[1...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2cc(C(=O)NC(CC(O)=O)C(O)=O)n(Cc3ccc4ccc(cc4c3)C(N)=N)c2c1 Show InChI InChI=1S/C32H34N6O6/c1-18(33)37-10-8-24(9-11-37)44-25-7-6-21-14-28(31(41)36-26(32(42)43)16-29(39)40)38(27(21)15-25)17-19-2-3-20-4-5-22(30(34)35)13-23(20)12-19/h2-7,12-15,24,26,33H,8-11,16-17H2,1H3,(H3,34,35)(H,36,41)(H,39,40)(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50088924

(1-(7-Carbamimidoyl-naphthalen-2-ylmethyl)-6-[1-(1-...)Show SMILES CCOC(=O)c1cc2ccc(OC3CCN(CC3)C(C)=N)cc2n1Cc1ccc2ccc(cc2c1)C(N)=N Show InChI InChI=1S/C30H33N5O3/c1-3-37-30(36)28-16-22-8-9-26(38-25-10-12-34(13-11-25)19(2)31)17-27(22)35(28)18-20-4-5-21-6-7-23(29(32)33)15-24(21)14-20/h4-9,14-17,25,31H,3,10-13,18H2,1-2H3,(H3,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against factor Xa (FXa) |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50077181
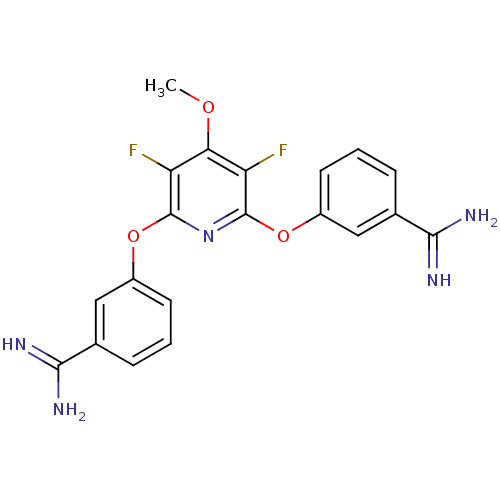
(3-{6-[3-amino(imino)methylphenoxy]-3,5-difluoro-4-...)Show SMILES COc1c(F)c(Oc2cccc(c2)C(N)=N)nc(Oc2cccc(c2)C(N)=N)c1F Show InChI InChI=1S/C20H17F2N5O3/c1-28-16-14(21)19(29-12-6-2-4-10(8-12)17(23)24)27-20(15(16)22)30-13-7-3-5-11(9-13)18(25)26/h2-9H,1H3,(H3,23,24)(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066640
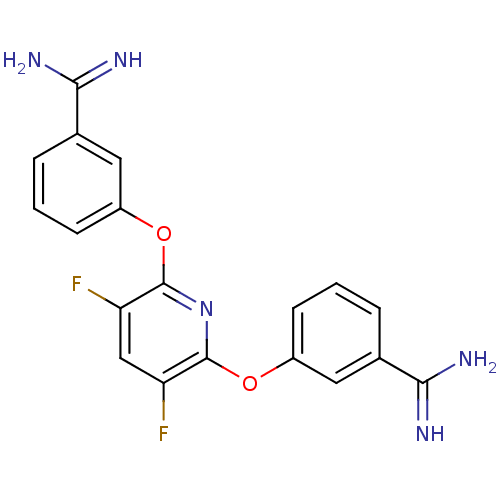
(3-{6-[3-amino(imino)methylphenoxy]-3,5-difluoro-2-...)Show SMILES NC(=N)c1cccc(Oc2nc(Oc3cccc(c3)C(N)=N)c(F)cc2F)c1 Show InChI InChI=1S/C19H15F2N5O2/c20-14-9-15(21)19(28-13-6-2-4-11(8-13)17(24)25)26-18(14)27-12-5-1-3-10(7-12)16(22)23/h1-9H,(H3,22,23)(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50088921

(2-({1-(7-Carbamimidoyl-naphthalen-2-ylmethyl)-6-[1...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2cc(C(=O)NC(CC(O)=O)C(O)=O)n(Cc3ccc4ccc(cc4c3)C(N)=N)c2c1 Show InChI InChI=1S/C32H34N6O6/c1-18(33)37-10-8-24(9-11-37)44-25-7-6-21-14-28(31(41)36-26(32(42)43)16-29(39)40)38(27(21)15-25)17-19-2-3-20-4-5-22(30(34)35)13-23(20)12-19/h2-7,12-15,24,26,33H,8-11,16-17H2,1H3,(H3,34,35)(H,36,41)(H,39,40)(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50088926
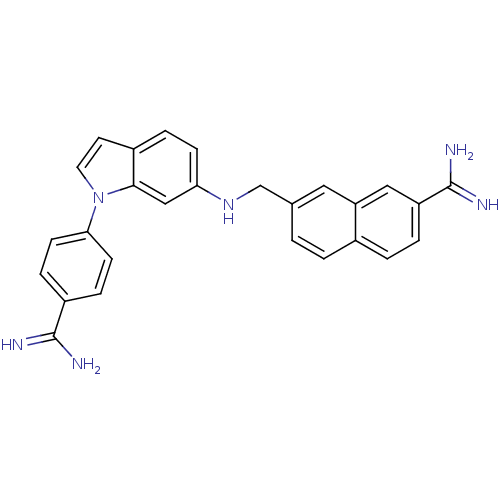
(7-{[1-(4-Carbamimidoyl-phenyl)-1H-indol-6-ylamino]...)Show SMILES NC(=N)c1ccc(cc1)-n1ccc2ccc(NCc3ccc4ccc(cc4c3)C(N)=N)cc12 Show InChI InChI=1S/C27H24N6/c28-26(29)20-6-9-24(10-7-20)33-12-11-19-5-8-23(15-25(19)33)32-16-17-1-2-18-3-4-21(27(30)31)14-22(18)13-17/h1-15,32H,16H2,(H3,28,29)(H3,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50088920

(7-[2-(Piperidin-4-yloxy)-carbazol-9-ylmethyl]-naph...)Show SMILES NC(=N)c1ccc2ccc(Cn3c4ccccc4c4ccc(OC5CCNCC5)cc34)cc2c1 Show InChI InChI=1S/C29H28N4O/c30-29(31)21-8-7-20-6-5-19(15-22(20)16-21)18-33-27-4-2-1-3-25(27)26-10-9-24(17-28(26)33)34-23-11-13-32-14-12-23/h1-10,15-17,23,32H,11-14,18H2,(H3,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50077169

(2,6-Bis-(3-carbamimidoyl-phenoxy)-N,N-dimethyl-nic...)Show SMILES CN(C)C(=O)c1ccc(Oc2cccc(c2)C(N)=N)nc1Oc1cccc(c1)C(N)=N Show InChI InChI=1S/C22H22N6O3/c1-28(2)22(29)17-9-10-18(30-15-7-3-5-13(11-15)19(23)24)27-21(17)31-16-8-4-6-14(12-16)20(25)26/h3-12H,1-2H3,(H3,23,24)(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM17277

((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 33 | -42.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine protease 1
(Bos taurus (bovine)) | BDBM50066614

((E,E)-2,7-Bis(4-amidinobenzylidine)cycloheptan-1-o...)Show SMILES NC(=N)c1ccc(\C=C2/CCCC\C(=C/c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13+,20-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against bovine trypsin. |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50077184
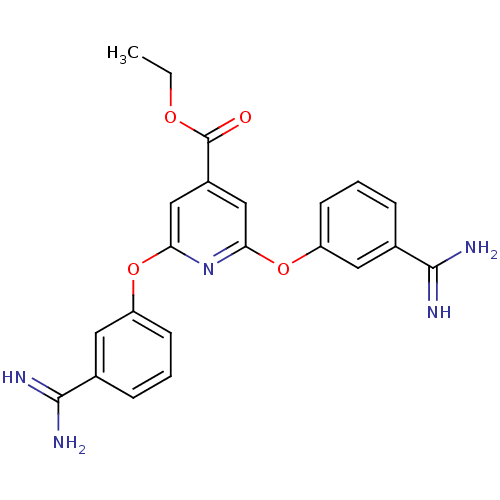
(2,6-Bis-(3-carbamimidoyl-phenoxy)-isonicotinic aci...)Show SMILES CCOC(=O)c1cc(Oc2cccc(c2)C(N)=N)nc(Oc2cccc(c2)C(N)=N)c1 Show InChI InChI=1S/C22H21N5O4/c1-2-29-22(28)15-11-18(30-16-7-3-5-13(9-16)20(23)24)27-19(12-15)31-17-8-4-6-14(10-17)21(25)26/h3-12H,2H2,1H3,(H3,23,24)(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM17281

(7-({2-[(1-ethanimidoylpiperidin-4-yl)oxy]-9H-carba...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2c(c1)n(Cc1ccc3ccc(cc3c1)C(N)=N)c1ccccc21 Show InChI InChI=1S/C31H31N5O/c1-20(32)35-14-12-25(13-15-35)37-26-10-11-28-27-4-2-3-5-29(27)36(30(28)18-26)19-21-6-7-22-8-9-23(31(33)34)17-24(22)16-21/h2-11,16-18,25,32H,12-15,19H2,1H3,(H3,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 36 | -42.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM17281

(7-({2-[(1-ethanimidoylpiperidin-4-yl)oxy]-9H-carba...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2c(c1)n(Cc1ccc3ccc(cc3c1)C(N)=N)c1ccccc21 Show InChI InChI=1S/C31H31N5O/c1-20(32)35-14-12-25(13-15-35)37-26-10-11-28-27-4-2-3-5-29(27)36(30(28)18-26)19-21-6-7-22-8-9-23(31(33)34)17-24(22)16-21/h2-11,16-18,25,32H,12-15,19H2,1H3,(H3,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor II. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM17281

(7-({2-[(1-ethanimidoylpiperidin-4-yl)oxy]-9H-carba...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2c(c1)n(Cc1ccc3ccc(cc3c1)C(N)=N)c1ccccc21 Show InChI InChI=1S/C31H31N5O/c1-20(32)35-14-12-25(13-15-35)37-26-10-11-28-27-4-2-3-5-29(27)36(30(28)18-26)19-21-6-7-22-8-9-23(31(33)34)17-24(22)16-21/h2-11,16-18,25,32H,12-15,19H2,1H3,(H3,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50077168
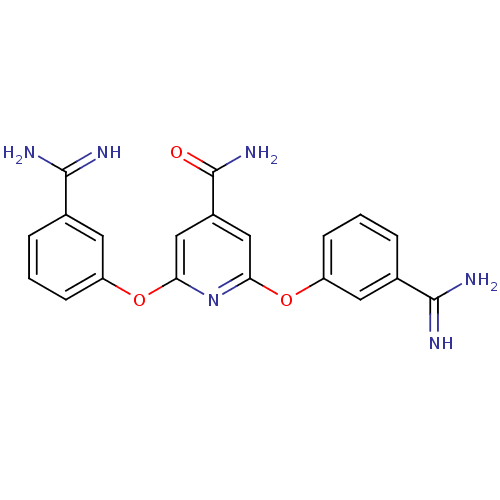
(2,6-Bis-(3-carbamimidoyl-phenoxy)-isonicotinamide ...)Show SMILES NC(=N)c1cccc(Oc2cc(cc(Oc3cccc(c3)C(N)=N)n2)C(N)=O)c1 Show InChI InChI=1S/C20H18N6O3/c21-18(22)11-3-1-5-14(7-11)28-16-9-13(20(25)27)10-17(26-16)29-15-6-2-4-12(8-15)19(23)24/h1-10H,(H3,21,22)(H3,23,24)(H2,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50088918

(2-[9-(7-Carbamimidoyl-naphthalen-2-ylmethyl)-9H-ca...)Show SMILES CN(C)C(=O)COc1ccc2c(c1)n(Cc1ccc3ccc(cc3c1)C(N)=N)c1ccccc21 Show InChI InChI=1S/C28H26N4O2/c1-31(2)27(33)17-34-22-11-12-24-23-5-3-4-6-25(23)32(26(24)15-22)16-18-7-8-19-9-10-20(28(29)30)14-21(19)13-18/h3-15H,16-17H2,1-2H3,(H3,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor II. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50077185
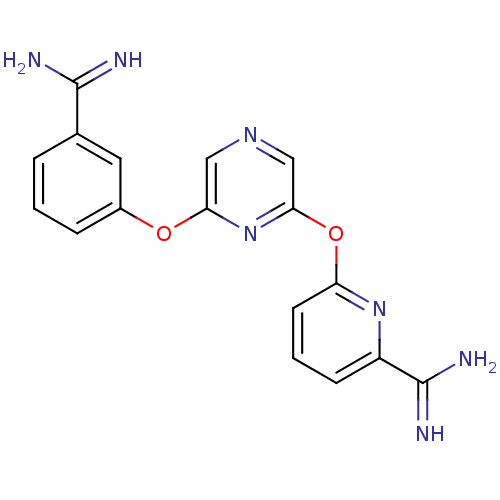
(6-[6-(3-Carbamimidoyl-phenoxy)-pyrazin-2-yloxy]-py...)Show SMILES NC(=N)c1cccc(Oc2cncc(Oc3cccc(n3)C(N)=N)n2)c1 Show InChI InChI=1S/C17H15N7O2/c18-16(19)10-3-1-4-11(7-10)25-14-8-22-9-15(24-14)26-13-6-2-5-12(23-13)17(20)21/h1-9H,(H3,18,19)(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50077172
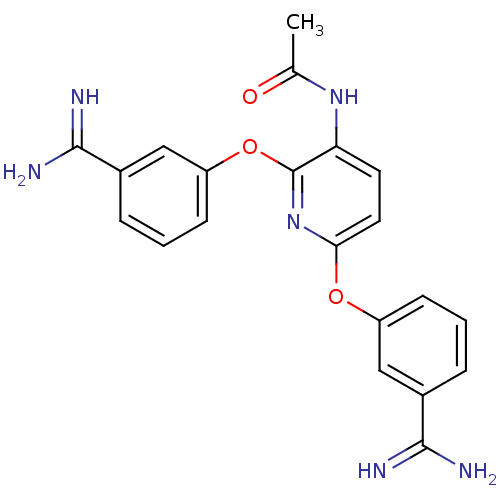
(CHEMBL49295 | N-[2,6-Bis-(3-carbamimidoyl-phenoxy)...)Show SMILES CC(=O)Nc1ccc(Oc2cccc(c2)C(N)=N)nc1Oc1cccc(c1)C(N)=N Show InChI InChI=1S/C21H20N6O3/c1-12(28)26-17-8-9-18(29-15-6-2-4-13(10-15)19(22)23)27-21(17)30-16-7-3-5-14(11-16)20(24)25/h2-11H,1H3,(H3,22,23)(H3,24,25)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50077186
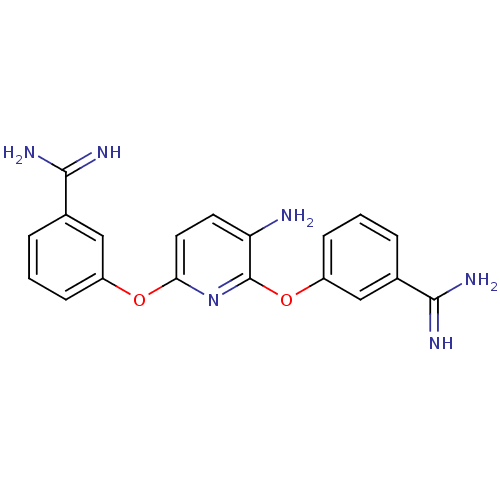
(2,6-di[3-amino(imino)methylphenoxy]-3-pyridinamine...)Show SMILES NC(=N)c1cccc(Oc2ccc(N)c(Oc3cccc(c3)C(N)=N)n2)c1 Show InChI InChI=1S/C19H18N6O2/c20-15-7-8-16(26-13-5-1-3-11(9-13)17(21)22)25-19(15)27-14-6-2-4-12(10-14)18(23)24/h1-10H,20H2,(H3,21,22)(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066626
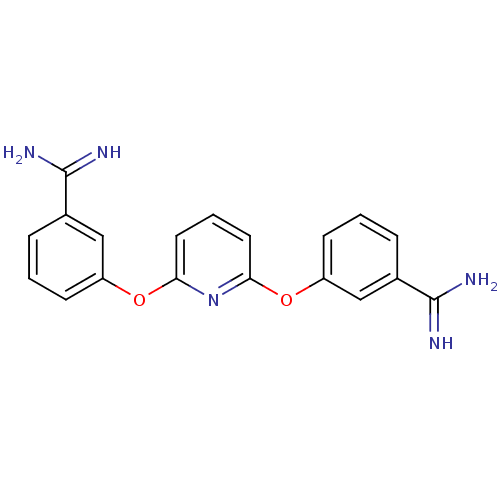
(3-{6-[3-amino(imino)methylphenoxy]-2-pyridyloxy}ph...)Show SMILES NC(=N)c1cccc(Oc2cccc(Oc3cccc(c3)C(N)=N)n2)c1 Show InChI InChI=1S/C19H17N5O2/c20-18(21)12-4-1-6-14(10-12)25-16-8-3-9-17(24-16)26-15-7-2-5-13(11-15)19(22)23/h1-11H,(H3,20,21)(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066620

(2,6-Bis-(3-carbamimidoyl-phenoxy)-isonicotinic aci...)Show SMILES NC(=N)c1cccc(Oc2cc(cc(Oc3cccc(c3)C(N)=N)n2)C(O)=O)c1 Show InChI InChI=1S/C20H17N5O4/c21-18(22)11-3-1-5-14(7-11)28-16-9-13(20(26)27)10-17(25-16)29-15-6-2-4-12(8-15)19(23)24/h1-10H,(H3,21,22)(H3,23,24)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50077171

(2,6-di[3-amino-N-methyl carboxamide-(imino)methylp...)Show SMILES CNC(=O)Nc1ccc(Oc2cccc(c2)C(N)=N)nc1Oc1cccc(c1)C(N)=N Show InChI InChI=1S/C21H21N7O3/c1-26-21(29)27-16-8-9-17(30-14-6-2-4-12(10-14)18(22)23)28-20(16)31-15-7-3-5-13(11-15)19(24)25/h2-11H,1H3,(H3,22,23)(H3,24,25)(H2,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50088925

(7-[9-(4-Carbamimidoyl-phenyl)-9H-carbazol-2-yloxym...)Show SMILES NC(=N)c1ccc(cc1)-n1c2ccccc2c2ccc(OCc3ccc4ccc(cc4c3)C(N)=N)cc12 Show InChI InChI=1S/C31H25N5O/c32-30(33)21-9-11-24(12-10-21)36-28-4-2-1-3-26(28)27-14-13-25(17-29(27)36)37-18-19-5-6-20-7-8-22(31(34)35)16-23(20)15-19/h1-17H,18H2,(H3,32,33)(H3,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50077180

(2,6-Bis-(3-carbamimidoyl-phenoxy)-N-methyl-isonico...)Show SMILES CNC(=O)c1cc(Oc2cccc(c2)C(N)=N)nc(Oc2cccc(c2)C(N)=N)c1 Show InChI InChI=1S/C21H20N6O3/c1-26-21(28)14-10-17(29-15-6-2-4-12(8-15)19(22)23)27-18(11-14)30-16-7-3-5-13(9-16)20(24)25/h2-11H,1H3,(H3,22,23)(H3,24,25)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50088932

(1-(7-Carbamimidoyl-naphthalen-2-ylmethyl)-6-[1-(1-...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2cc(C(O)=O)n(Cc3ccc4ccc(cc4c3)C(N)=N)c2c1 Show InChI InChI=1S/C28H29N5O3/c1-17(29)32-10-8-23(9-11-32)36-24-7-6-20-14-26(28(34)35)33(25(20)15-24)16-18-2-3-19-4-5-21(27(30)31)13-22(19)12-18/h2-7,12-15,23,29H,8-11,16H2,1H3,(H3,30,31)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50077170

(2,6-di[3-amino-phenyl carboxamide-(imino)methylphe...)Show SMILES NC(=N)c1cccc(Oc2ccc(NC(=O)Nc3ccccc3)c(Oc3cccc(c3)C(N)=N)n2)c1 Show InChI InChI=1S/C26H23N7O3/c27-23(28)16-6-4-10-19(14-16)35-22-13-12-21(32-26(34)31-18-8-2-1-3-9-18)25(33-22)36-20-11-5-7-17(15-20)24(29)30/h1-15H,(H3,27,28)(H3,29,30)(H2,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition activity against Coagulation factor X |
J Med Chem 42: 1749-56 (1999)
Article DOI: 10.1021/jm980667k
BindingDB Entry DOI: 10.7270/Q2FX78NF |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50088917

(7-[2-(3-Carbamimidoyl-phenoxy)-carbazol-9-ylmethyl...)Show SMILES NC(=N)c1cccc(Oc2ccc3c(c2)n(Cc2ccc4ccc(cc4c2)C(N)=N)c2ccccc32)c1 Show InChI InChI=1S/C31H25N5O/c32-30(33)21-4-3-5-24(16-21)37-25-12-13-27-26-6-1-2-7-28(26)36(29(27)17-25)18-19-8-9-20-10-11-22(31(34)35)15-23(20)14-19/h1-17H,18H2,(H3,32,33)(H3,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17279

(2-(5-carbamimidoyl-2-hydroxyphenoxy)-6-[3-(4,5-dih...)Show SMILES NC(=N)c1ccc(O)c(Oc2cc(cc(Oc3cccc(c3)C3=NCCN3)n2)C(O)=O)c1 |t:23| Show InChI InChI=1S/C22H19N5O5/c23-20(24)12-4-5-16(28)17(9-12)32-19-11-14(22(29)30)10-18(27-19)31-15-3-1-2-13(8-15)21-25-6-7-26-21/h1-5,8-11,28H,6-7H2,(H3,23,24)(H,25,26)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 85 | -40.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50088918

(2-[9-(7-Carbamimidoyl-naphthalen-2-ylmethyl)-9H-ca...)Show SMILES CN(C)C(=O)COc1ccc2c(c1)n(Cc1ccc3ccc(cc3c1)C(N)=N)c1ccccc21 Show InChI InChI=1S/C28H26N4O2/c1-31(2)27(33)17-34-22-11-12-24-23-5-3-4-6-25(23)32(26(24)15-22)16-18-7-8-19-9-10-20(28(29)30)14-21(19)13-18/h3-15H,16-17H2,1-2H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin. |
Bioorg Med Chem Lett 10: 957-61 (2000)
BindingDB Entry DOI: 10.7270/Q2MG7NRD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data