 Found 128 hits with Last Name = 'krief' and Initial = 's'
Found 128 hits with Last Name = 'krief' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492784
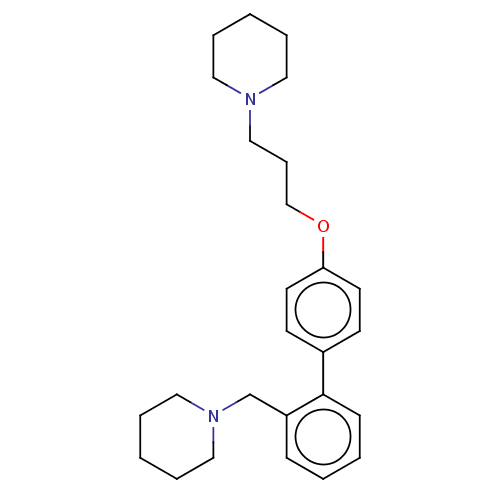
(CHEMBL2413837)Show InChI InChI=1S/C26H36N2O/c1-5-16-27(17-6-1)20-9-21-29-25-14-12-23(13-15-25)26-11-4-3-10-24(26)22-28-18-7-2-8-19-28/h3-4,10-15H,1-2,5-9,16-22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492781
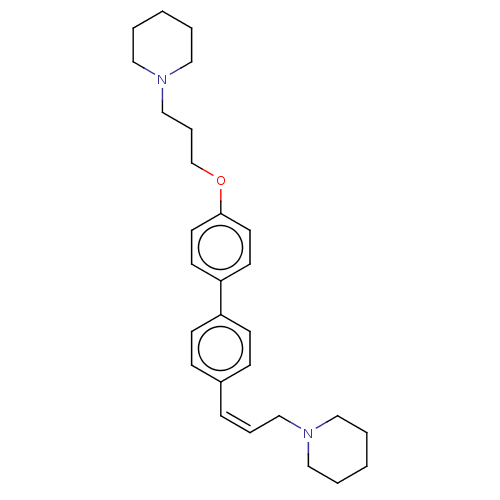
(CHEMBL2413835)Show SMILES C(COc1ccc(cc1)-c1ccc(\C=C/CN2CCCCC2)cc1)CN1CCCCC1 Show InChI InChI=1S/C28H38N2O/c1-3-18-29(19-4-1)22-7-9-25-10-12-26(13-11-25)27-14-16-28(17-15-27)31-24-8-23-30-20-5-2-6-21-30/h7,9-17H,1-6,8,18-24H2/b9-7- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492783
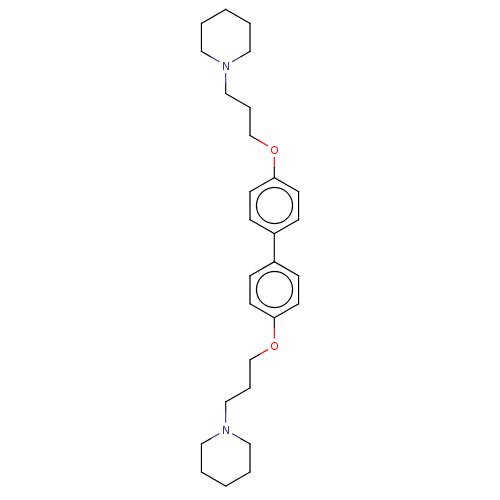
(CHEMBL2413824)Show SMILES C(COc1ccc(cc1)-c1ccc(OCCCN2CCCCC2)cc1)CN1CCCCC1 Show InChI InChI=1S/C28H40N2O2/c1-3-17-29(18-4-1)21-7-23-31-27-13-9-25(10-14-27)26-11-15-28(16-12-26)32-24-8-22-30-19-5-2-6-20-30/h9-16H,1-8,17-24H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492772

(CHEMBL2413828)Show SMILES C(CCN1CCCCC1)COc1ccc(cc1)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C29H42N2O2/c1-3-18-30(19-4-1)22-7-8-24-32-28-14-10-26(11-15-28)27-12-16-29(17-13-27)33-25-9-23-31-20-5-2-6-21-31/h10-17H,1-9,18-25H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492769

(CHEMBL2413836)Show SMILES C(COc1ccc(cc1)-c1ccc(CCCN2CCCCC2)cc1)CN1CCCCC1 Show InChI InChI=1S/C28H40N2O/c1-3-18-29(19-4-1)22-7-9-25-10-12-26(13-11-25)27-14-16-28(17-15-27)31-24-8-23-30-20-5-2-6-21-30/h10-17H,1-9,18-24H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492780
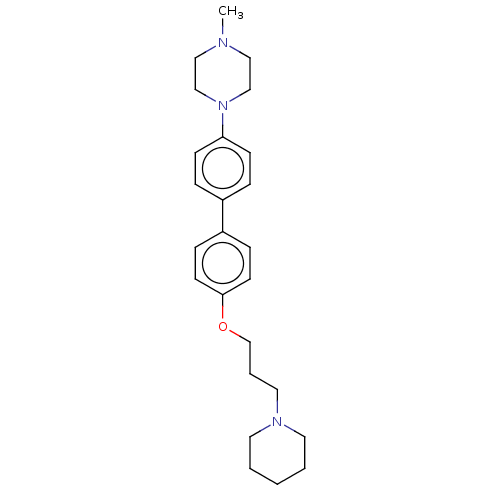
(CHEMBL2413833)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C25H35N3O/c1-26-17-19-28(20-18-26)24-10-6-22(7-11-24)23-8-12-25(13-9-23)29-21-5-16-27-14-3-2-4-15-27/h6-13H,2-5,14-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186290
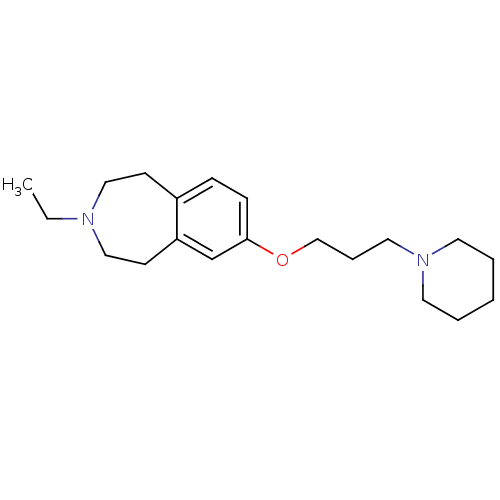
(3-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...)Show InChI InChI=1S/C20H32N2O/c1-2-21-14-9-18-7-8-20(17-19(18)10-15-21)23-16-6-13-22-11-4-3-5-12-22/h7-8,17H,2-6,9-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H3 receptor (unknown origin) |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50412447
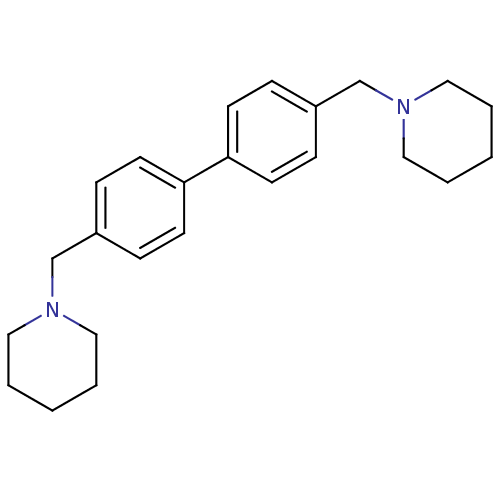
(CHEMBL380101)Show InChI InChI=1S/C24H32N2/c1-3-15-25(16-4-1)19-21-7-11-23(12-8-21)24-13-9-22(10-14-24)20-26-17-5-2-6-18-26/h7-14H,1-6,15-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H3 receptor (unknown origin) |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186309

(2-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...)Show InChI InChI=1S/C20H32N2O/c1-2-21-13-6-8-18-16-20(10-9-19(18)17-21)23-15-7-14-22-11-4-3-5-12-22/h9-10,16H,2-8,11-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H3 receptor (unknown origin) |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186269
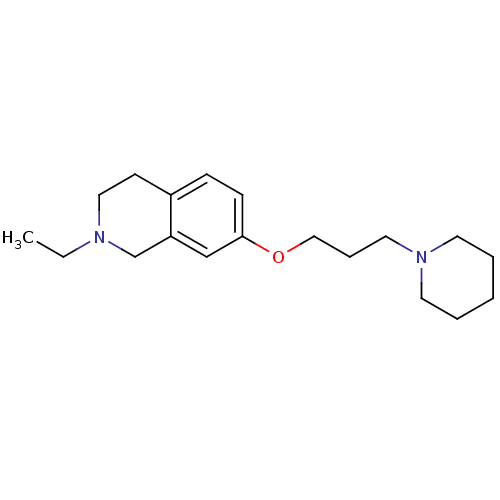
(2-ethyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-20-13-9-17-7-8-19(15-18(17)16-20)22-14-6-12-21-10-4-3-5-11-21/h7-8,15H,2-6,9-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H3 receptor (unknown origin) |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492782
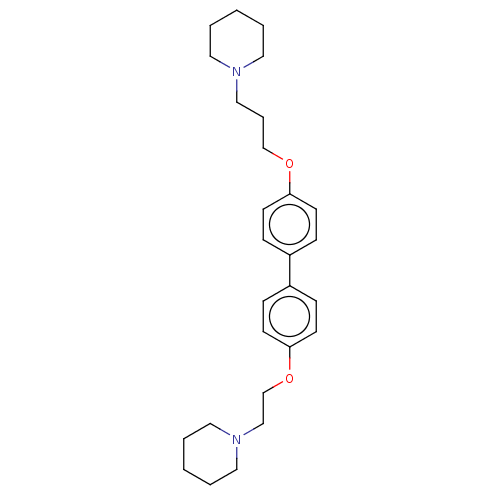
(CHEMBL2413829)Show SMILES C(COc1ccc(cc1)-c1ccc(OCCN2CCCCC2)cc1)CN1CCCCC1 Show InChI InChI=1S/C27H38N2O2/c1-3-16-28(17-4-1)20-7-22-30-26-12-8-24(9-13-26)25-10-14-27(15-11-25)31-23-21-29-18-5-2-6-19-29/h8-15H,1-7,16-23H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50259267
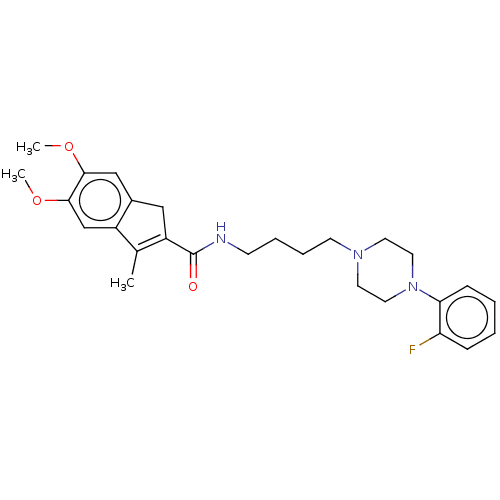
(CHEMBL4099779)Show SMILES COc1cc2CC(C(=O)NCCCCN3CCN(CC3)c3ccccc3F)=C(C)c2cc1OC |t:28| Show InChI InChI=1S/C27H34FN3O3/c1-19-21-18-26(34-3)25(33-2)17-20(21)16-22(19)27(32)29-10-6-7-11-30-12-14-31(15-13-30)24-9-5-4-8-23(24)28/h4-5,8-9,17-18H,6-7,10-16H2,1-3H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at D3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492774
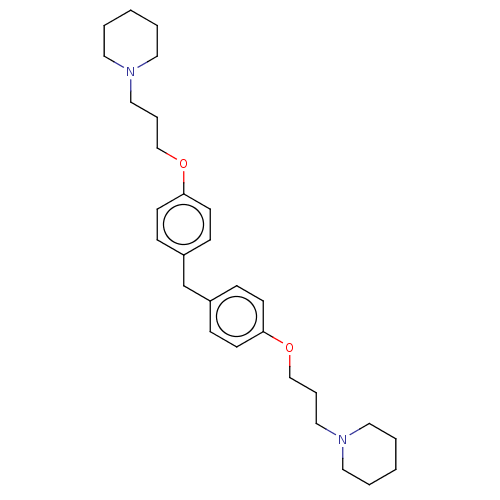
(CHEMBL2413825)Show SMILES C(COc1ccc(Cc2ccc(OCCCN3CCCCC3)cc2)cc1)CN1CCCCC1 Show InChI InChI=1S/C29H42N2O2/c1-3-17-30(18-4-1)21-7-23-32-28-13-9-26(10-14-28)25-27-11-15-29(16-12-27)33-24-8-22-31-19-5-2-6-20-31/h9-16H,1-8,17-25H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492773
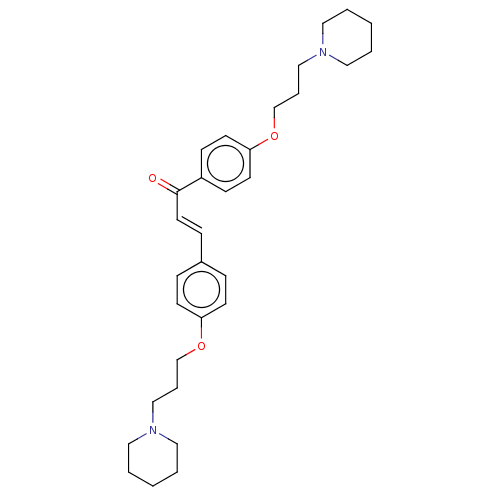
(CHEMBL2413827)Show SMILES O=C(\C=C\c1ccc(OCCCN2CCCCC2)cc1)c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C31H42N2O3/c34-31(28-12-16-30(17-13-28)36-26-8-24-33-21-5-2-6-22-33)18-11-27-9-14-29(15-10-27)35-25-7-23-32-19-3-1-4-20-32/h9-18H,1-8,19-26H2/b18-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186283
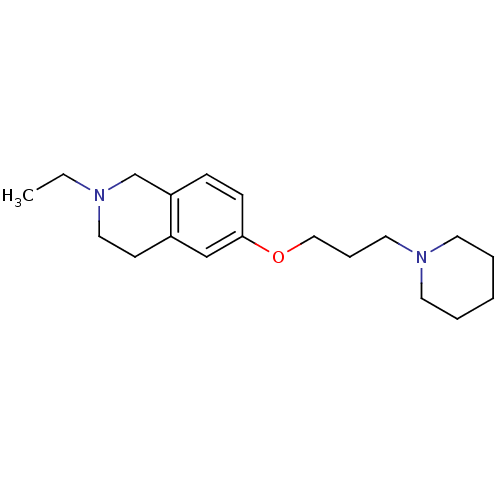
(2-ethyl-6-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-20-13-9-17-15-19(8-7-18(17)16-20)22-14-6-12-21-10-4-3-5-11-21/h7-8,15H,2-6,9-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H3 receptor (unknown origin) |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492768
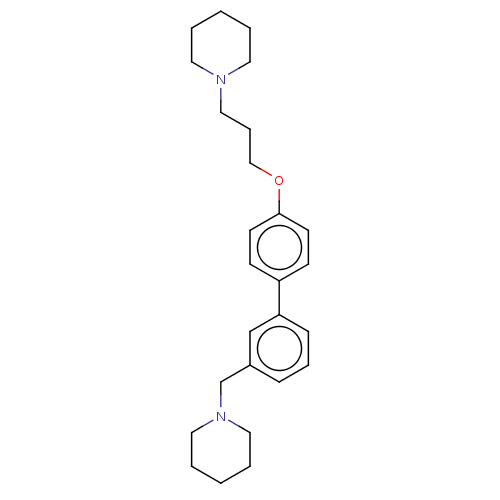
(CHEMBL2413838)Show SMILES C(COc1ccc(cc1)-c1cccc(CN2CCCCC2)c1)CN1CCCCC1 Show InChI InChI=1S/C26H36N2O/c1-3-15-27(16-4-1)19-8-20-29-26-13-11-24(12-14-26)25-10-7-9-23(21-25)22-28-17-5-2-6-18-28/h7,9-14,21H,1-6,8,15-20,22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.549 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50325744

(CHEMBL1223683 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Clc1cccc(N2CCN(CCCCNc3ccc4ccccc4n3)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4/c24-19-7-5-9-21(23(19)25)29-16-14-28(15-17-29)13-4-3-12-26-22-11-10-18-6-1-2-8-20(18)27-22/h1-2,5-11H,3-4,12-17H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at D3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492767
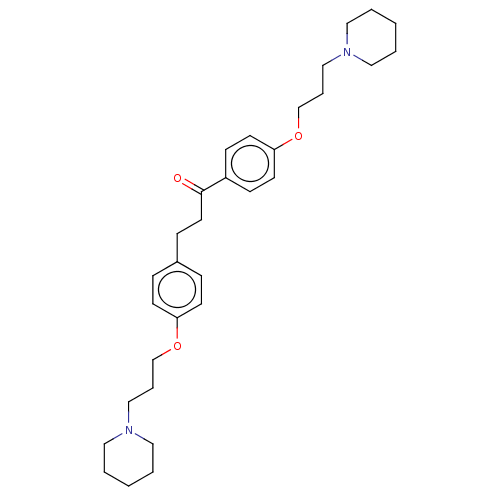
(CHEMBL2413839)Show SMILES O=C(CCc1ccc(OCCCN2CCCCC2)cc1)c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C31H44N2O3/c34-31(28-12-16-30(17-13-28)36-26-8-24-33-21-5-2-6-22-33)18-11-27-9-14-29(15-10-27)35-25-7-23-32-19-3-1-4-20-32/h9-10,12-17H,1-8,11,18-26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492770
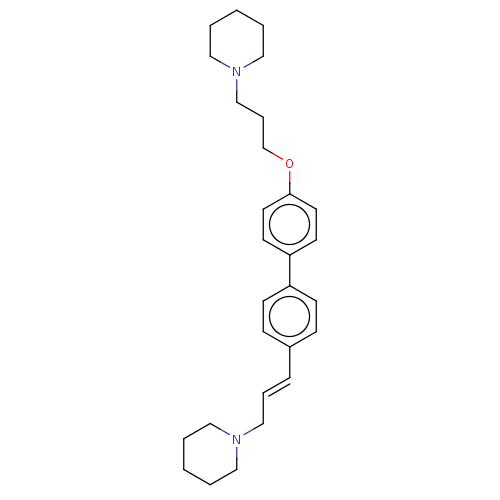
(CHEMBL2413832)Show SMILES C(COc1ccc(cc1)-c1ccc(\C=C\CN2CCCCC2)cc1)CN1CCCCC1 Show InChI InChI=1S/C28H38N2O/c1-3-18-29(19-4-1)22-7-9-25-10-12-26(13-11-25)27-14-16-28(17-15-27)31-24-8-23-30-20-5-2-6-21-30/h7,9-17H,1-6,8,18-24H2/b9-7+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.655 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492777
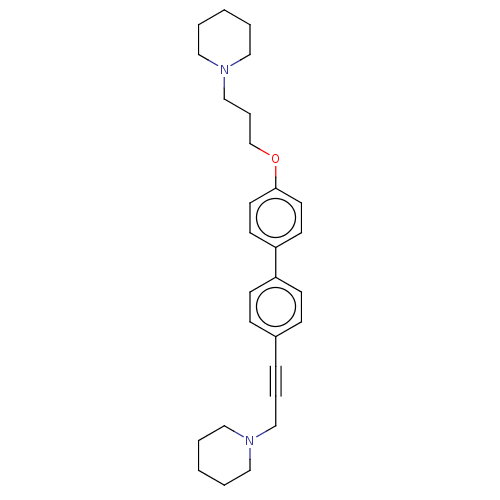
(CHEMBL2413834)Show SMILES C(COc1ccc(cc1)-c1ccc(cc1)C#CCN1CCCCC1)CN1CCCCC1 Show InChI InChI=1S/C28H36N2O/c1-3-18-29(19-4-1)22-7-9-25-10-12-26(13-11-25)27-14-16-28(17-15-27)31-24-8-23-30-20-5-2-6-21-30/h10-17H,1-6,8,18-24H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492766
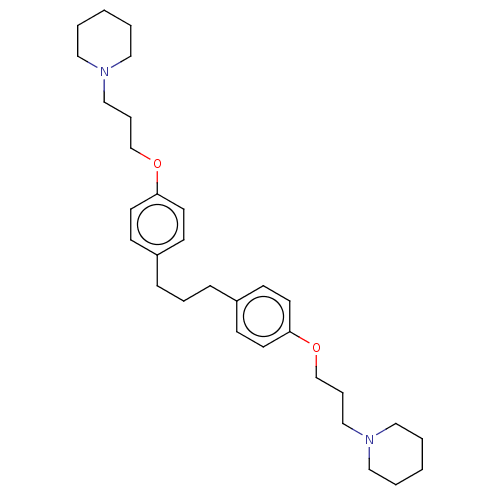
(CHEMBL2413840)Show SMILES C(COc1ccc(CCCc2ccc(OCCCN3CCCCC3)cc2)cc1)CN1CCCCC1 Show InChI InChI=1S/C31H46N2O2/c1-3-20-32(21-4-1)24-8-26-34-30-16-12-28(13-17-30)10-7-11-29-14-18-31(19-15-29)35-27-9-25-33-22-5-2-6-23-33/h12-19H,1-11,20-27H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50325746
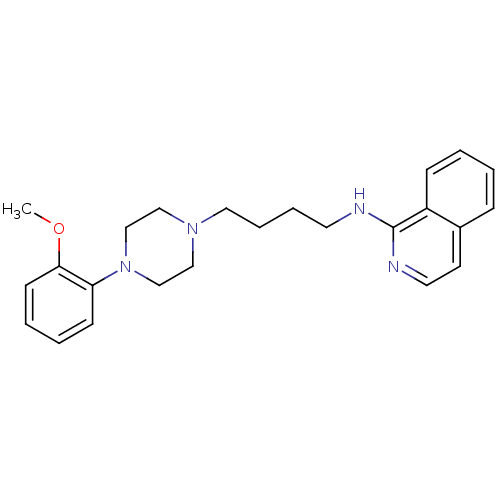
(CHEMBL1223748 | N-(4-(4-(2-methoxyphenyl)piperazin...)Show InChI InChI=1S/C24H30N4O/c1-29-23-11-5-4-10-22(23)28-18-16-27(17-19-28)15-7-6-13-25-24-21-9-3-2-8-20(21)12-14-26-24/h2-5,8-12,14H,6-7,13,15-19H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at D3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50325748

(CHEMBL1223750 | N-(4-(4-(3-(trifluoromethyl)phenyl...)Show SMILES FC(F)(F)c1cccc(c1)N1CCN(CCCCNc2nccc3ccccc23)CC1 Show InChI InChI=1S/C24H27F3N4/c25-24(26,27)20-7-5-8-21(18-20)31-16-14-30(15-17-31)13-4-3-11-28-23-22-9-2-1-6-19(22)10-12-29-23/h1-2,5-10,12,18H,3-4,11,13-17H2,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at D3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50412467

(CHEMBL378178)Show SMILES C(Cc1ccc(cc1)-c1ccc(CCN2CCCCC2)cc1)N1CCCCC1 Show InChI InChI=1S/C26H36N2/c1-3-17-27(18-4-1)21-15-23-7-11-25(12-8-23)26-13-9-24(10-14-26)16-22-28-19-5-2-6-20-28/h7-14H,1-6,15-22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H3 receptor (unknown origin) |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492778
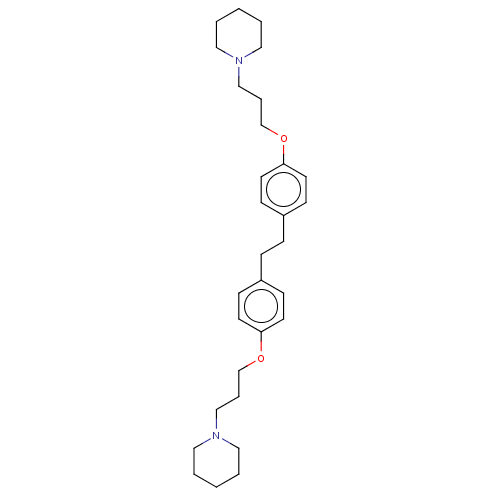
(CHEMBL2413826)Show SMILES C(COc1ccc(CCc2ccc(OCCCN3CCCCC3)cc2)cc1)CN1CCCCC1 Show InChI InChI=1S/C30H44N2O2/c1-3-19-31(20-4-1)23-7-25-33-29-15-11-27(12-16-29)9-10-28-13-17-30(18-14-28)34-26-8-24-32-21-5-2-6-22-32/h11-18H,1-10,19-26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492771
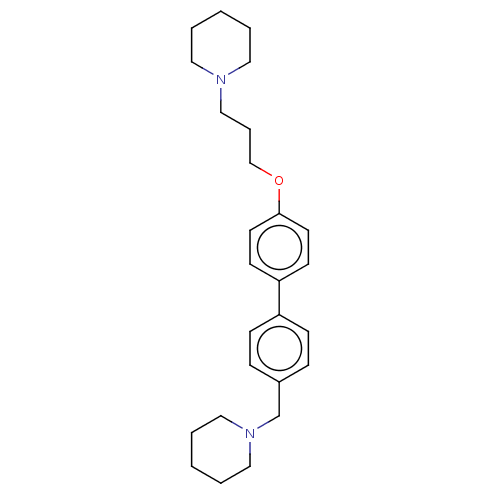
(CHEMBL2413831)Show SMILES C(COc1ccc(cc1)-c1ccc(CN2CCCCC2)cc1)CN1CCCCC1 Show InChI InChI=1S/C26H36N2O/c1-3-16-27(17-4-1)20-7-21-29-26-14-12-25(13-15-26)24-10-8-23(9-11-24)22-28-18-5-2-6-19-28/h8-15H,1-7,16-22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50121205

(CHEBI:18295 | Histamine)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H4 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 5263-5266 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.049
BindingDB Entry DOI: 10.7270/Q2Q52RMS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50412469
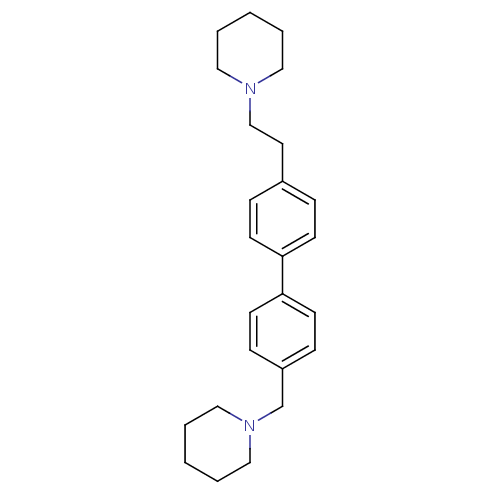
(CHEMBL459756)Show InChI InChI=1S/C25H34N2/c1-3-16-26(17-4-1)20-15-22-7-11-24(12-8-22)25-13-9-23(10-14-25)21-27-18-5-2-6-19-27/h7-14H,1-6,15-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H3 receptor (unknown origin) |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50237878
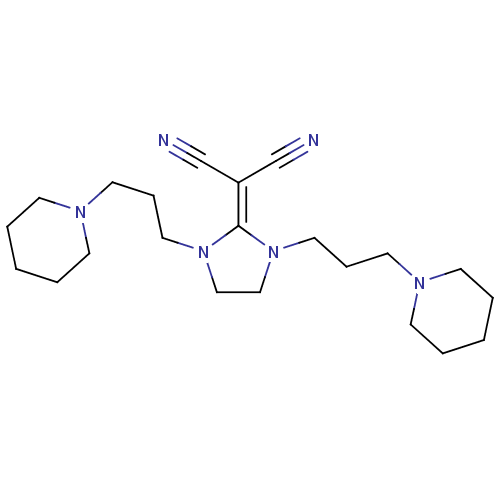
(2-(1,3-bis(3-(piperidin-1-yl)propyl)imidazolidin-2...)Show SMILES N#C\[#6](C#N)=[#6]-1\[#7](-[#6]-[#6]-[#6]-[#7]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2)-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1 Show InChI InChI=1S/C22H36N6/c23-19-21(20-24)22-27(15-7-13-25-9-3-1-4-10-25)17-18-28(22)16-8-14-26-11-5-2-6-12-26/h1-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H3 receptor (unknown origin) |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259291

(CHEMBL4102970)Show SMILES [O-][N+](=O)c1cccc(c1)C(OCCCc1c[nH]cn1)c1ccccc1 Show InChI InChI=1S/C19H19N3O3/c23-22(24)18-10-4-8-16(12-18)19(15-6-2-1-3-7-15)25-11-5-9-17-13-20-14-21-17/h1-4,6-8,10,12-14,19H,5,9,11H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259291

(CHEMBL4102970)Show SMILES [O-][N+](=O)c1cccc(c1)C(OCCCc1c[nH]cn1)c1ccccc1 Show InChI InChI=1S/C19H19N3O3/c23-22(24)18-10-4-8-16(12-18)19(15-6-2-1-3-7-15)25-11-5-9-17-13-20-14-21-17/h1-4,6-8,10,12-14,19H,5,9,11H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50259290
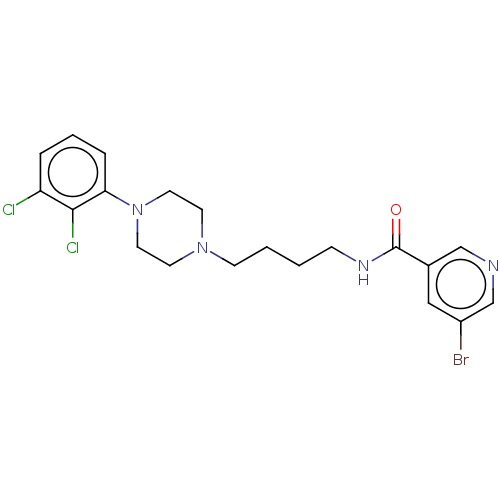
(CHEMBL4078910)Show SMILES Clc1cccc(N2CCN(CCCCNC(=O)c3cncc(Br)c3)CC2)c1Cl Show InChI InChI=1S/C20H23BrCl2N4O/c21-16-12-15(13-24-14-16)20(28)25-6-1-2-7-26-8-10-27(11-9-26)18-5-3-4-17(22)19(18)23/h3-5,12-14H,1-2,6-11H2,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at D3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259257

(CHEMBL4065099)Show InChI InChI=1S/C19H19IN2O/c20-17-10-8-16(9-11-17)19(15-5-2-1-3-6-15)23-12-4-7-18-13-21-14-22-18/h1-3,5-6,8-11,13-14,19H,4,7,12H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259257

(CHEMBL4065099)Show InChI InChI=1S/C19H19IN2O/c20-17-10-8-16(9-11-17)19(15-5-2-1-3-6-15)23-12-4-7-18-13-21-14-22-18/h1-3,5-6,8-11,13-14,19H,4,7,12H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50325747

(CHEMBL1223749 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Clc1cccc(N2CCN(CCCCNc3nccc4ccccc34)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4/c24-20-8-5-9-21(22(20)25)29-16-14-28(15-17-29)13-4-3-11-26-23-19-7-2-1-6-18(19)10-12-27-23/h1-2,5-10,12H,3-4,11,13-17H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at D3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259258
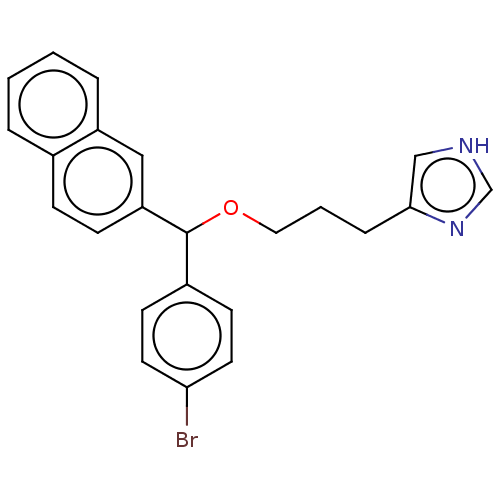
(CHEMBL4098585)Show SMILES Brc1ccc(cc1)C(OCCCc1c[nH]cn1)c1ccc2ccccc2c1 Show InChI InChI=1S/C23H21BrN2O/c24-21-11-9-18(10-12-21)23(27-13-3-6-22-15-25-16-26-22)20-8-7-17-4-1-2-5-19(17)14-20/h1-2,4-5,7-12,14-16,23H,3,6,13H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259258

(CHEMBL4098585)Show SMILES Brc1ccc(cc1)C(OCCCc1c[nH]cn1)c1ccc2ccccc2c1 Show InChI InChI=1S/C23H21BrN2O/c24-21-11-9-18(10-12-21)23(27-13-3-6-22-15-25-16-26-22)20-8-7-17-4-1-2-5-19(17)14-20/h1-2,4-5,7-12,14-16,23H,3,6,13H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50259268

(CHEMBL4084820)Show InChI InChI=1S/C21H30N4O/c22-19-7-10-21(24-17-19)23-12-11-18-5-8-20(9-6-18)26-16-4-15-25-13-2-1-3-14-25/h5-10,17H,1-4,11-16,22H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50325747

(CHEMBL1223749 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Clc1cccc(N2CCN(CCCCNc3nccc4ccccc34)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4/c24-20-8-5-9-21(22(20)25)29-16-14-28(15-17-29)13-4-3-11-26-23-19-7-2-1-6-18(19)10-12-27-23/h1-2,5-10,12H,3-4,11,13-17H2,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50325747

(CHEMBL1223749 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Clc1cccc(N2CCN(CCCCNc3nccc4ccccc34)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4/c24-20-8-5-9-21(22(20)25)29-16-14-28(15-17-29)13-4-3-11-26-23-19-7-2-1-6-18(19)10-12-27-23/h1-2,5-10,12H,3-4,11,13-17H2,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50325748

(CHEMBL1223750 | N-(4-(4-(3-(trifluoromethyl)phenyl...)Show SMILES FC(F)(F)c1cccc(c1)N1CCN(CCCCNc2nccc3ccccc23)CC1 Show InChI InChI=1S/C24H27F3N4/c25-24(26,27)20-7-5-8-21(18-20)31-16-14-30(15-17-31)13-4-3-11-28-23-22-9-2-1-6-19(22)10-12-29-23/h1-2,5-10,12,18H,3-4,11,13-17H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50325748

(CHEMBL1223750 | N-(4-(4-(3-(trifluoromethyl)phenyl...)Show SMILES FC(F)(F)c1cccc(c1)N1CCN(CCCCNc2nccc3ccccc23)CC1 Show InChI InChI=1S/C24H27F3N4/c25-24(26,27)20-7-5-8-21(18-20)31-16-14-30(15-17-31)13-4-3-11-28-23-22-9-2-1-6-19(22)10-12-29-23/h1-2,5-10,12,18H,3-4,11,13-17H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50237871
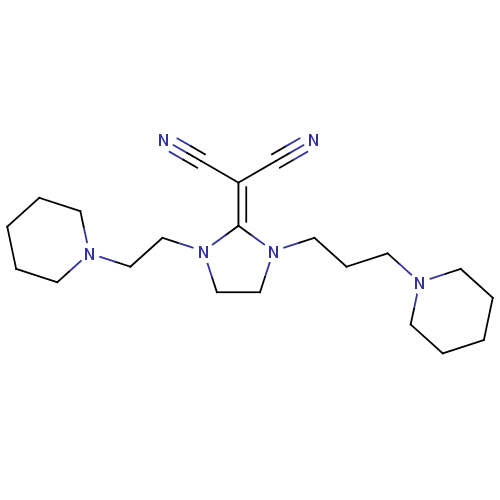
(2-(1-(2-(piperidin-1-yl)ethyl)-3-(3-(piperidin-1-y...)Show SMILES N#C\[#6](C#N)=[#6]-1/[#7](-[#6]-[#6]-[#6]-[#7]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2)-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1 Show InChI InChI=1S/C21H34N6/c22-18-20(19-23)21-26(13-7-12-24-8-3-1-4-9-24)16-17-27(21)15-14-25-10-5-2-6-11-25/h1-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H3 receptor (unknown origin) |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from recombinant human histamine H3 receptor stably transfected in HEK-293T cells membrane incubated for 9... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112150
BindingDB Entry DOI: 10.7270/Q2C53QGV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50259293
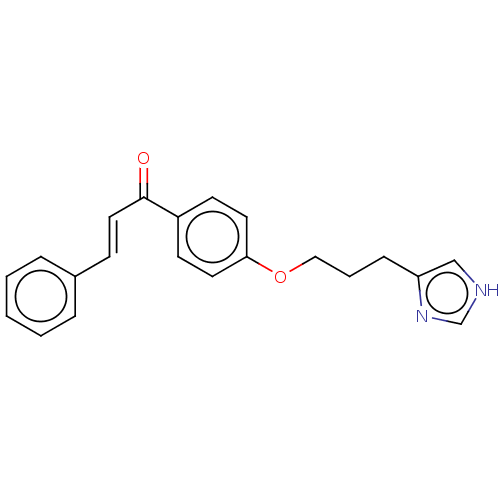
(CHEMBL4063170)Show InChI InChI=1S/C21H20N2O2/c24-21(13-8-17-5-2-1-3-6-17)18-9-11-20(12-10-18)25-14-4-7-19-15-22-16-23-19/h1-3,5-6,8-13,15-16H,4,7,14H2,(H,22,23)/b13-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50492776
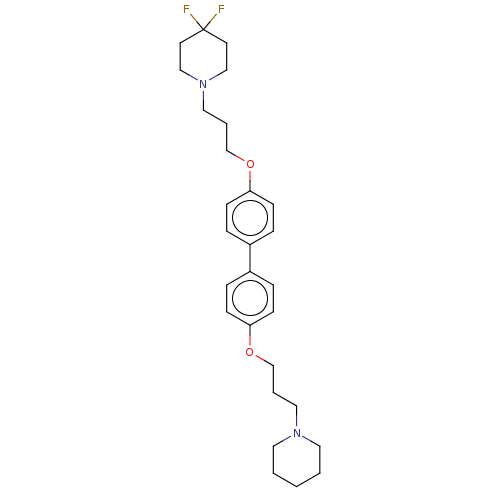
(CHEMBL2413830)Show SMILES FC1(F)CCN(CCCOc2ccc(cc2)-c2ccc(OCCCN3CCCCC3)cc2)CC1 Show InChI InChI=1S/C28H38F2N2O2/c29-28(30)14-20-32(21-15-28)19-5-23-34-27-12-8-25(9-13-27)24-6-10-26(11-7-24)33-22-4-18-31-16-2-1-3-17-31/h6-13H,1-5,14-23H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells |
Bioorg Med Chem 21: 4526-9 (2013)
Article DOI: 10.1016/j.bmc.2013.05.035
BindingDB Entry DOI: 10.7270/Q2J9699Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50259291

(CHEMBL4102970)Show SMILES [O-][N+](=O)c1cccc(c1)C(OCCCc1c[nH]cn1)c1ccccc1 Show InChI InChI=1S/C19H19N3O3/c23-22(24)18-10-4-8-16(12-18)19(15-6-2-1-3-7-15)25-11-5-9-17-13-20-14-21-17/h1-4,6-8,10,12-14,19H,5,9,11H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50325744

(CHEMBL1223683 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Clc1cccc(N2CCN(CCCCNc3ccc4ccccc4n3)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4/c24-19-7-5-9-21(23(19)25)29-16-14-28(15-17-29)13-4-3-12-26-22-11-10-18-6-1-2-8-20(18)27-22/h1-2,5-11H,3-4,12-17H2,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259293

(CHEMBL4063170)Show InChI InChI=1S/C21H20N2O2/c24-21(13-8-17-5-2-1-3-6-17)18-9-11-20(12-10-18)25-14-4-7-19-15-22-16-23-19/h1-3,5-6,8-13,15-16H,4,7,14H2,(H,22,23)/b13-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259293

(CHEMBL4063170)Show InChI InChI=1S/C21H20N2O2/c24-21(13-8-17-5-2-1-3-6-17)18-9-11-20(12-10-18)25-14-4-7-19-15-22-16-23-19/h1-3,5-6,8-13,15-16H,4,7,14H2,(H,22,23)/b13-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data