 Found 13 hits with Last Name = 'krysiak' and Initial = 'k'
Found 13 hits with Last Name = 'krysiak' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50225571
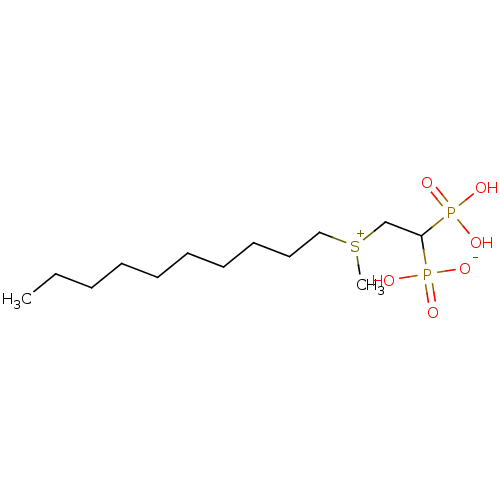
(2-(methyl, decylsulfonium-1-yl)ethylidene-1,1-bisp...)Show InChI InChI=1S/C13H30O6P2S/c1-3-4-5-6-7-8-9-10-11-22(2)12-13(20(14,15)16)21(17,18)19/h13H,3-12H2,1-2H3,(H3-,14,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25272
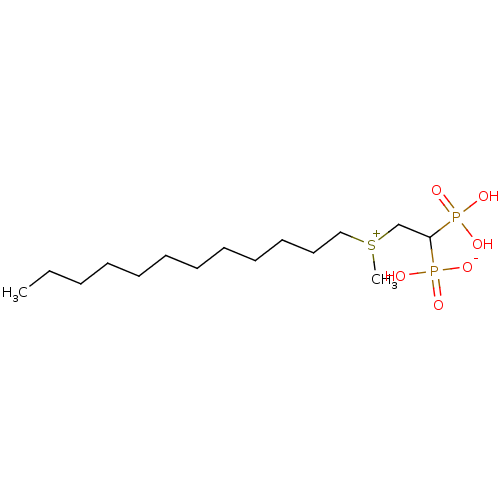
(CHEMBL238046 | bisphosphonate, 25 | hydrogen {2-[d...)Show SMILES CCCCCCCCCCCC[S+](C)CC(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C15H34O6P2S/c1-3-4-5-6-7-8-9-10-11-12-13-24(2)14-15(22(16,17)18)23(19,20)21/h15H,3-14H2,1-2H3,(H3-,16,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25287
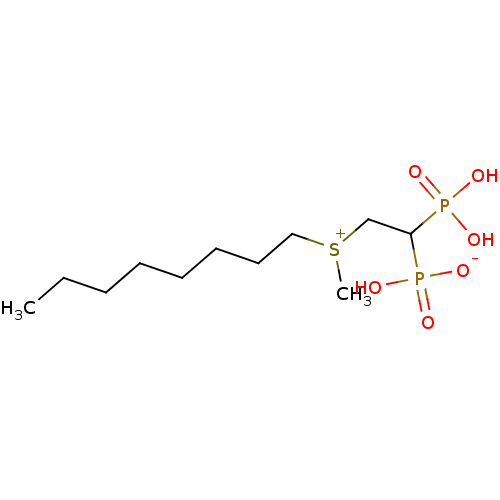
(CHEMBL235690 | bisphosphonate, 37 | hydrogen {2-[m...)Show InChI InChI=1S/C11H26O6P2S/c1-3-4-5-6-7-8-9-20(2)10-11(18(12,13)14)19(15,16)17/h11H,3-10H2,1-2H3,(H3-,12,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25303

(CHEMBL235059 | bisphosphonate, 50 | hydrogen {2-[m...)Show InChI InChI=1S/C6H16O6P2S/c1-3-4-15(2)5-6(13(7,8)9)14(10,11)12/h6H,3-5H2,1-2H3,(H3-,7,8,9,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25294
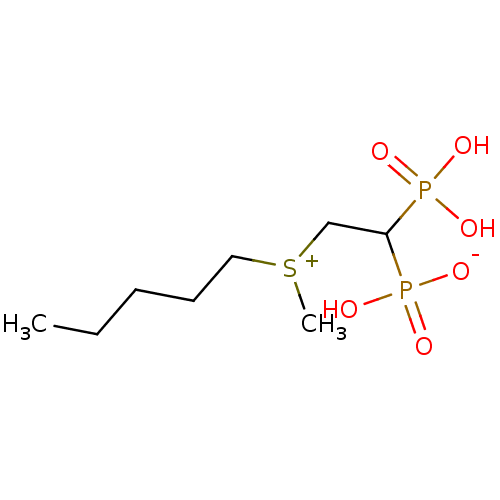
(CHEMBL392884 | bisphosphonate, 43 | hydrogen {2-[m...)Show InChI InChI=1S/C8H20O6P2S/c1-3-4-5-6-17(2)7-8(15(9,10)11)16(12,13)14/h8H,3-7H2,1-2H3,(H3-,9,10,11,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25302
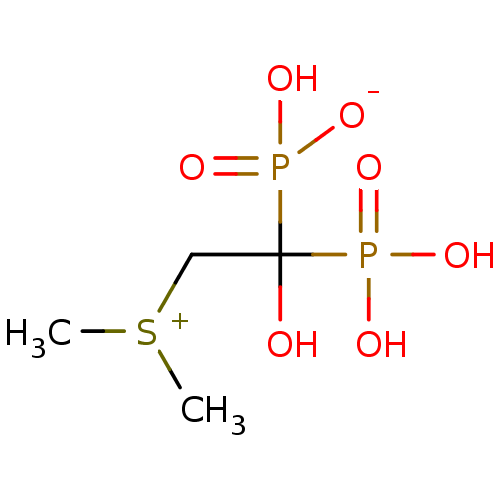
(CHEMBL277580 | bisphosphonate, 49 | hydrogen [2-(d...)Show InChI InChI=1S/C4H12O7P2S/c1-14(2)3-4(5,12(6,7)8)13(9,10)11/h5H,3H2,1-2H3,(H3-,6,7,8,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50225572
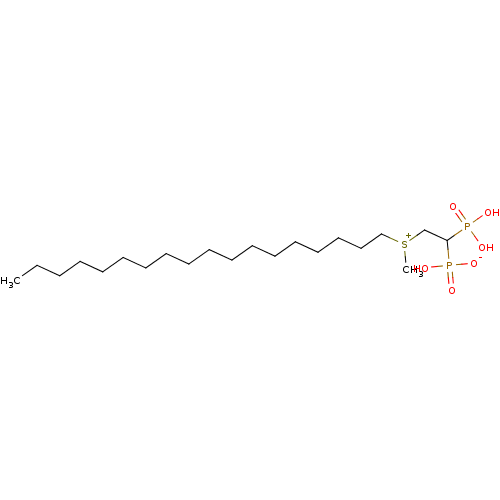
(2-(methyl, octadecylsulfonium-1-yl)ethylidene-1,1-...)Show SMILES CCCCCCCCCCCCCCCCCC[S+](C)CC(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C21H46O6P2S/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-30(2)20-21(28(22,23)24)29(25,26)27/h21H,3-20H2,1-2H3,(H3-,22,23,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25275

(CHEMBL237808 | bisphosphonate, 28 | hydrogen {2-[m...)Show SMILES CCCCCCCCCCCCCC[S+](C)CC(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C17H38O6P2S/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-26(2)16-17(24(18,19)20)25(21,22)23/h17H,3-16H2,1-2H3,(H3-,18,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50138040
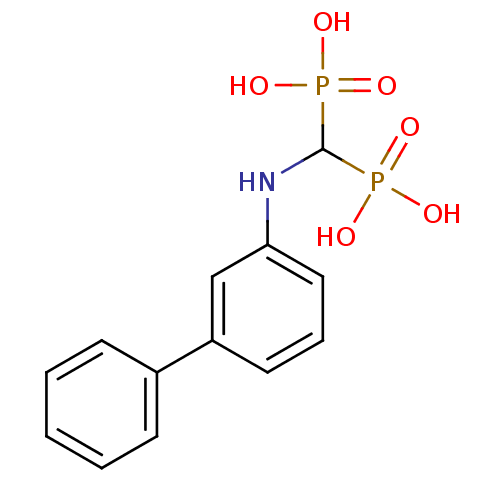
((biphenyl-3-ylamino)methylenediphosphonic acid | B...)Show InChI InChI=1S/C13H15NO6P2/c15-21(16,17)13(22(18,19)20)14-12-8-4-7-11(9-12)10-5-2-1-3-6-10/h1-9,13-14H,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
Bioorg Med Chem 16: 8959-67 (2008)
Article DOI: 10.1016/j.bmc.2008.08.047
BindingDB Entry DOI: 10.7270/Q2639PKT |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50225559
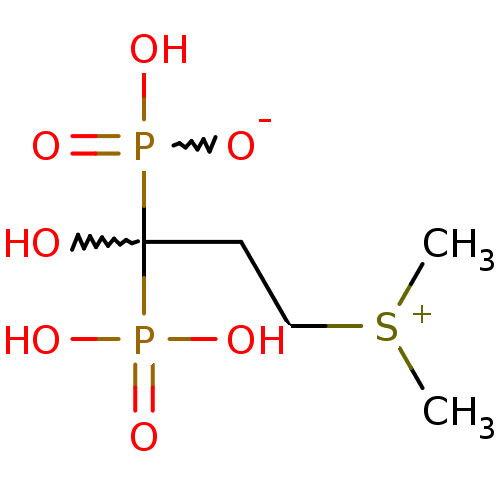
(1-hydroxy-3-(dimethylsulfonium-1-yl)propylidene-1,...)Show SMILES C[S+](C)CCC(O)(P(O)(O)=O)P(O)([O-])=O |w:5.5,11.12| Show InChI InChI=1S/C5H14O7P2S/c1-15(2)4-3-5(6,13(7,8)9)14(10,11)12/h6H,3-4H2,1-2H3,(H3-,7,8,9,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50225563
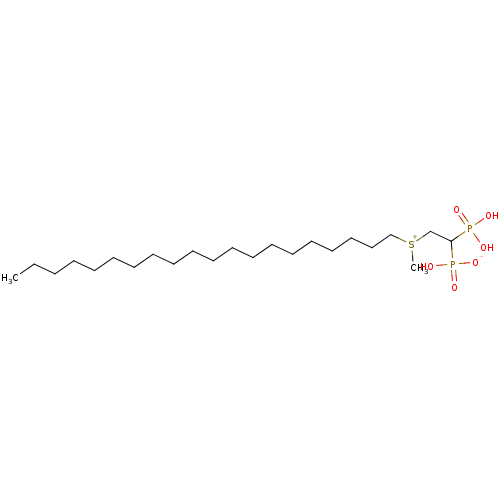
(2-(methyl, licosylsulfonium-1-yl)ethylidene-1,1-bi...)Show SMILES CCCCCCCCCCCCCCCCCCCC[S+](C)CC(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C23H50O6P2S/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-32(2)22-23(30(24,25)26)31(27,28)29/h23H,3-22H2,1-2H3,(H3-,24,25,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50240448

(2-(3,4-dichlorophenyl)-1-hydroxyethane-1,1-diyldip...)Show InChI InChI=1S/C8H10Cl2O7P2/c9-6-2-1-5(3-7(6)10)4-8(11,18(12,13)14)19(15,16)17/h1-3,11H,4H2,(H2,12,13,14)(H2,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
Bioorg Med Chem 16: 8959-67 (2008)
Article DOI: 10.1016/j.bmc.2008.08.047
BindingDB Entry DOI: 10.7270/Q2639PKT |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50225562

(2-(methyl, hexadecylsulfonium-1-yl)ethylidene-1,1-...)Show SMILES CCCCCCCCCCCCCCCC[S+](C)CC(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C19H42O6P2S/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-28(2)18-19(26(20,21)22)27(23,24)25/h19H,3-18H2,1-2H3,(H3-,20,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data