

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50609728 (CHEMBL5267350) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50609742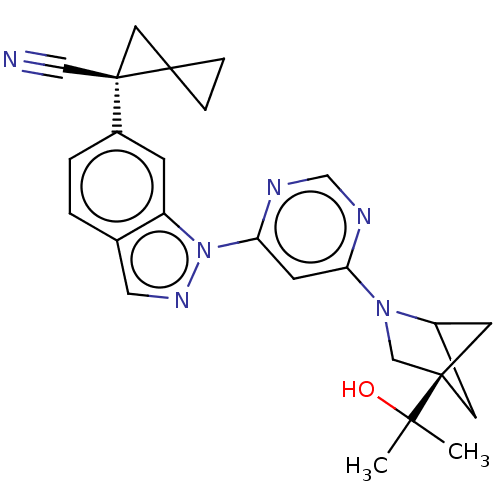 (CHEMBL5286106) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50609742 (CHEMBL5286106) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50609741 (CHEMBL5284341) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359544 (4-{(6R or S)-1-({2- chloro-6-[1- (difluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359566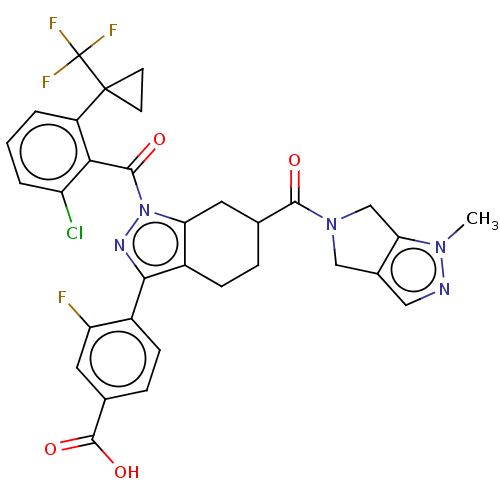 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359568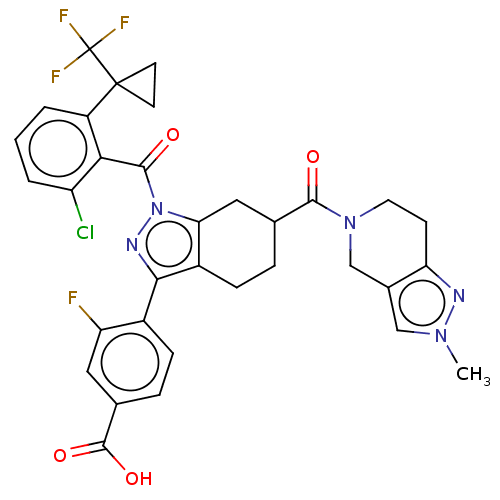 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359569 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM257207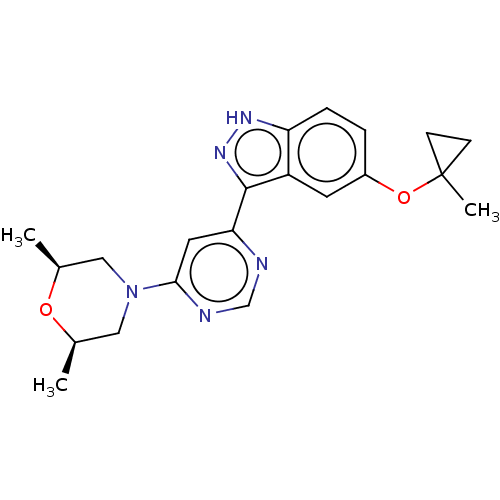 (US9493440, 51) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523028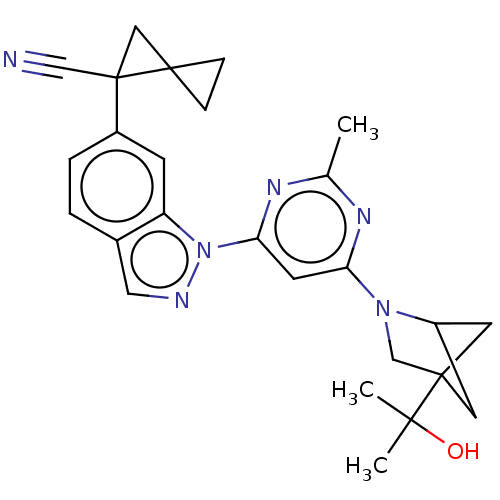 (US11161854, Example 2.4-1 | US11161854, Example 2....) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523096 (US11161854, Example 4.20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523095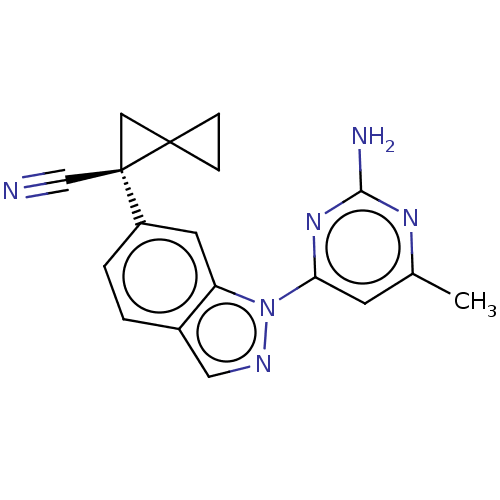 (US11161854, Example 4.19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523093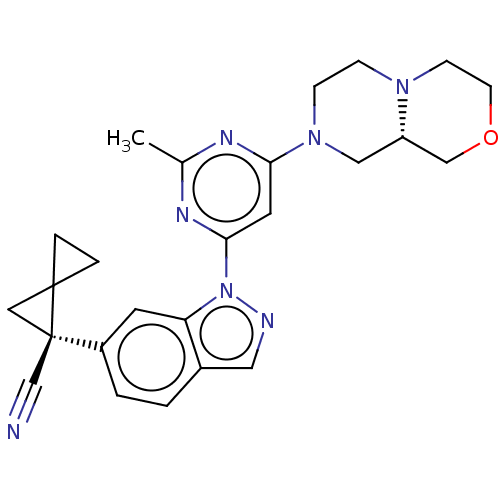 (US11161854, Example 4.17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523092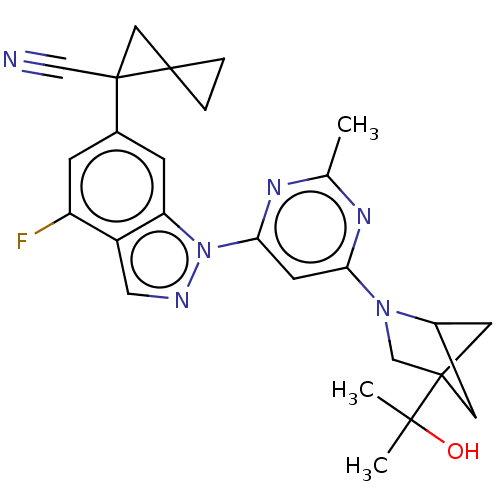 (US11161854, Example 4.16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523088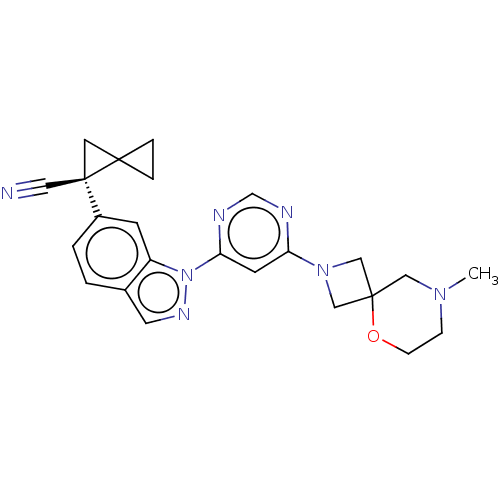 (US11161854, Example 4.13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523087 (US11161854, Example 4.12-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523040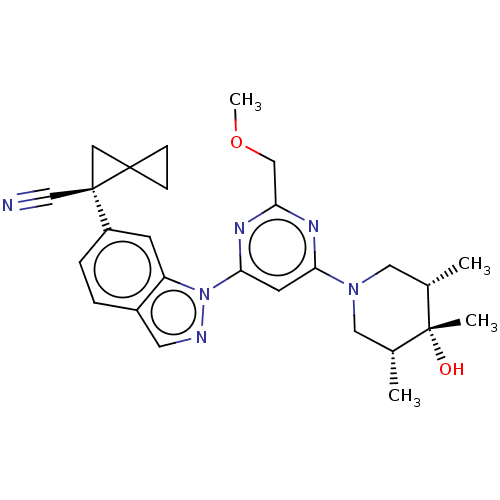 (US11161854, Example 3.3 | US11161854, Example 4.10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523081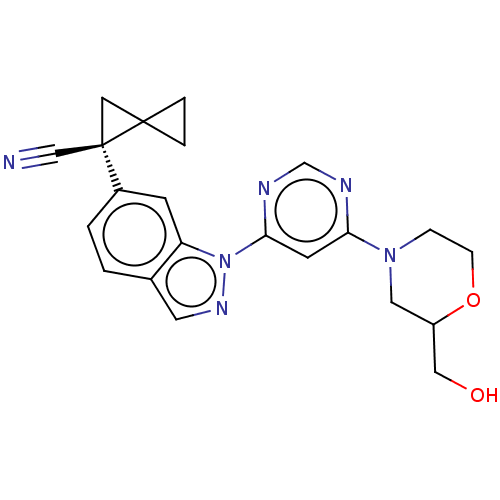 (US11161854, Example 4.9-1 | US11161854, Example 4....) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523081 (US11161854, Example 4.9-1 | US11161854, Example 4....) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523076 (US11161854, Example 4.4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523074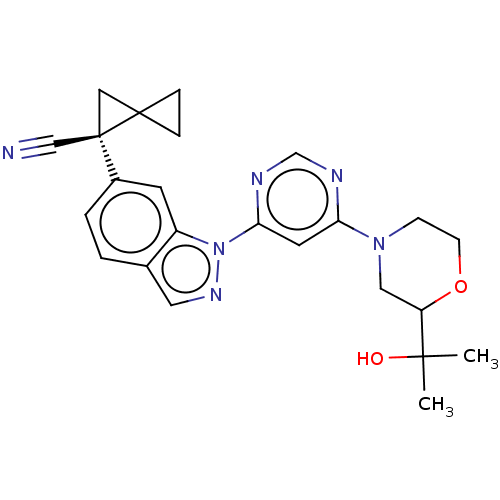 (US11161854, Example 4.3-1 | US11161854, Example 4....) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523072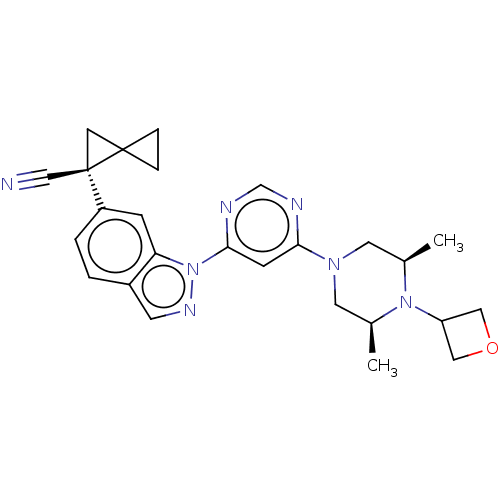 (US11161854, Example 4.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523069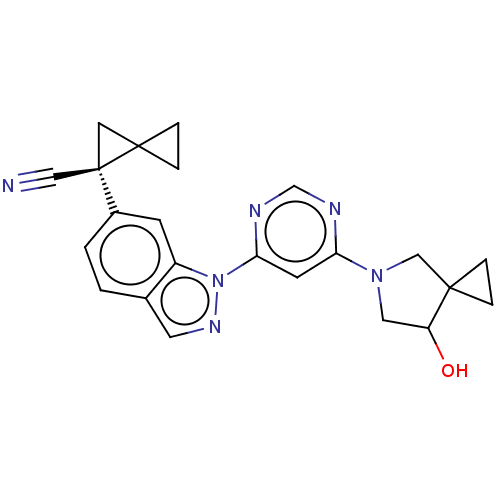 (US11161854, Example 3.11-2 | US11161854, Example 3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523061 (US11161854, Example 3.8-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523051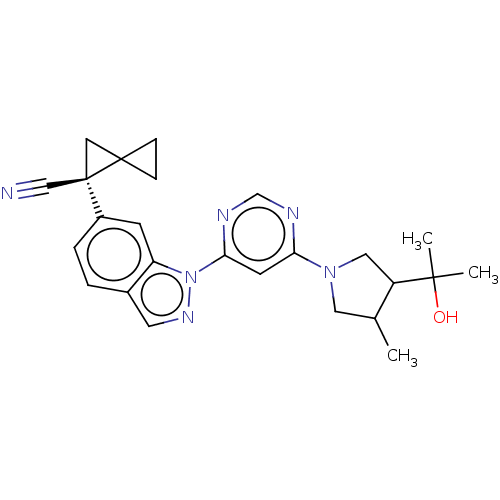 (US11161854, Example 3.6-2 | US11161854, Example 3....) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523042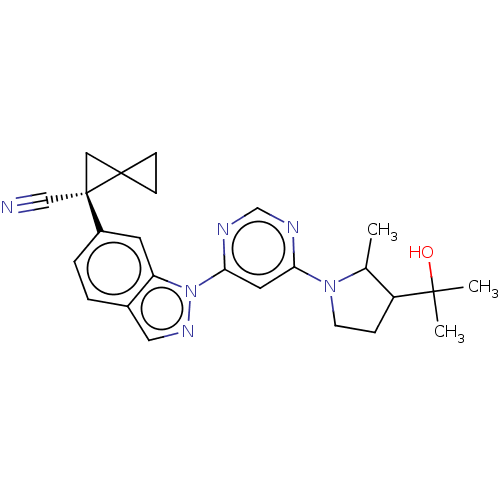 (US11161854, Example 3.5-1 | US11161854, Example 3....) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523038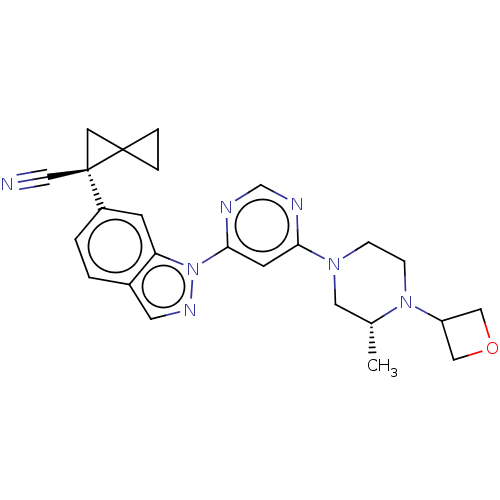 (US11161854, Example 3.1-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523034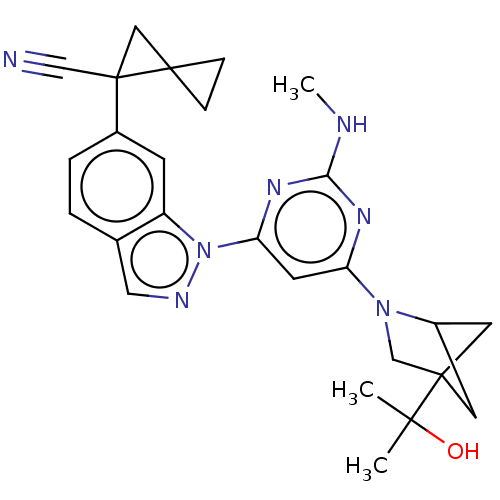 (US11161854, Example 2.7-1 | US11161854, Example 2....) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523131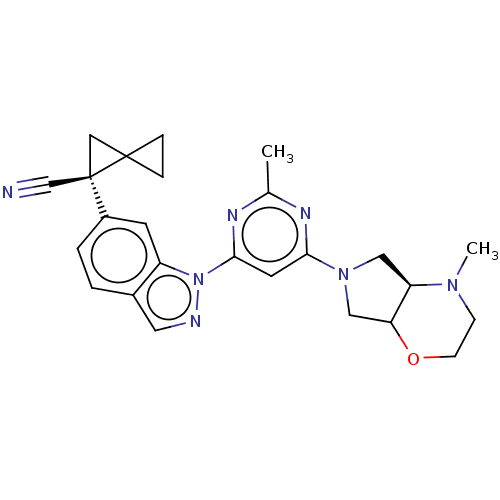 (US11161854, Example 11.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359571 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359543 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359545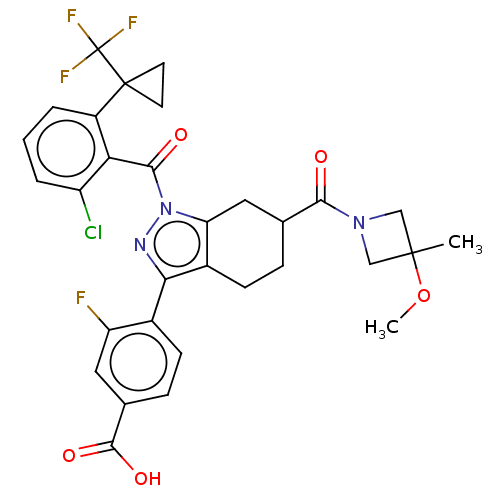 (4-{(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359548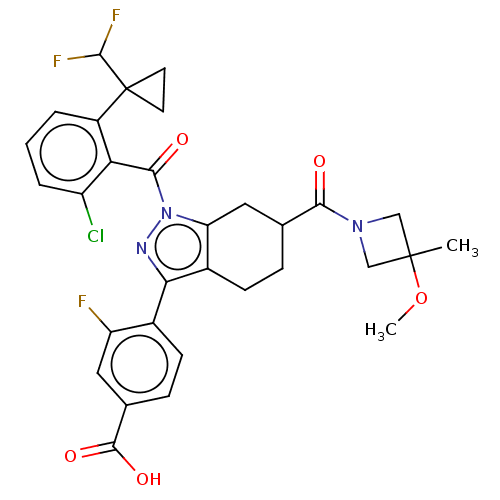 (4-{(6R or S)-1-({2- chloro-6-[1- (difluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359567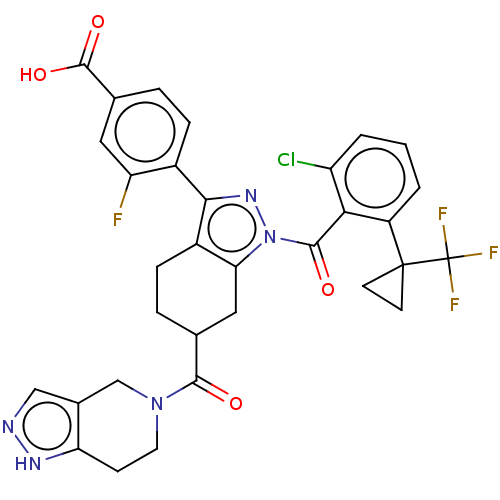 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359570 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523106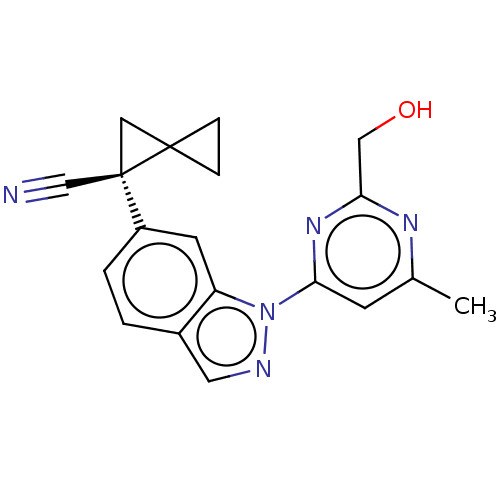 (US11161854, Example 4.30) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.625 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523104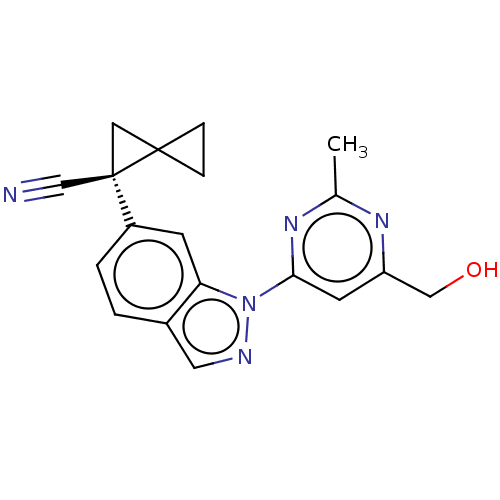 (US11161854, Example 4.28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.625 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359540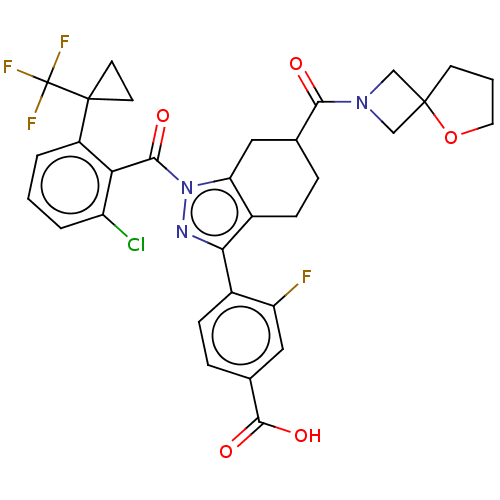 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359582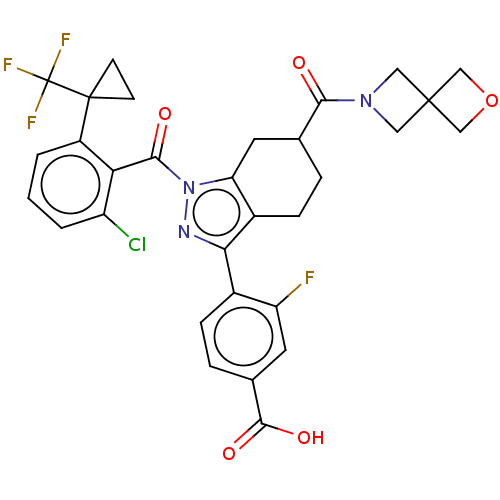 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50609741 (CHEMBL5284341) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359547 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523036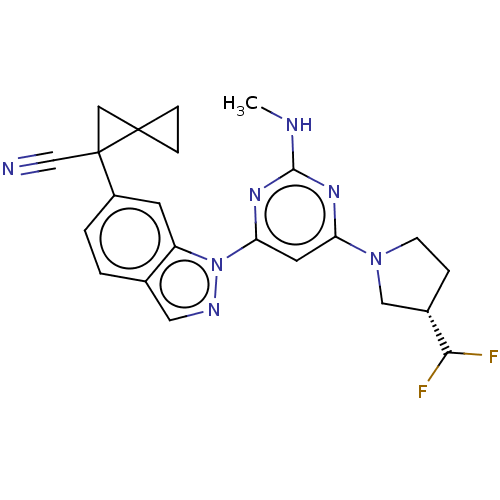 (US11161854, Example 2.8-1 | US11161854, Example 2....) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359577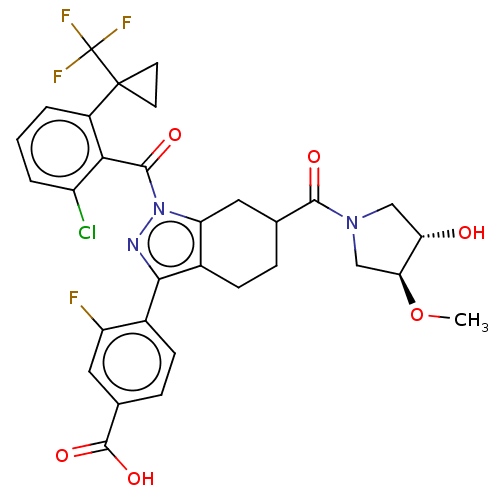 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523109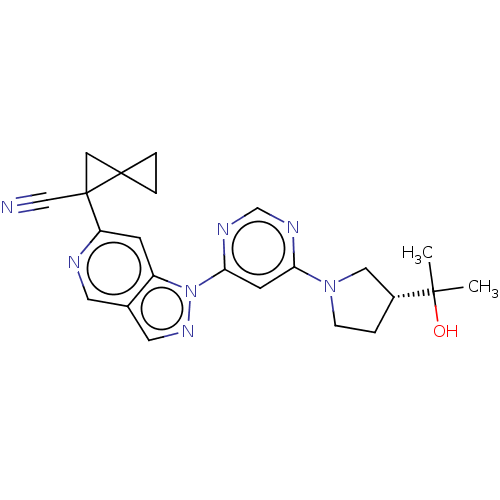 (US11161854, Example 5.3-1 | US11161854, Example 5....) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359535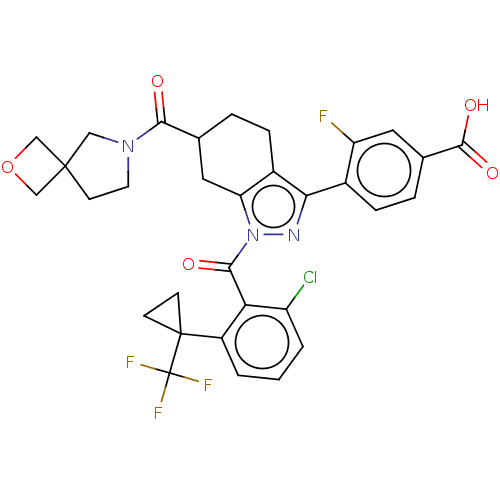 (US10221142, Example 21A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523085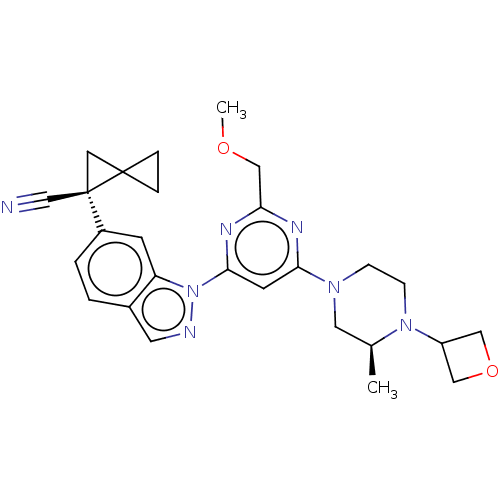 (US11161854, Example 4.11-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359539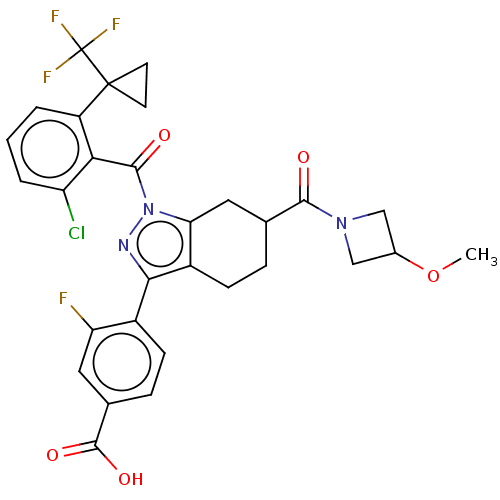 (4-{(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [1-1200,1251-2527,G2019S] (Homo sapiens (Human)) | BDBM523032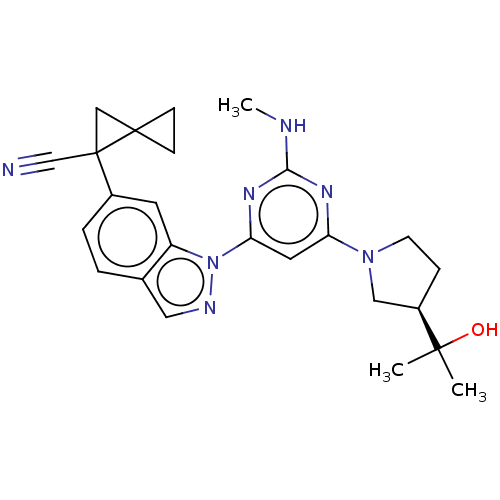 (US11161854, Example 2.6-1 | US11161854, Example 2....) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The LRRK2 kinase activity reported herein as IC50 values was determined with LanthaScreen™ technology from Life Technologies Corporation (Carlsbad, C... | Citation and Details BindingDB Entry DOI: 10.7270/Q29K4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359550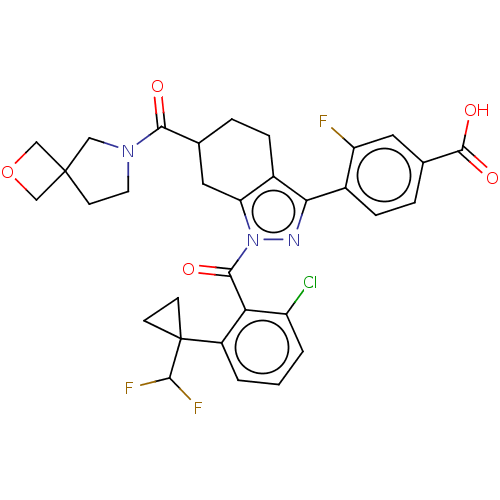 (4-[(6R or S)-1-({2- chloro-6-[1- (difluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359551 (4-[(6R or S)-1-({2- chloro-6-[1- (difluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3197 total ) | Next | Last >> |