

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (MOUSE) | BDBM50059997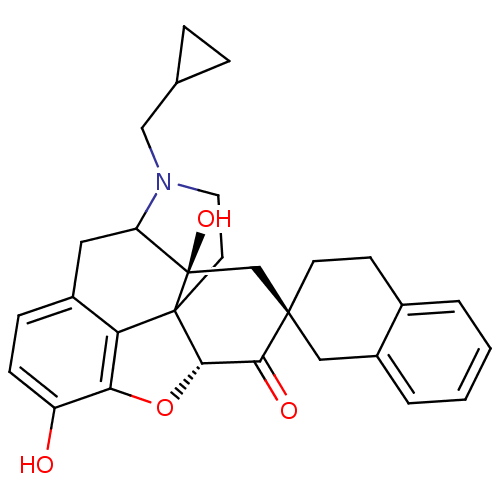 (7 beta-Spirobenzocyclohexylnaltrexone | CHEMBL1016...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-NTI binding to Opioid receptor delta 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50059994 (7 alpha-Spirobenzocyclohexylnaltrexone | CHEMBL105...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-NTI binding to Opioid receptor delta 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50058154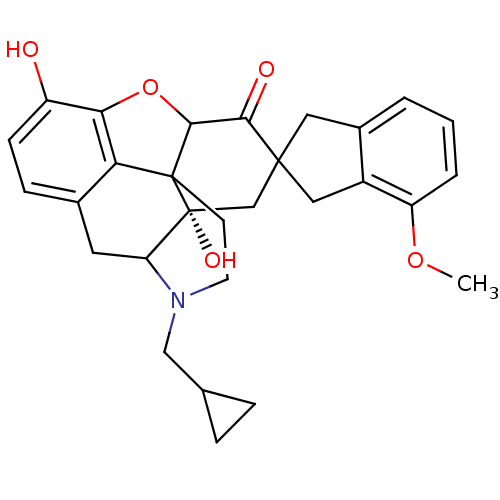 (7-(4'-Methoxy-2'-spiroindanyl)naltrexone | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor delta 1 using [3H]- NT1 as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound was evaluated for binding affinity against Opioid receptor delta 1 in guinea pig brain membrane using [3H]- DPDPE as ligand | J Med Chem 35: 4325-9 (1992) BindingDB Entry DOI: 10.7270/Q2DF6RTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087456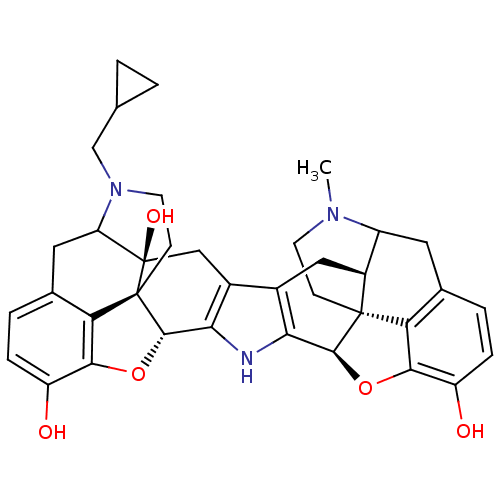 (17-Methyl-17'-cyclopropylmethyl-3'-hydroxy-6,6',7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against K303E Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50058152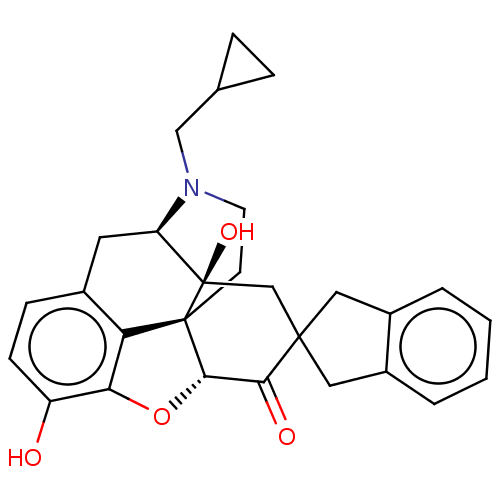 (7-(2''-spiroindanyl)naltrexone | CHEMBL610293) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor delta 1 using [3H]- NT1 as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50001714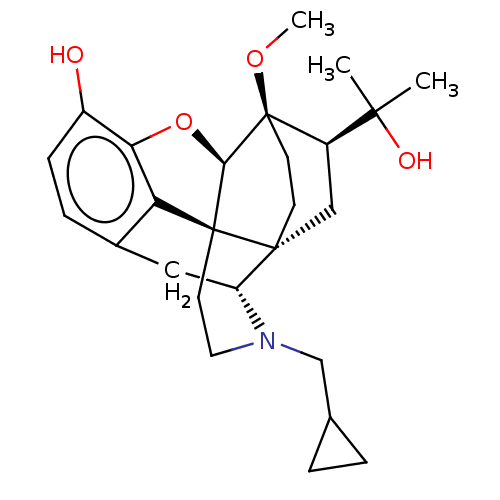 (2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50001714 (2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087456 (17-Methyl-17'-cyclopropylmethyl-3'-hydroxy-6,6',7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50001714 (2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against K303E Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50001714 (2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against E297K mutant Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50059997 (7 beta-Spirobenzocyclohexylnaltrexone | CHEMBL1016...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50001714 (2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against E297A mutant Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087460 (17-cyclopropylmethyl-17'-cyclopropylmethyl-3'-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor kappa 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50058154 (7-(4'-Methoxy-2'-spiroindanyl)naltrexone | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor mu 1 using [3H]- DAMGO as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087458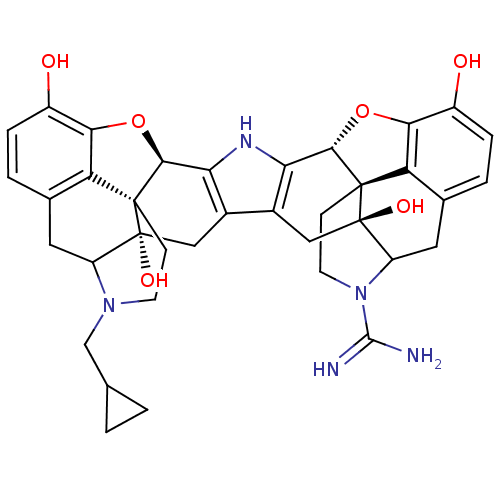 (17-Cyclopropylmethyl-17'-guanidinyl-6,6',7,7'-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against wild-type Opioid receptor kappa 1r in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50221416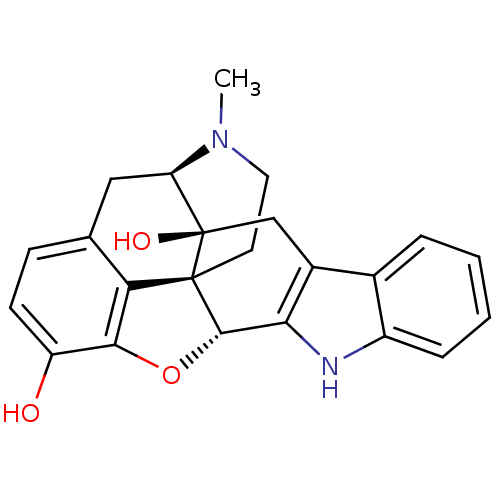 (22-methyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound was evaluated for binding affinity against Opioid receptor delta 1 in guinea pig brain membrane using [3H]- DPDPE as ligand | J Med Chem 35: 4325-9 (1992) BindingDB Entry DOI: 10.7270/Q2DF6RTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50059994 (7 alpha-Spirobenzocyclohexylnaltrexone | CHEMBL105...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 in Guinea pig brain membranes using [3H]DAMGO | J Med Chem 39: 1816-22 (1996) Article DOI: 10.1021/jm950807f BindingDB Entry DOI: 10.7270/Q20865Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against K303E Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50049563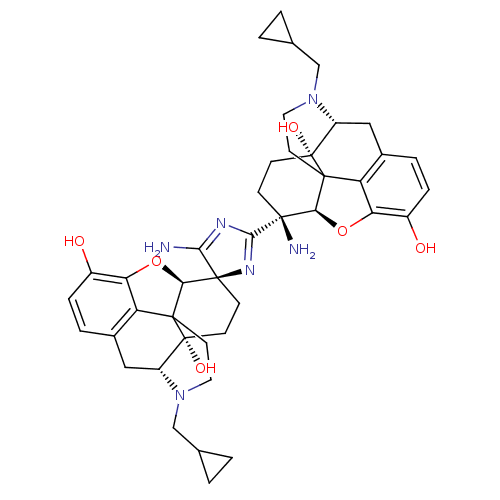 (5-amino-2,4(Bis-4-cyclopropylmethyl-10,17-dihydrox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 in Guinea pig brain membranes | J Med Chem 39: 1816-22 (1996) Article DOI: 10.1021/jm950807f BindingDB Entry DOI: 10.7270/Q20865Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50021498 (1N,4N-di[4-cyclopropylmethyl-10,17-dihydroxy-(13R,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DADLE from Opioid receptor kappa 1 in guinea pig brain membrane | J Med Chem 29: 1855-61 (1986) BindingDB Entry DOI: 10.7270/Q21Z43DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50049560 ((4S,5'R,13'R,17'S)-2-[(5R,13R,14R,17S)-14-amino-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Delta Opioid receptor in Guinea pig brain membranes | J Med Chem 39: 1816-22 (1996) Article DOI: 10.1021/jm950807f BindingDB Entry DOI: 10.7270/Q20865Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50021500 (1N-[4-cyclopropylmethyl-10,17-dihydroxy-(13R,14R,1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from Opioid receptor mu 1 in guinea pig brain membrane | J Med Chem 29: 1855-61 (1986) BindingDB Entry DOI: 10.7270/Q21Z43DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50058152 (7-(2''-spiroindanyl)naltrexone | CHEMBL610293) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-NTI binding to Opioid receptor delta 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50058152 (7-(2''-spiroindanyl)naltrexone | CHEMBL610293) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor mu 1 using [3H]- DAMGO as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50058152 (7-(2''-spiroindanyl)naltrexone | CHEMBL610293) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50021495 (1N,4N-di[4-cyclopropylmethyl-10,17-dihydroxy-(13R,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from Opioid receptor mu 1 in guinea pig brain membrane | J Med Chem 29: 1855-61 (1986) BindingDB Entry DOI: 10.7270/Q21Z43DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50021493 (1N,4N-di[4-cyclopropylmethyl-10,17-dihydroxy-(13R,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from Opioid receptor mu 1 in guinea pig brain membrane | J Med Chem 29: 1855-61 (1986) BindingDB Entry DOI: 10.7270/Q21Z43DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50021498 (1N,4N-di[4-cyclopropylmethyl-10,17-dihydroxy-(13R,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from Opioid receptor mu 1 in guinea pig brain membrane | J Med Chem 29: 1855-61 (1986) BindingDB Entry DOI: 10.7270/Q21Z43DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50016457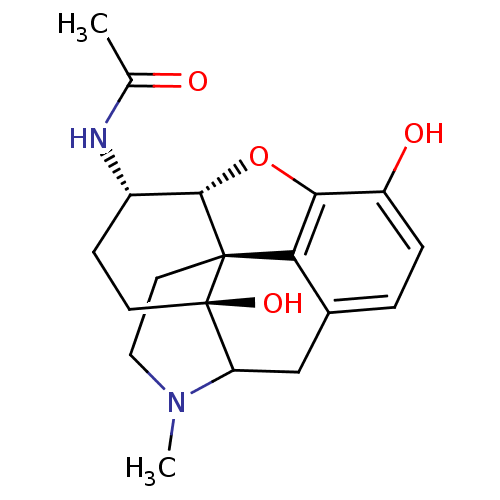 (1N-[10,17-dihydroxy-4-methyl-(13R,14S,17S)-12-oxa-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity of Opioid receptor mu 1 by the displacement of mu-selective [3H]DAGO from brain membrane preparations. | J Med Chem 30: 1991-4 (1987) BindingDB Entry DOI: 10.7270/Q24T6JZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50021499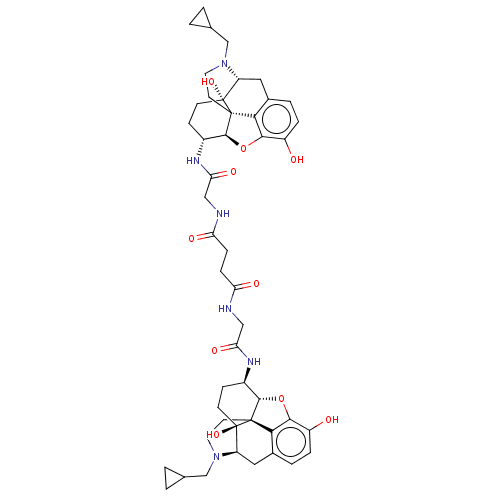 (1N,4N-di[4-cyclopropylmethyl-10,17-dihydroxy-(13R,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from Opioid receptor mu 1 in guinea pig brain membrane | J Med Chem 29: 1855-61 (1986) BindingDB Entry DOI: 10.7270/Q21Z43DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50021499 (1N,4N-di[4-cyclopropylmethyl-10,17-dihydroxy-(13R,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DADLE from Opioid receptor kappa 1 in guinea pig brain membrane | J Med Chem 29: 1855-61 (1986) BindingDB Entry DOI: 10.7270/Q21Z43DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50021495 (1N,4N-di[4-cyclopropylmethyl-10,17-dihydroxy-(13R,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DADLE from Opioid receptor kappa 1 in guinea pig brain membrane | J Med Chem 29: 1855-61 (1986) BindingDB Entry DOI: 10.7270/Q21Z43DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001465 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity of Opioid receptor delta 1 by the displacement of delta-selective [3H]DSLET from brain membrane preparations. | J Med Chem 30: 1991-4 (1987) BindingDB Entry DOI: 10.7270/Q24T6JZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50021493 (1N,4N-di[4-cyclopropylmethyl-10,17-dihydroxy-(13R,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DADLE from Opioid receptor kappa 1 in guinea pig brain membrane | J Med Chem 29: 1855-61 (1986) BindingDB Entry DOI: 10.7270/Q21Z43DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087460 (17-cyclopropylmethyl-17'-cyclopropylmethyl-3'-hydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity using [3H]diprenorphine as the radioligand against K303E Opioid receptor mu 1 in COS-7 cells | J Med Chem 43: 1573-6 (2000) BindingDB Entry DOI: 10.7270/Q2FF3T23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50297170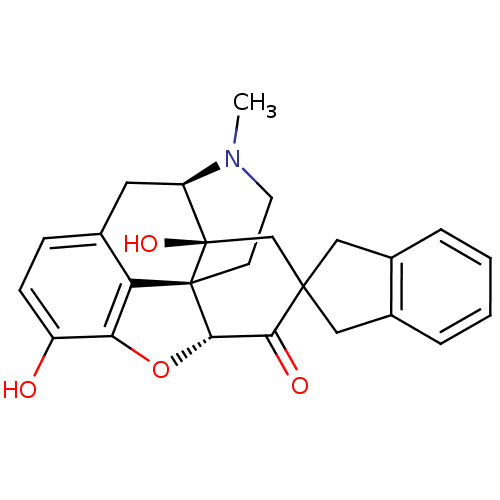 (7-(2'-spiroindanyl)oxymorphone | 7-(Spiroindano)Ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-NTI binding to Opioid receptor delta 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50059996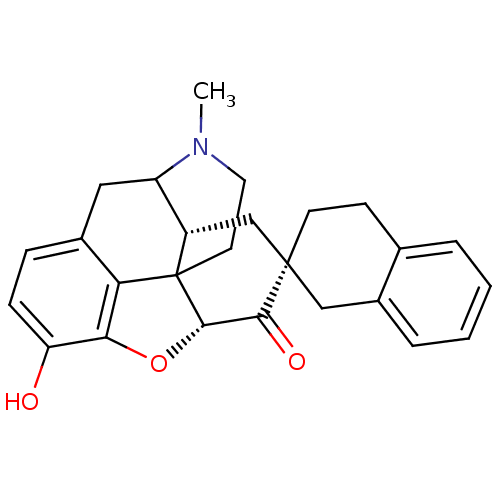 (7 beta-Spirobenzocyclohexylhydromorphone | CHEMBL1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50297170 (7-(2'-spiroindanyl)oxymorphone | 7-(Spiroindano)Ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor delta 1 using [3H]- NT1 as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound was evaluated for binding affinity against opioid receptor mu in guinea pig brain membranes using [3H]- DAMGO as ligand | J Med Chem 35: 4325-9 (1992) BindingDB Entry DOI: 10.7270/Q2DF6RTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50059995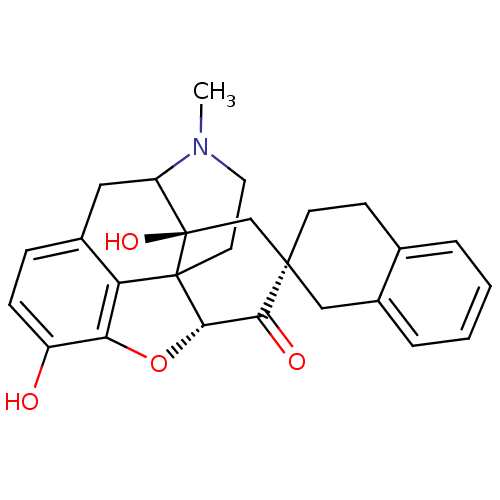 (7 beta-Spirobenzocyclohexyloxymorphone | CHEMBL104...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-NTI binding to Opioid receptor delta 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50021495 (1N,4N-di[4-cyclopropylmethyl-10,17-dihydroxy-(13R,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-EK from Opioid receptor delta 1 in guinea pig brain membrane | J Med Chem 29: 1855-61 (1986) BindingDB Entry DOI: 10.7270/Q21Z43DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001674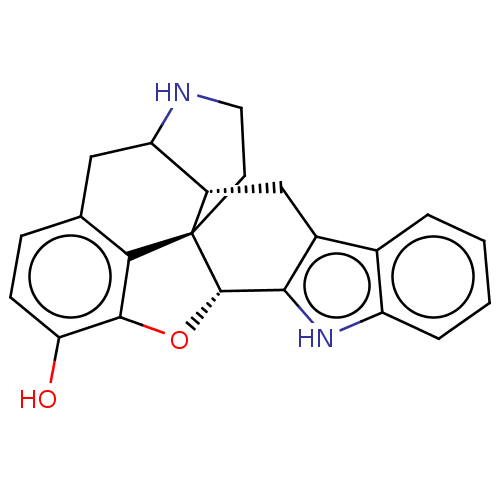 (14-oxa-11,22-diazaheptacyclo[13.9.1.01,13.02,21.04...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound was evaluated for binding affinity against the opioid receptor kappa in guinea pig brain membranes using [3H]- U-69,593 | J Med Chem 35: 4325-9 (1992) BindingDB Entry DOI: 10.7270/Q2DF6RTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50058149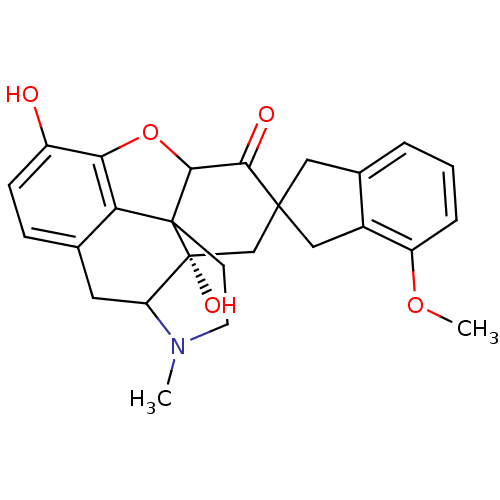 (7-(4'-Methoxy-2'-spiroindanyl)oxymorphone | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor delta 1 using [3H]- NT1 as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50016455 (1N-[10,17-dihydroxy-4-methyl-(13R,17S)-12-oxa-4-az...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity of Opioid receptor mu 1 by the displacement of mu-selective [3H]DAGO from brain membrane preparations. | J Med Chem 30: 1991-4 (1987) BindingDB Entry DOI: 10.7270/Q24T6JZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50049560 ((4S,5'R,13'R,17'S)-2-[(5R,13R,14R,17S)-14-amino-4-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 in Guinea pig brain membranes | J Med Chem 39: 1816-22 (1996) Article DOI: 10.1021/jm950807f BindingDB Entry DOI: 10.7270/Q20865Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50021493 (1N,4N-di[4-cyclopropylmethyl-10,17-dihydroxy-(13R,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-EK from Opioid receptor delta 1 in guinea pig brain membrane | J Med Chem 29: 1855-61 (1986) BindingDB Entry DOI: 10.7270/Q21Z43DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50058150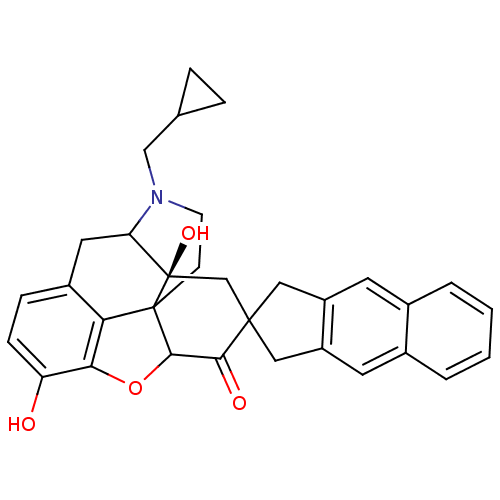 (7-(5',6'-Benzo-2'-spiroindanyl)naltrexone | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor mu 1 using [3H]- DAMGO as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 191 total ) | Next | Last >> |