

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149478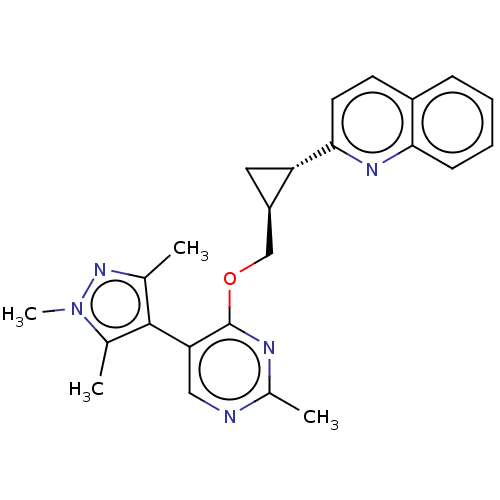 (US8975261, I-46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149477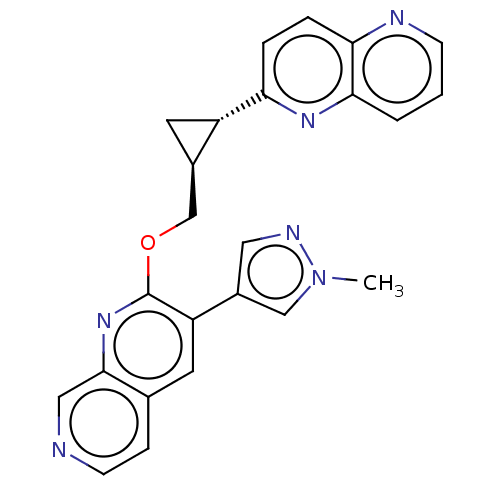 (US8975261, I-42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Rattus norvegicus (Rat)) | BDBM104700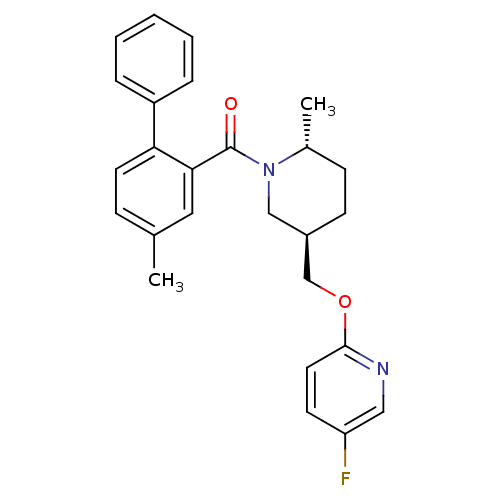 (US8569311, 1-10) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp. US Patent | Assay Description Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. | US Patent US8569311 (2013) BindingDB Entry DOI: 10.7270/Q2TT4PM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM120778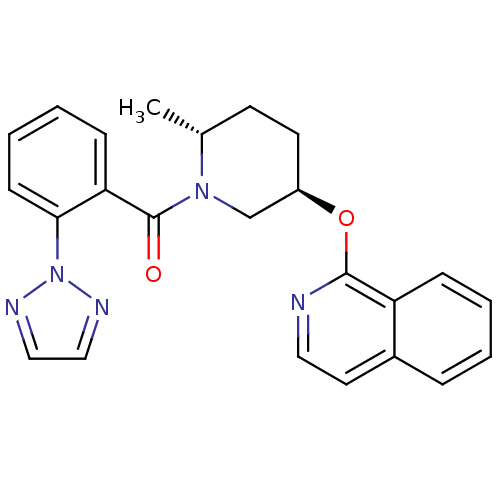 (US8710076, F-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Compound potency can be assessed by a radioligand binding assay (described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430) in which ... | US Patent US8710076 (2014) BindingDB Entry DOI: 10.7270/Q2QN65DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149487 (US8975261, I-31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318697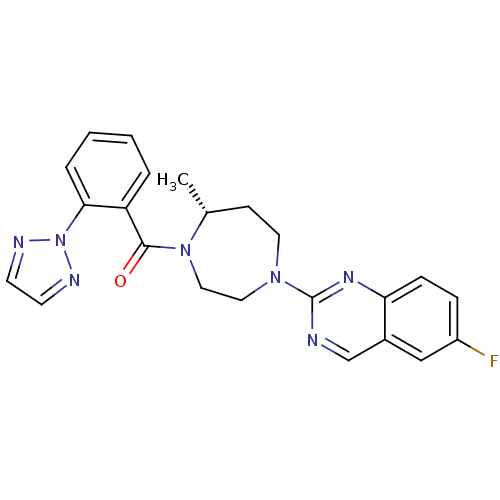 (6-Fluoro-2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50202406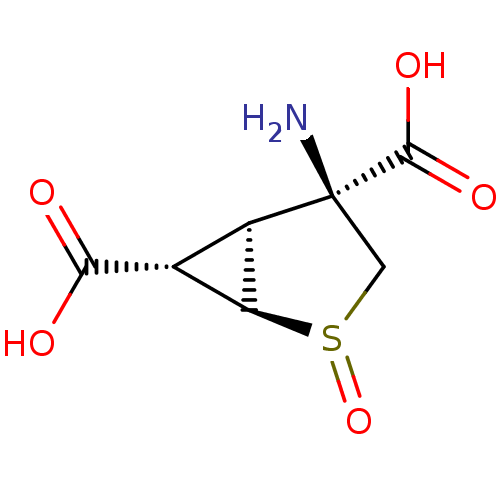 ((+)-(1R,2R,4S,5S,6S)-4-amino-2-thiabicyclo[3.1.0]h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]LY341495 from human recombinant mGluR3 in RGT cells | J Med Chem 50: 233-40 (2007) Article DOI: 10.1021/jm060917u BindingDB Entry DOI: 10.7270/Q21Z442N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50202406 ((+)-(1R,2R,4S,5S,6S)-4-amino-2-thiabicyclo[3.1.0]h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]LY341495 from human recombinant mGluR3 in RGT cells | J Med Chem 50: 233-40 (2007) Article DOI: 10.1021/jm060917u BindingDB Entry DOI: 10.7270/Q21Z442N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149485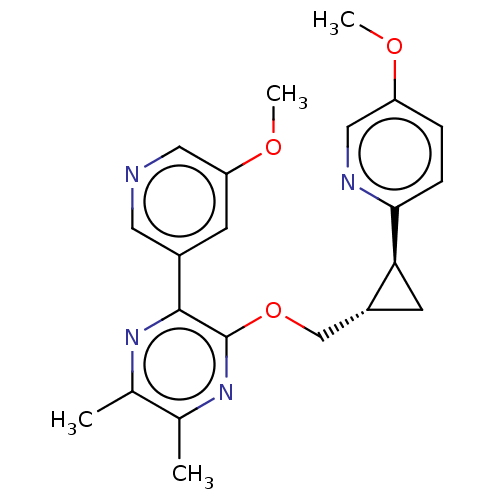 (US8975261, I-36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149475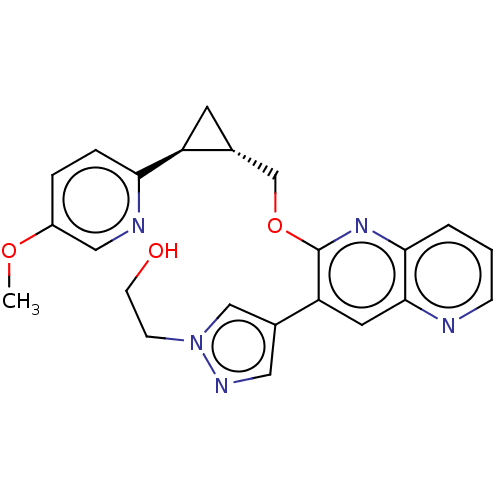 (US8975261, LL3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Rattus norvegicus (Rat)) | BDBM104689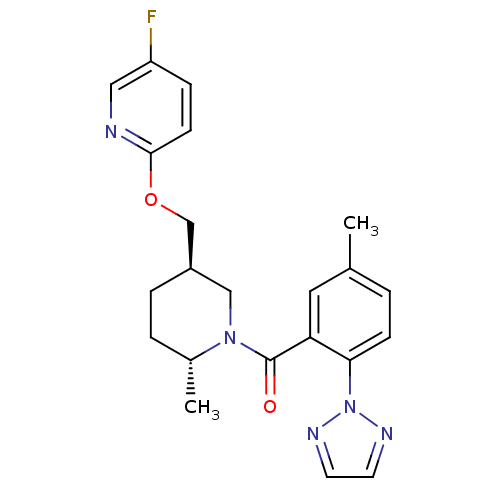 (US8569311, A-9) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp. US Patent | Assay Description Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. | US Patent US8569311 (2013) BindingDB Entry DOI: 10.7270/Q2TT4PM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Rattus norvegicus (Rat)) | BDBM104697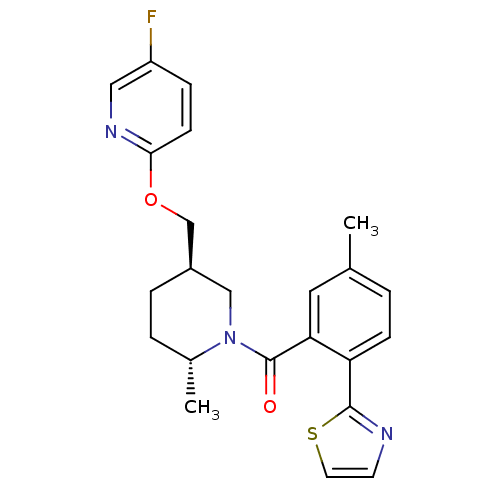 (US8569311, 1-1) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp. US Patent | Assay Description Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. | US Patent US8569311 (2013) BindingDB Entry DOI: 10.7270/Q2TT4PM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50060938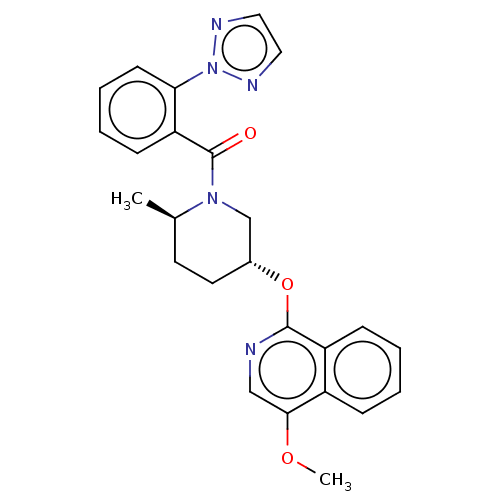 (CHEMBL3394847) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human OX2R by radioligand displacement assay | Bioorg Med Chem Lett 25: 444-50 (2015) Article DOI: 10.1016/j.bmcl.2014.12.056 BindingDB Entry DOI: 10.7270/Q2KD20MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318698 (6,7-Fluoro-2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149476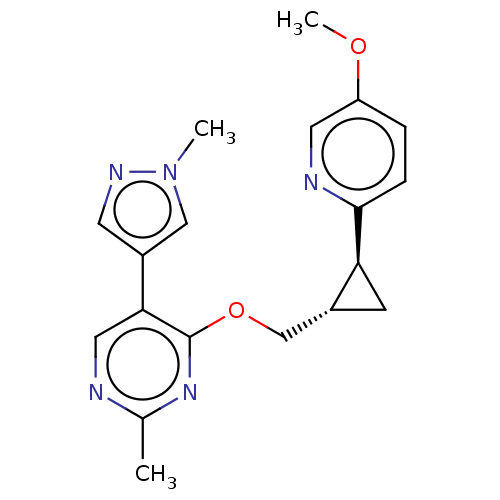 (US8975261, MM4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177911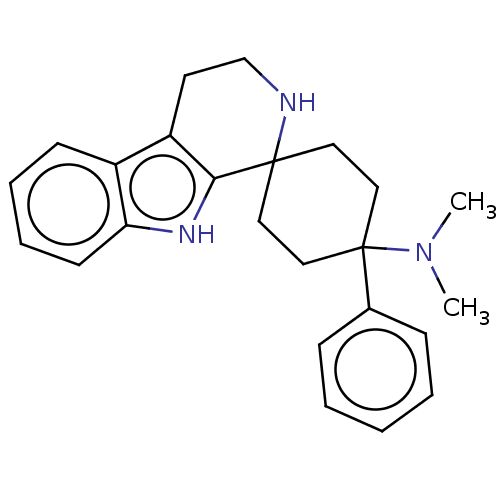 (US9120797, 10 | US9120797, 9) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.260 | -54.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318699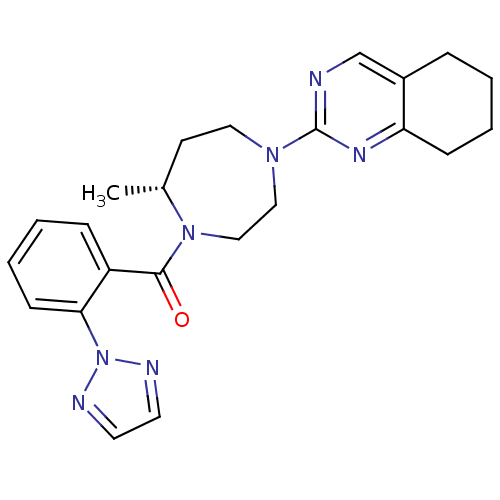 (2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM120778 (US8710076, F-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human OX2R by radioligand displacement assay | Bioorg Med Chem Lett 25: 444-50 (2015) Article DOI: 10.1016/j.bmcl.2014.12.056 BindingDB Entry DOI: 10.7270/Q2KD20MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM104692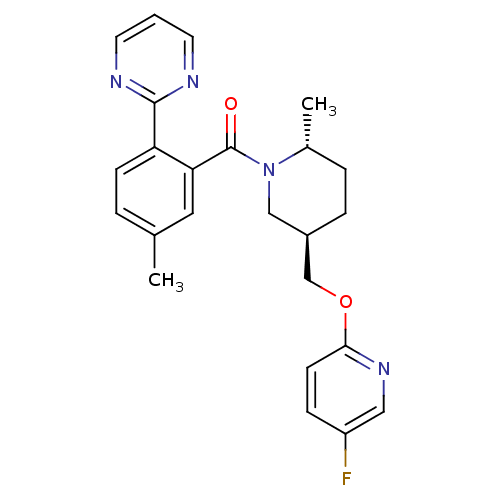 (US8569311, E-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human OX2R by radioligand displacement assay | Bioorg Med Chem Lett 25: 444-50 (2015) Article DOI: 10.1016/j.bmcl.2014.12.056 BindingDB Entry DOI: 10.7270/Q2KD20MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Rattus norvegicus (Rat)) | BDBM104692 (US8569311, E-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp. US Patent | Assay Description Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. | US Patent US8569311 (2013) BindingDB Entry DOI: 10.7270/Q2TT4PM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149486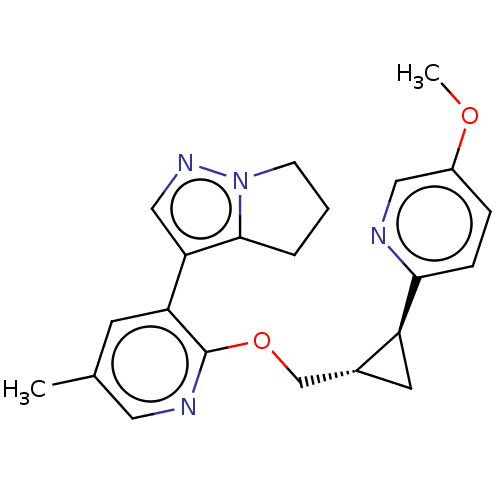 (US8975261, I-39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM50403547 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-methylscopolamine ([3H]-NMS) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318701 (CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Rattus norvegicus (Rat)) | BDBM104701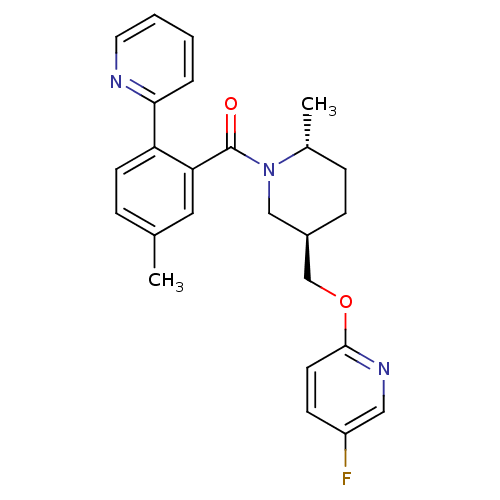 (US8569311, 1-15) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp. US Patent | Assay Description Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. | US Patent US8569311 (2013) BindingDB Entry DOI: 10.7270/Q2TT4PM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50403547 (ATROPEN | ATROPINE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity by displacing [3H]oxotremorine from mouse cerebral cortex tissue. | J Med Chem 35: 1102-8 (1992) BindingDB Entry DOI: 10.7270/Q28K79QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177911 (US9120797, 10 | US9120797, 9) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.360 | -53.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318695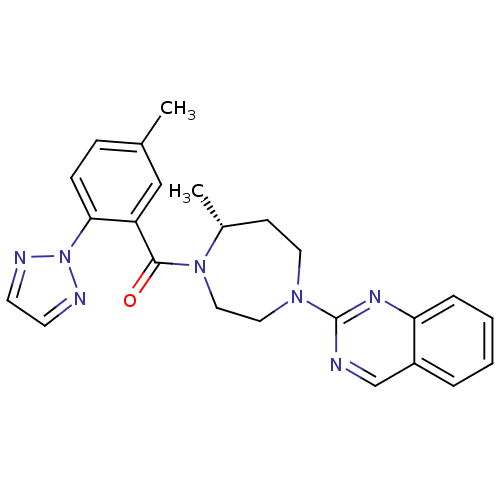 (2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113787 (CHEMBL81056 | S-(2-{2-[2-(4-Carbamimidoyl-phenoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Rattus norvegicus (Rat)) | BDBM104698 (US8569311, 1-4) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp. US Patent | Assay Description Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. | US Patent US8569311 (2013) BindingDB Entry DOI: 10.7270/Q2TT4PM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318695 (2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318695 (2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50060938 (CHEMBL3394847) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human OX1R by radioligand displacement assay | Bioorg Med Chem Lett 25: 444-50 (2015) Article DOI: 10.1016/j.bmcl.2014.12.056 BindingDB Entry DOI: 10.7270/Q2KD20MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98538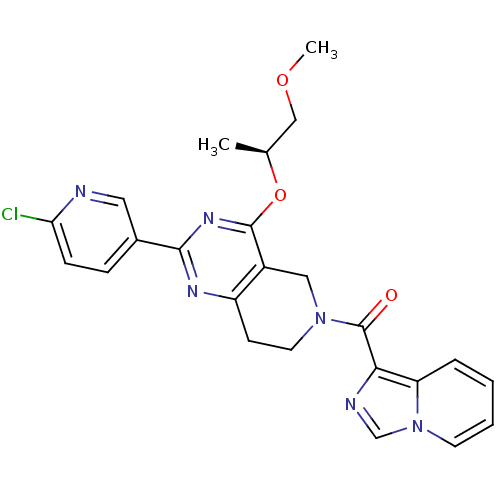 (US8492392, T-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.430 | -53.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318696 (2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Rattus norvegicus (Rat)) | BDBM104703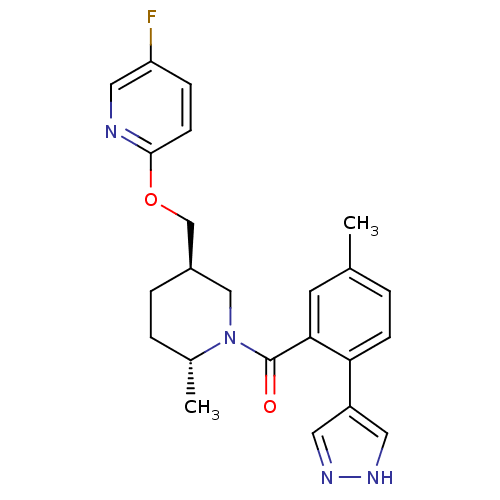 (US8569311, 1-21) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp. US Patent | Assay Description Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. | US Patent US8569311 (2013) BindingDB Entry DOI: 10.7270/Q2TT4PM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Rattus norvegicus (Rat)) | BDBM104699 (US8569311, 1-8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp. US Patent | Assay Description Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. | US Patent US8569311 (2013) BindingDB Entry DOI: 10.7270/Q2TT4PM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177935 (US9120797, 33) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177935 (US9120797, 33) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Rattus norvegicus (Rat)) | BDBM104702 (US8569311, 1-19) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp. US Patent | Assay Description Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. | US Patent US8569311 (2013) BindingDB Entry DOI: 10.7270/Q2TT4PM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113790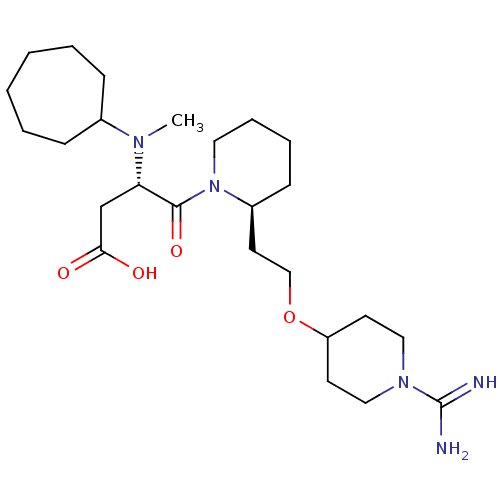 (CHEMBL84389 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Rattus norvegicus (Rat)) | BDBM104690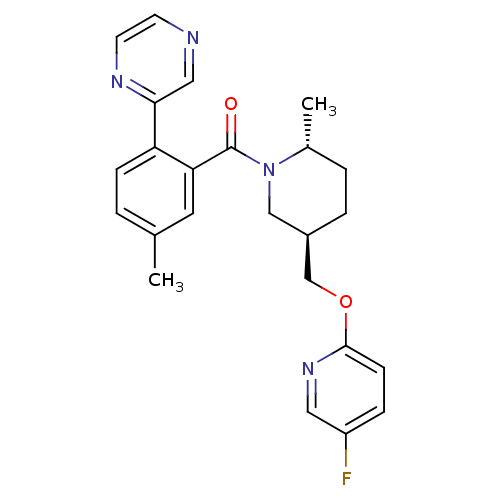 (US8569311, B-3) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp. US Patent | Assay Description Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. | US Patent US8569311 (2013) BindingDB Entry DOI: 10.7270/Q2TT4PM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318701 (CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM120777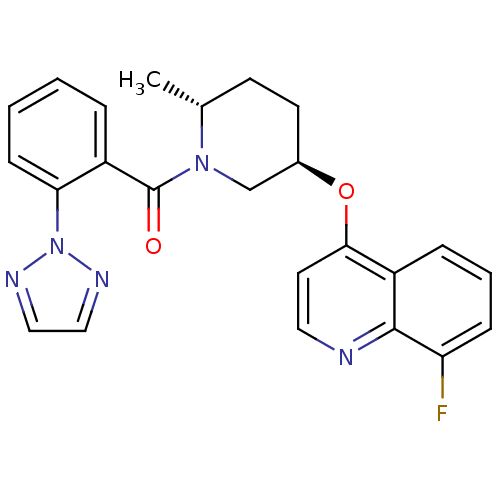 (US8710076, E-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Compound potency can be assessed by a radioligand binding assay (described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430) in which ... | US Patent US8710076 (2014) BindingDB Entry DOI: 10.7270/Q2QN65DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318701 (CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM120779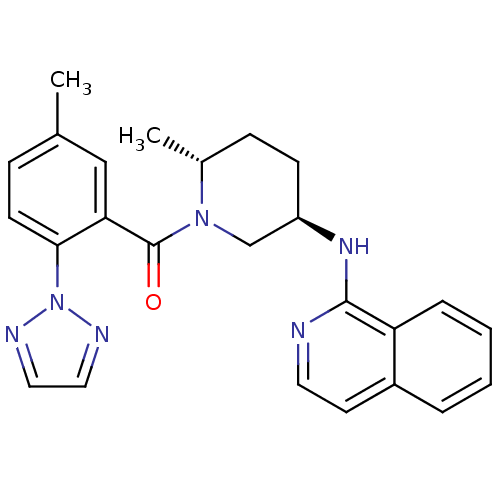 (US8710076, H-4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Compound potency can be assessed by a radioligand binding assay (described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430) in which ... | US Patent US8710076 (2014) BindingDB Entry DOI: 10.7270/Q2QN65DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50258741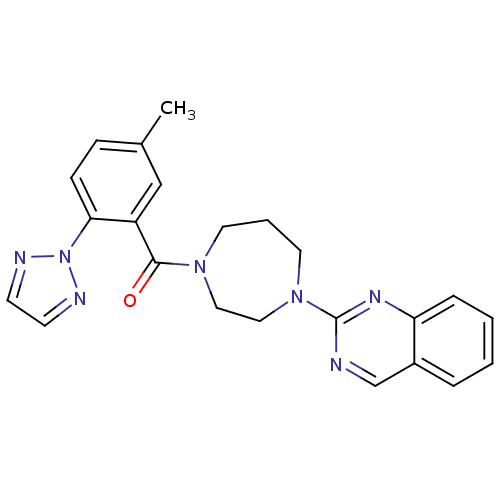 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM98540 (US8492392, 1-11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... | US Patent US8492392 (2013) BindingDB Entry DOI: 10.7270/Q2SN07M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177955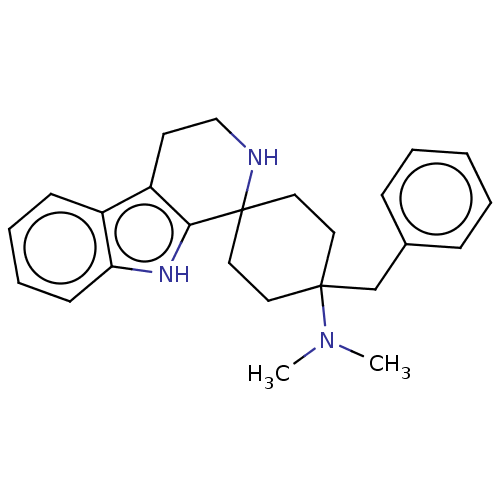 (US9120797, 53) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 11496 total ) | Next | Last >> |