 Found 309 hits with Last Name = 'li' and Initial = 'ym'
Found 309 hits with Last Name = 'li' and Initial = 'ym' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50486237

(3,4-Dihydroxy-Benzoic Acid Heptyl Ester | HEPTYL 3...)Show InChI InChI=1S/C14H20O4/c1-2-3-4-5-6-9-18-14(17)11-7-8-12(15)13(16)10-11/h7-8,10,15-16H,2-6,9H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xiamen University
Curated by ChEMBL
| Assay Description
Inhibition of Agaricus bisporus (mushroom) tyrosinase using L-DOPA as substrate by secondary plot analysis |
J Agric Food Chem 59: 6645-9 (2011)
Article DOI: 10.1021/jf200990g
BindingDB Entry DOI: 10.7270/Q2KP851B |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50486238
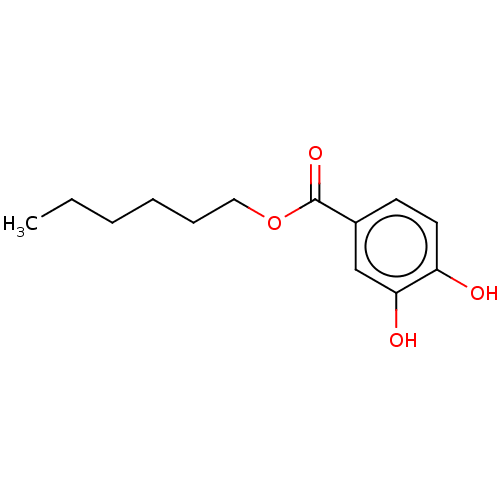
(3,4-Dihydroxy-Benzoic Acid Hexyl Ester | HEXYL 3,4...)Show InChI InChI=1S/C13H18O4/c1-2-3-4-5-8-17-13(16)10-6-7-11(14)12(15)9-10/h6-7,9,14-15H,2-5,8H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xiamen University
Curated by ChEMBL
| Assay Description
Inhibition of Agaricus bisporus (mushroom) tyrosinase using L-DOPA as substrate by secondary plot analysis |
J Agric Food Chem 59: 6645-9 (2011)
Article DOI: 10.1021/jf200990g
BindingDB Entry DOI: 10.7270/Q2KP851B |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50245492
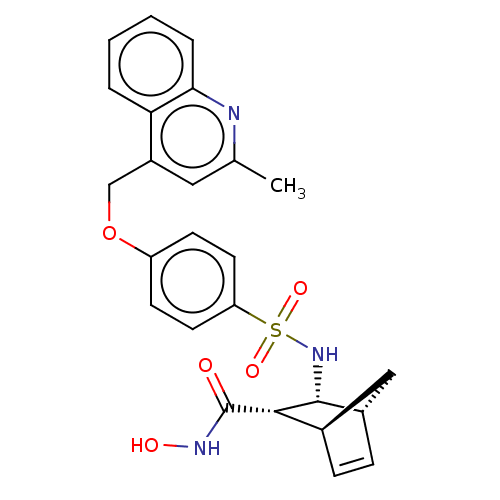
(CHEMBL4082554)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2NS(=O)(=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r,c:5| Show InChI InChI=1S/C25H25N3O5S/c1-15-12-18(21-4-2-3-5-22(21)26-15)14-33-19-8-10-20(11-9-19)34(31,32)28-24-17-7-6-16(13-17)23(24)25(29)27-30/h2-12,16-17,23-24,28,30H,13-14H2,1H3,(H,27,29)/t16-,17+,23+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TACE catalytic domain (unknown origin) using pro-TNFalpha peptide Abz-LAQAVRSSSR-Dpa as substrate preincubated for 10 mins ... |
J Med Chem 60: 527-553 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00935
BindingDB Entry DOI: 10.7270/Q29C70V2 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50496623

(CHEMBL3134498)Show SMILES C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1)C(=O)N[C@@H]1N=C(C2CCCCC2)c2ccccc2NC1=O |r,t:38| Show InChI InChI=1S/C42H53N5O6/c1-27(38(49)47-37-40(51)44-33-23-15-14-22-32(33)36(46-37)30-20-12-7-13-21-30)43-39(50)31(24-28-16-8-5-9-17-28)26-35(48)34(25-29-18-10-6-11-19-29)45-41(52)53-42(2,3)4/h5-6,8-11,14-19,22-23,27,30-31,34-35,37,48H,7,12-13,20-21,24-26H2,1-4H3,(H,43,50)(H,44,51)(H,45,52)(H,47,49)/t27-,31+,34-,35+,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase (unknown origin) using Notch1 substrate assessed as Notch1-NICD production |
Medchemcomm 5: 338-341 (2014)
Article DOI: 10.1039/c3md00281k
BindingDB Entry DOI: 10.7270/Q2ZP493S |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50236621
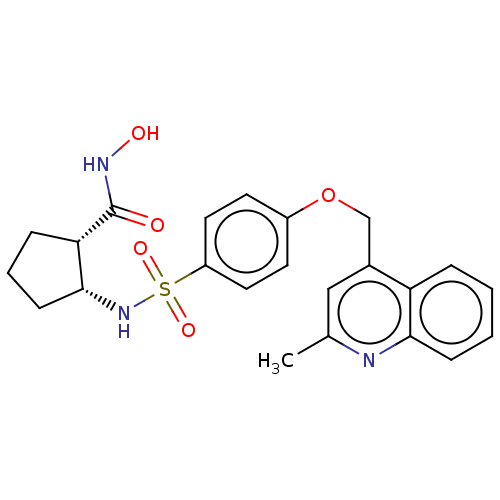
(CHEMBL4078142)Show SMILES Cc1cc(COc2ccc(cc2)S(=O)(=O)N[C@@H]2CCC[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C23H25N3O5S/c1-15-13-16(19-5-2-3-7-21(19)24-15)14-31-17-9-11-18(12-10-17)32(29,30)26-22-8-4-6-20(22)23(27)25-28/h2-3,5,7,9-13,20,22,26,28H,4,6,8,14H2,1H3,(H,25,27)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TACE catalytic domain (unknown origin) using pro-TNFalpha peptide Abz-LAQAVRSSSR-Dpa as substrate preincubated for 10 mins ... |
J Med Chem 60: 527-553 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00935
BindingDB Entry DOI: 10.7270/Q29C70V2 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50479671

(CHEMBL448909)Show SMILES [H][C@@]12CSC(CCCCC(=O)NCCCCCNC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C[C@@H](O)[C@H](Cc3ccc(cc3)C(=O)c3ccccc3)NC(=O)OC(C)(C)C)Cc3ccccc3)[C@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C61H81N7O9S/c1-40(2)34-48(58(74)65-49(37-42-22-12-7-13-23-42)57(73)63-33-19-9-18-32-62-53(70)27-17-16-26-52-54-50(39-78-52)66-59(75)68-54)64-56(72)46(35-41-20-10-6-11-21-41)38-51(69)47(67-60(76)77-61(3,4)5)36-43-28-30-45(31-29-43)55(71)44-24-14-8-15-25-44/h6-8,10-15,20-25,28-31,40,46-52,54,69H,9,16-19,26-27,32-39H2,1-5H3,(H,62,70)(H,63,73)(H,64,72)(H,65,74)(H,67,76)(H2,66,68,75)/t46-,47+,48+,49+,50-,51-,52?,54-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of human gamma secretase assessed as effect on C100Flag substrate cleavage |
Bioorg Med Chem Lett 19: 922-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.117
BindingDB Entry DOI: 10.7270/Q2BC429W |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50496623

(CHEMBL3134498)Show SMILES C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1)C(=O)N[C@@H]1N=C(C2CCCCC2)c2ccccc2NC1=O |r,t:38| Show InChI InChI=1S/C42H53N5O6/c1-27(38(49)47-37-40(51)44-33-23-15-14-22-32(33)36(46-37)30-20-12-7-13-21-30)43-39(50)31(24-28-16-8-5-9-17-28)26-35(48)34(25-29-18-10-6-11-19-29)45-41(52)53-42(2,3)4/h5-6,8-11,14-19,22-23,27,30-31,34-35,37,48H,7,12-13,20-21,24-26H2,1-4H3,(H,43,50)(H,44,51)(H,45,52)(H,47,49)/t27-,31+,34-,35+,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase (unknown origin) using APP substrate assessed as amyloid-beta40 production |
Medchemcomm 5: 338-341 (2014)
Article DOI: 10.1039/c3md00281k
BindingDB Entry DOI: 10.7270/Q2ZP493S |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50479672

(CHEMBL448449)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccc(cc1)C(=O)c1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 |r| Show InChI InChI=1S/C47H57N3O8/c1-31(2)26-39(44(54)49-40(45(55)57-6)29-33-18-12-8-13-19-33)48-43(53)37(27-32-16-10-7-11-17-32)30-41(51)38(50-46(56)58-47(3,4)5)28-34-22-24-36(25-23-34)42(52)35-20-14-9-15-21-35/h7-25,31,37-41,51H,26-30H2,1-6H3,(H,48,53)(H,49,54)(H,50,56)/t37-,38+,39+,40+,41-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of human gamma secretase assessed as effect on C100Flag substrate cleavage |
Bioorg Med Chem Lett 19: 922-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.117
BindingDB Entry DOI: 10.7270/Q2BC429W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 5
(Homo sapiens (Human)) | BDBM50245498
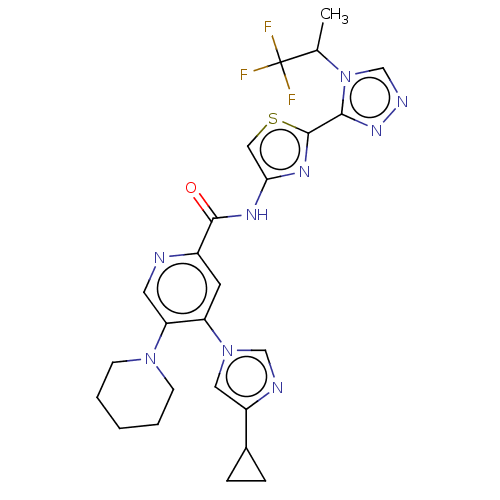
(CHEMBL4083562)Show SMILES CC(n1cnnc1-c1nc(NC(=O)c2cc(c(cn2)N2CCCCC2)-n2cnc(c2)C2CC2)cs1)C(F)(F)F Show InChI InChI=1S/C25H26F3N9OS/c1-15(25(26,27)28)37-14-31-34-22(37)24-33-21(12-39-24)32-23(38)17-9-19(36-11-18(30-13-36)16-5-6-16)20(10-29-17)35-7-3-2-4-8-35/h9-16H,2-8H2,1H3,(H,32,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ASK1 using STK3 peptide as substrate incubated for 30 mins followed by ATP addition measured for 3 hrs by TR-FRET ass... |
J Med Chem 60: 527-553 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00935
BindingDB Entry DOI: 10.7270/Q29C70V2 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50496624

(CHEMBL3134497)Show SMILES C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)C(=O)c1ccc(OCC#C)cc1)Cc1ccccc1)C(=O)N[C@@H]1N=C(C2CCCCC2)c2ccccc2NC1=O |r,t:53| Show InChI InChI=1S/C54H55N5O7/c1-3-31-66-43-29-27-40(28-30-43)49(61)39-23-25-41(26-24-39)52(63)57-46(33-37-17-9-5-10-18-37)47(60)34-42(32-36-15-7-4-8-16-36)53(64)55-35(2)51(62)59-50-54(65)56-45-22-14-13-21-44(45)48(58-50)38-19-11-6-12-20-38/h1,4-5,7-10,13-18,21-30,35,38,42,46-47,50,60H,6,11-12,19-20,31-34H2,2H3,(H,55,64)(H,56,65)(H,57,63)(H,59,62)/t35-,42+,46-,47+,50-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase (unknown origin) using APP substrate assessed as amyloid-beta40 production |
Medchemcomm 5: 338-341 (2014)
Article DOI: 10.1039/c3md00281k
BindingDB Entry DOI: 10.7270/Q2ZP493S |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50496624

(CHEMBL3134497)Show SMILES C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)C(=O)c1ccc(OCC#C)cc1)Cc1ccccc1)C(=O)N[C@@H]1N=C(C2CCCCC2)c2ccccc2NC1=O |r,t:53| Show InChI InChI=1S/C54H55N5O7/c1-3-31-66-43-29-27-40(28-30-43)49(61)39-23-25-41(26-24-39)52(63)57-46(33-37-17-9-5-10-18-37)47(60)34-42(32-36-15-7-4-8-16-36)53(64)55-35(2)51(62)59-50-54(65)56-45-22-14-13-21-44(45)48(58-50)38-19-11-6-12-20-38/h1,4-5,7-10,13-18,21-30,35,38,42,46-47,50,60H,6,11-12,19-20,31-34H2,2H3,(H,55,64)(H,56,65)(H,57,63)(H,59,62)/t35-,42+,46-,47+,50-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase (unknown origin) using Notch1 substrate assessed as Notch1-NICD production |
Medchemcomm 5: 338-341 (2014)
Article DOI: 10.1039/c3md00281k
BindingDB Entry DOI: 10.7270/Q2ZP493S |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50386657
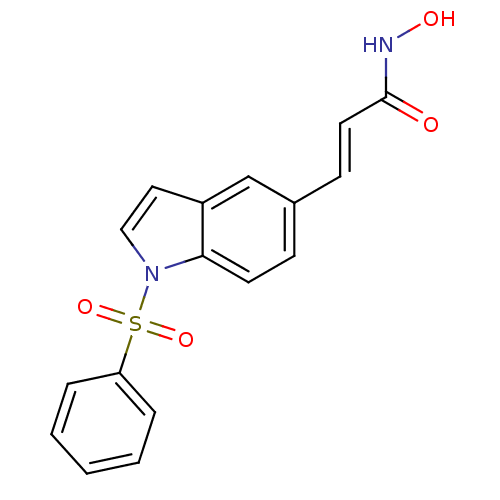
(CHEMBL2048746)Show SMILES ONC(=O)\C=C\c1ccc2n(ccc2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C17H14N2O4S/c20-17(18-21)9-7-13-6-8-16-14(12-13)10-11-19(16)24(22,23)15-4-2-1-3-5-15/h1-12,21H,(H,18,20)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 after 30 mins by fluorometric assay |
J Med Chem 55: 3777-91 (2012)
Article DOI: 10.1021/jm300197a
BindingDB Entry DOI: 10.7270/Q2CR5VD7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 after 30 mins by fluorometric assay |
J Med Chem 55: 3777-91 (2012)
Article DOI: 10.1021/jm300197a
BindingDB Entry DOI: 10.7270/Q2CR5VD7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50386659
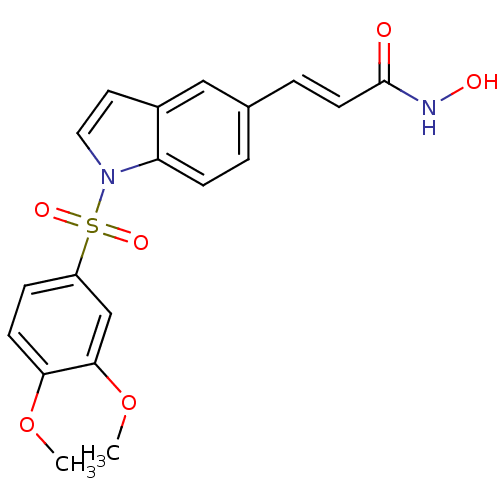
(CHEMBL2048750)Show SMILES COc1ccc(cc1OC)S(=O)(=O)n1ccc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C19H18N2O6S/c1-26-17-7-5-15(12-18(17)27-2)28(24,25)21-10-9-14-11-13(3-6-16(14)21)4-8-19(22)20-23/h3-12,23H,1-2H3,(H,20,22)/b8-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 after 30 mins by fluorometric assay |
J Med Chem 55: 3777-91 (2012)
Article DOI: 10.1021/jm300197a
BindingDB Entry DOI: 10.7270/Q2CR5VD7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50056985

(CHEMBL3329991)Show SMILES Cc1[nH]c2ccccc2c1CCNS(=O)(=O)c1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C20H21N3O4S/c1-14-17(18-4-2-3-5-19(18)22-14)12-13-21-28(26,27)16-9-6-15(7-10-16)8-11-20(24)23-25/h2-11,21-22,25H,12-13H2,1H3,(H,23,24)/b11-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells nuclear extracts incubated for 30 mins by fluorescent assay |
Eur J Med Chem 85: 468-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.020
BindingDB Entry DOI: 10.7270/Q2NK3GQQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50386658
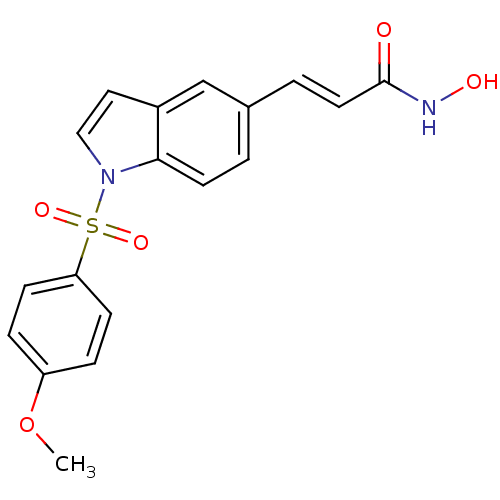
(CHEMBL2048749)Show SMILES COc1ccc(cc1)S(=O)(=O)n1ccc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C18H16N2O5S/c1-25-15-4-6-16(7-5-15)26(23,24)20-11-10-14-12-13(2-8-17(14)20)3-9-18(21)19-22/h2-12,22H,1H3,(H,19,21)/b9-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 after 30 mins by fluorometric assay |
J Med Chem 55: 3777-91 (2012)
Article DOI: 10.1021/jm300197a
BindingDB Entry DOI: 10.7270/Q2CR5VD7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50056984
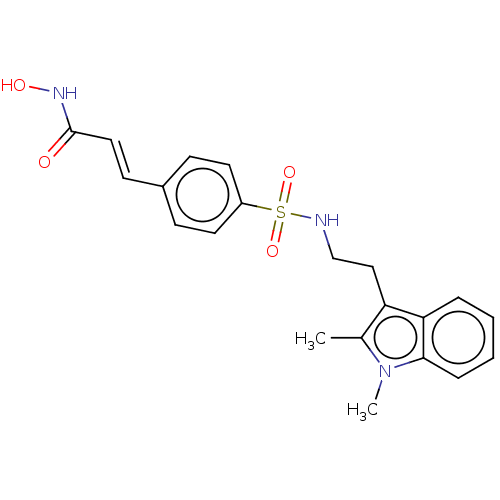
(CHEMBL3329990)Show SMILES Cc1c(CCNS(=O)(=O)c2ccc(\C=C\C(=O)NO)cc2)c2ccccc2n1C Show InChI InChI=1S/C21H23N3O4S/c1-15-18(19-5-3-4-6-20(19)24(15)2)13-14-22-29(27,28)17-10-7-16(8-11-17)9-12-21(25)23-26/h3-12,22,26H,13-14H2,1-2H3,(H,23,25)/b12-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells nuclear extracts incubated for 30 mins by fluorescent assay |
Eur J Med Chem 85: 468-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.020
BindingDB Entry DOI: 10.7270/Q2NK3GQQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50056983

(CHEMBL3329992)Show SMILES CCn1c(C)c(CCNS(=O)(=O)c2ccc(\C=C\C(=O)NO)cc2)c2ccccc12 Show InChI InChI=1S/C22H25N3O4S/c1-3-25-16(2)19(20-6-4-5-7-21(20)25)14-15-23-30(28,29)18-11-8-17(9-12-18)10-13-22(26)24-27/h4-13,23,27H,3,14-15H2,1-2H3,(H,24,26)/b13-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells nuclear extracts incubated for 30 mins by fluorescent assay |
Eur J Med Chem 85: 468-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.020
BindingDB Entry DOI: 10.7270/Q2NK3GQQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 using RHKKAc as substrate by fluorescence assay |
Eur J Med Chem 162: 612-630 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.066
BindingDB Entry DOI: 10.7270/Q2057KBH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50386657

(CHEMBL2048746)Show SMILES ONC(=O)\C=C\c1ccc2n(ccc2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C17H14N2O4S/c20-17(18-21)9-7-13-6-8-16-14(12-13)10-11-19(16)24(22,23)15-4-2-1-3-5-15/h1-12,21H,(H,18,20)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 after 30 mins by fluorometric assay |
J Med Chem 55: 3777-91 (2012)
Article DOI: 10.1021/jm300197a
BindingDB Entry DOI: 10.7270/Q2CR5VD7 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50484974
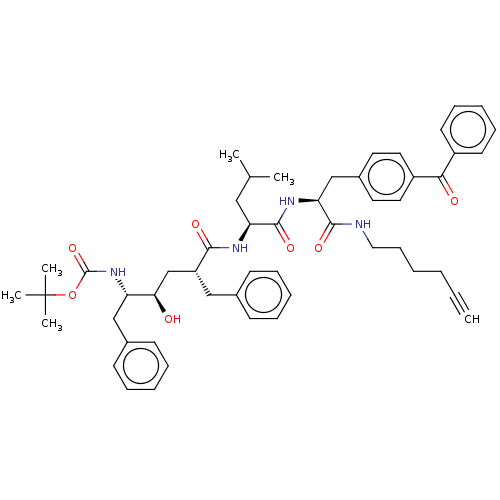
(CHEMBL2017499)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)C(=O)c1ccccc1)C(=O)NCCCCC#C |r| Show InChI InChI=1S/C52H64N4O7/c1-7-8-9-19-30-53-49(60)45(34-39-26-28-41(29-27-39)47(58)40-24-17-12-18-25-40)55-50(61)44(31-36(2)3)54-48(59)42(32-37-20-13-10-14-21-37)35-46(57)43(33-38-22-15-11-16-23-38)56-51(62)63-52(4,5)6/h1,10-18,20-29,36,42-46,57H,8-9,19,30-35H2,2-6H3,(H,53,60)(H,54,59)(H,55,61)(H,56,62)/t42-,43+,44+,45+,46-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in cell-free human HeLa membrane assessed as amyloid beta-42 production using APP as substrate |
Bioorg Med Chem Lett 22: 2997-3000 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.027
BindingDB Entry DOI: 10.7270/Q2SB48KN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50439674
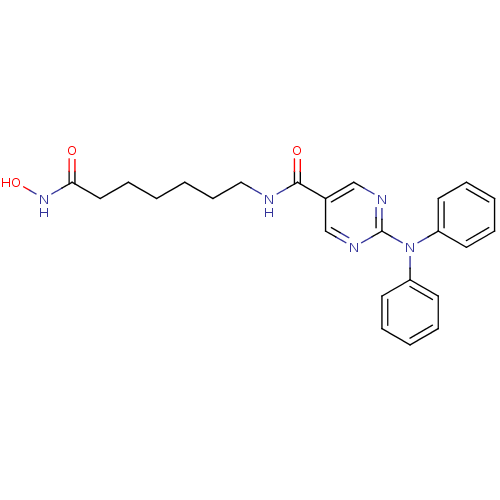
(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac) (379 to 382) p53 peptide as substrate by fluorescence assay |
J Med Chem 57: 4009-22 (2014)
Article DOI: 10.1021/jm401899x
BindingDB Entry DOI: 10.7270/Q28917F9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50551464

(CHEMBL4793318)Show SMILES Oc1ccc2c(Oc3ccc(Nc4nccc5[nH]cc(-c6ccc(F)cc6)c(=O)c45)cc3F)ccnc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MET (unknown origin) using biotin as substrate preincubated for 5 mins followed by substrate addition and measured after 30 to 60 mins ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112174
BindingDB Entry DOI: 10.7270/Q23N2716 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 after 30 mins by fluorometric assay |
J Med Chem 55: 3777-91 (2012)
Article DOI: 10.1021/jm300197a
BindingDB Entry DOI: 10.7270/Q2CR5VD7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50015233
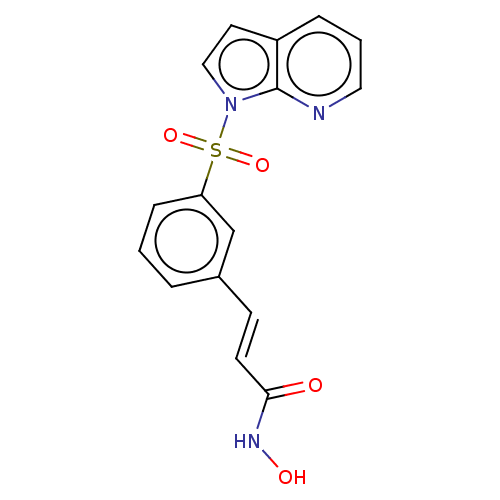
(CHEMBL3262727)Show SMILES ONC(=O)\C=C\c1cccc(c1)S(=O)(=O)n1ccc2cccnc12 Show InChI InChI=1S/C16H13N3O4S/c20-15(18-21)7-6-12-3-1-5-14(11-12)24(22,23)19-10-8-13-4-2-9-17-16(13)19/h1-11,21H,(H,18,20)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac) (379 to 382) p53 peptide as substrate by fluorescence assay |
J Med Chem 57: 4009-22 (2014)
Article DOI: 10.1021/jm401899x
BindingDB Entry DOI: 10.7270/Q28917F9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50551465
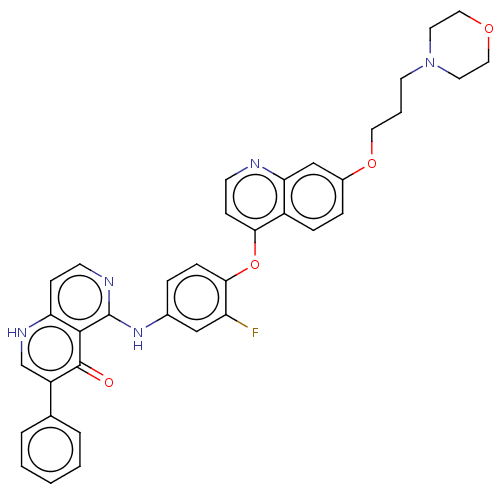
(CHEMBL4791178)Show SMILES Fc1cc(Nc2nccc3[nH]cc(-c4ccccc4)c(=O)c23)ccc1Oc1ccnc2cc(OCCCN3CCOCC3)ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MET (unknown origin) using biotin as substrate preincubated for 5 mins followed by substrate addition and measured after 30 to 60 mins ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112174
BindingDB Entry DOI: 10.7270/Q23N2716 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50551449
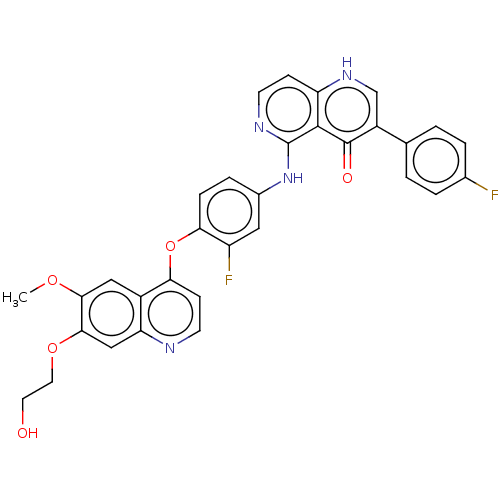
(CHEMBL4745046)Show SMILES COc1cc2c(Oc3ccc(Nc4nccc5[nH]cc(-c6ccc(F)cc6)c(=O)c45)cc3F)ccnc2cc1OCCO | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MET (unknown origin) using biotin as substrate preincubated for 5 mins followed by substrate addition and measured after 30 to 60 mins ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112174
BindingDB Entry DOI: 10.7270/Q23N2716 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50056975
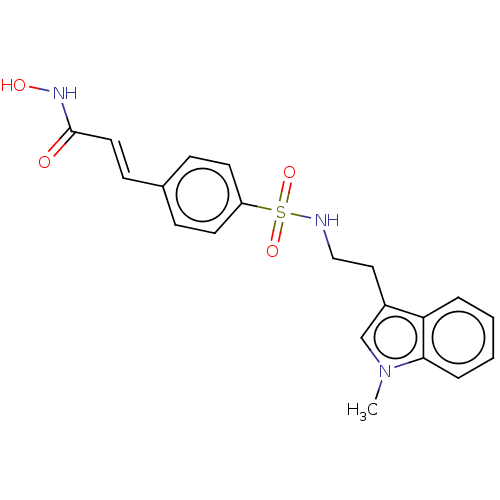
(CHEMBL3329999)Show SMILES Cn1cc(CCNS(=O)(=O)c2ccc(\C=C\C(=O)NO)cc2)c2ccccc12 Show InChI InChI=1S/C20H21N3O4S/c1-23-14-16(18-4-2-3-5-19(18)23)12-13-21-28(26,27)17-9-6-15(7-10-17)8-11-20(24)22-25/h2-11,14,21,25H,12-13H2,1H3,(H,22,24)/b11-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells nuclear extracts incubated for 30 mins by fluorescent assay |
Eur J Med Chem 85: 468-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.020
BindingDB Entry DOI: 10.7270/Q2NK3GQQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50056974

(CHEMBL3329998)Show SMILES ONC(=O)\C=C\c1ccc(cc1)S(=O)(=O)NCCc1c[nH]c2ccccc12 Show InChI InChI=1S/C19H19N3O4S/c23-19(22-24)10-7-14-5-8-16(9-6-14)27(25,26)21-12-11-15-13-20-18-4-2-1-3-17(15)18/h1-10,13,20-21,24H,11-12H2,(H,22,23)/b10-7+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells nuclear extracts incubated for 30 mins by fluorescent assay |
Eur J Med Chem 85: 468-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.020
BindingDB Entry DOI: 10.7270/Q2NK3GQQ |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50551459

(CHEMBL4760632)Show SMILES OCCOc1ccc2c(Oc3ccc(Nc4nccc5[nH]cc(-c6ccc(F)cc6)c(=O)c45)cc3F)ccnc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MET (unknown origin) using biotin as substrate preincubated for 5 mins followed by substrate addition and measured after 30 to 60 mins ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112174
BindingDB Entry DOI: 10.7270/Q23N2716 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50056982

(CHEMBL3329994)Show SMILES CCN(CC)CCn1c(C)c(CCNS(=O)(=O)c2ccc(\C=C\C(=O)NO)cc2)c2ccccc12 Show InChI InChI=1S/C26H34N4O4S/c1-4-29(5-2)18-19-30-20(3)23(24-8-6-7-9-25(24)30)16-17-27-35(33,34)22-13-10-21(11-14-22)12-15-26(31)28-32/h6-15,27,32H,4-5,16-19H2,1-3H3,(H,28,31)/b15-12+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells nuclear extracts incubated for 30 mins by fluorescent assay |
Eur J Med Chem 85: 468-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.020
BindingDB Entry DOI: 10.7270/Q2NK3GQQ |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM387242
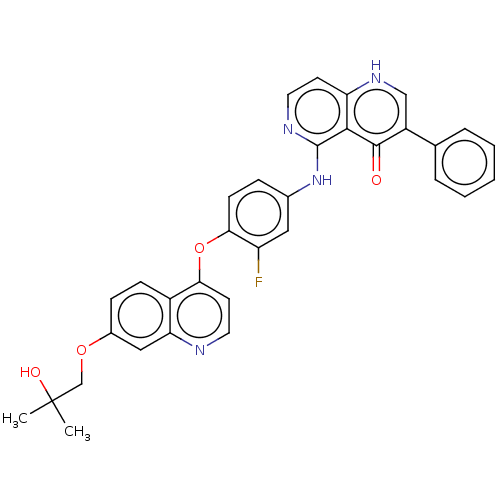
(US9938274, 7)Show SMILES CC(C)(O)COc1ccc2c(Oc3ccc(Nc4nccc5[nH]cc(-c6ccccc6)c(=O)c45)cc3F)ccnc2c1 Show InChI InChI=1S/C33H27FN4O4/c1-33(2,40)19-41-22-9-10-23-27(17-22)35-15-13-28(23)42-29-11-8-21(16-25(29)34)38-32-30-26(12-14-36-32)37-18-24(31(30)39)20-6-4-3-5-7-20/h3-18,40H,19H2,1-2H3,(H,36,38)(H,37,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MET (unknown origin) using biotin as substrate preincubated for 5 mins followed by substrate addition and measured after 30 to 60 mins ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112174
BindingDB Entry DOI: 10.7270/Q23N2716 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50056978

(CHEMBL3330002)Show InChI InChI=1S/C21H25N3O/c1-16-19(20-6-2-3-7-21(20)24-16)12-14-22-15-18-10-8-17(9-11-18)5-4-13-23-25/h2-11,22-25H,12-15H2,1H3/b5-4+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells nuclear extracts incubated for 30 mins by fluorescent assay |
Eur J Med Chem 85: 468-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.020
BindingDB Entry DOI: 10.7270/Q2NK3GQQ |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM387238
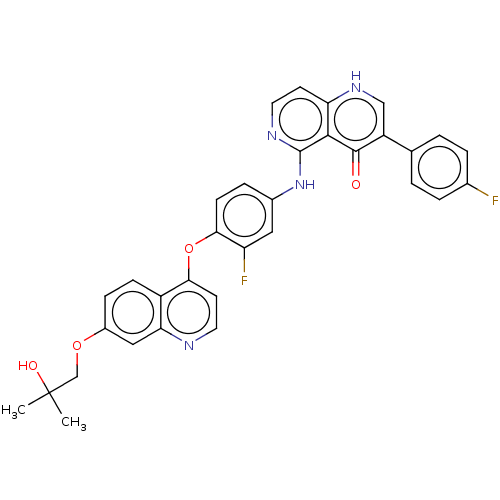
(US9938274, 4)Show SMILES CC(C)(O)COc1ccc2c(Oc3ccc(Nc4nccc5[nH]cc(-c6ccc(F)cc6)c(=O)c45)cc3F)ccnc2c1 Show InChI InChI=1S/C33H26F2N4O4/c1-33(2,41)18-42-22-8-9-23-27(16-22)36-14-12-28(23)43-29-10-7-21(15-25(29)35)39-32-30-26(11-13-37-32)38-17-24(31(30)40)19-3-5-20(34)6-4-19/h3-17,41H,18H2,1-2H3,(H,37,39)(H,38,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MET (unknown origin) using biotin as substrate preincubated for 5 mins followed by substrate addition and measured after 30 to 60 mins ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112174
BindingDB Entry DOI: 10.7270/Q23N2716 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50551454

(CHEMBL4799428)Show SMILES COCCOc1cc2nccc(Oc3ccc(Nc4nccc5[nH]cc(-c6ccccc6)c(=O)c45)cc3F)c2cc1OC | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MET (unknown origin) using biotin as substrate preincubated for 5 mins followed by substrate addition and measured after 30 to 60 mins ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112174
BindingDB Entry DOI: 10.7270/Q23N2716 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50056986

(CHEMBL3329993)Show SMILES Cc1[nH]c2ccccc2c1CCNS(=O)(=O)c1cccc(\C=C\C(=O)NO)c1 Show InChI InChI=1S/C20H21N3O4S/c1-14-17(18-7-2-3-8-19(18)22-14)11-12-21-28(26,27)16-6-4-5-15(13-16)9-10-20(24)23-25/h2-10,13,21-22,25H,11-12H2,1H3,(H,23,24)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells nuclear extracts incubated for 30 mins by fluorescent assay |
Eur J Med Chem 85: 468-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.020
BindingDB Entry DOI: 10.7270/Q2NK3GQQ |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50551460
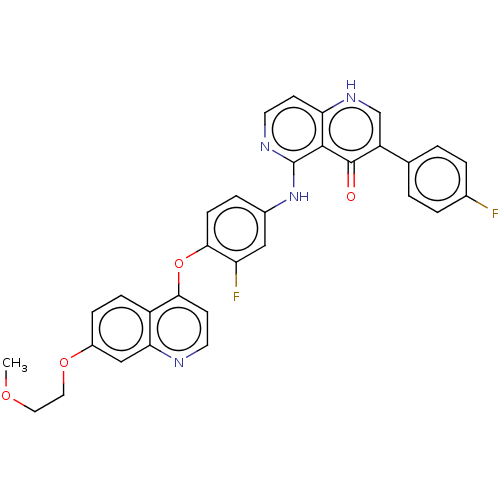
(CHEMBL4740288)Show SMILES COCCOc1ccc2c(Oc3ccc(Nc4nccc5[nH]cc(-c6ccc(F)cc6)c(=O)c45)cc3F)ccnc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MET (unknown origin) using biotin as substrate preincubated for 5 mins followed by substrate addition and measured after 30 to 60 mins ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112174
BindingDB Entry DOI: 10.7270/Q23N2716 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
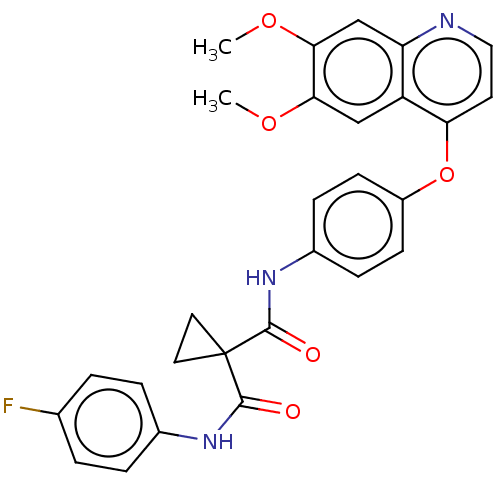
(Homo sapiens (Human)) | BDBM50021574
(BMS-907351 | CABOZANTINIB | CHEBI:72317 | Cabomety...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3)c2cc1OC Show InChI InChI=1S/C28H24FN3O5/c1-35-24-15-21-22(16-25(24)36-2)30-14-11-23(21)37-20-9-7-19(8-10-20)32-27(34)28(12-13-28)26(33)31-18-5-3-17(29)4-6-18/h3-11,14-16H,12-13H2,1-2H3,(H,31,33)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MET (unknown origin) using biotin as substrate preincubated for 5 mins followed by substrate addition and measured after 30 to 60 mins ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112174
BindingDB Entry DOI: 10.7270/Q23N2716 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 30 mins by fluorometric assay |
J Med Chem 55: 3777-91 (2012)
Article DOI: 10.1021/jm300197a
BindingDB Entry DOI: 10.7270/Q2CR5VD7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50056979

(CHEMBL3329997)Show SMILES CC(C)N(CCc1c(C)[nH]c2ccccc12)S(=O)(=O)c1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H27N3O4S/c1-16(2)26(15-14-20-17(3)24-22-7-5-4-6-21(20)22)31(29,30)19-11-8-18(9-12-19)10-13-23(27)25-28/h4-13,16,24,28H,14-15H2,1-3H3,(H,25,27)/b13-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells nuclear extracts incubated for 30 mins by fluorescent assay |
Eur J Med Chem 85: 468-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.020
BindingDB Entry DOI: 10.7270/Q2NK3GQQ |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50551457
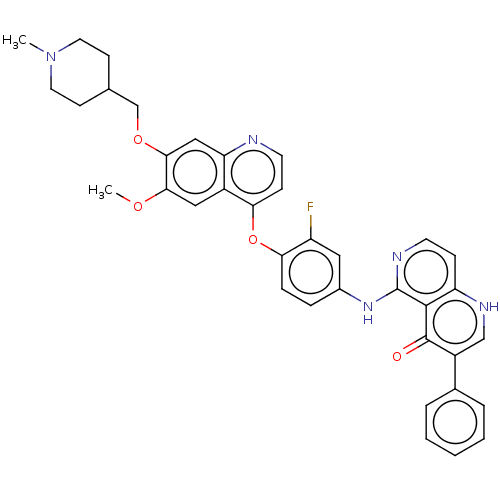
(CHEMBL4762933)Show SMILES COc1cc2c(Oc3ccc(Nc4nccc5[nH]cc(-c6ccccc6)c(=O)c45)cc3F)ccnc2cc1OCC1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MET (unknown origin) using biotin as substrate preincubated for 5 mins followed by substrate addition and measured after 30 to 60 mins ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112174
BindingDB Entry DOI: 10.7270/Q23N2716 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac) (379 to 382) p53 peptide as substrate by fluorescence assay |
J Med Chem 57: 4009-22 (2014)
Article DOI: 10.1021/jm401899x
BindingDB Entry DOI: 10.7270/Q28917F9 |
More data for this
Ligand-Target Pair | |
G-protein coupled receptor 84
(Homo sapiens (Human)) | BDBM50245480
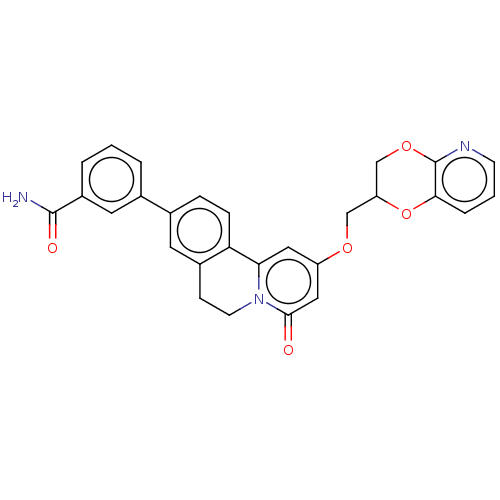
(CHEMBL4082477)Show SMILES NC(=O)c1cccc(c1)-c1ccc-2c(CCn3c-2cc(OCC2COc4ncccc4O2)cc3=O)c1 Show InChI InChI=1S/C28H23N3O5/c29-27(33)20-4-1-3-17(12-20)18-6-7-23-19(11-18)8-10-31-24(23)13-21(14-26(31)32)34-15-22-16-35-28-25(36-22)5-2-9-30-28/h1-7,9,11-14,22H,8,10,15-16H2,(H2,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Antagonist activity at GPR84 (unknown origin) expressed in cell membranes after 90 mins by [35S]GTPgammaS binding assay |
J Med Chem 60: 527-553 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00935
BindingDB Entry DOI: 10.7270/Q29C70V2 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50551453

(CHEMBL4785176)Show SMILES COc1cc2c(Oc3ccc(Nc4nccc5[nH]cc(-c6ccc(F)cc6)c(=O)c45)cc3F)ccnc2cc1OCC1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MET (unknown origin) using biotin as substrate preincubated for 5 mins followed by substrate addition and measured after 30 to 60 mins ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112174
BindingDB Entry DOI: 10.7270/Q23N2716 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50551456
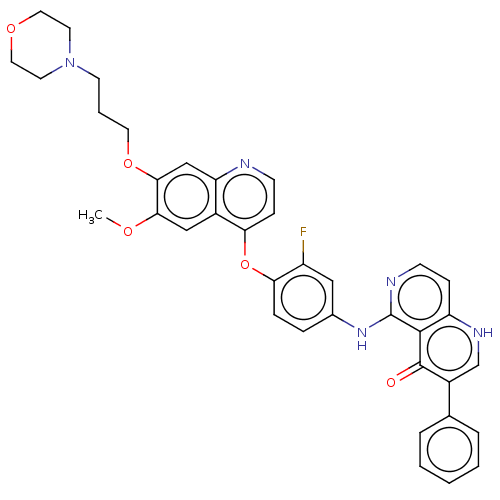
(CHEMBL4792128)Show SMILES COc1cc2c(Oc3ccc(Nc4nccc5[nH]cc(-c6ccccc6)c(=O)c45)cc3F)ccnc2cc1OCCCN1CCOCC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MET (unknown origin) using biotin as substrate preincubated for 5 mins followed by substrate addition and measured after 30 to 60 mins ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112174
BindingDB Entry DOI: 10.7270/Q23N2716 |
More data for this
Ligand-Target Pair | |
G-protein coupled receptor 84
(Homo sapiens (Human)) | BDBM50245516
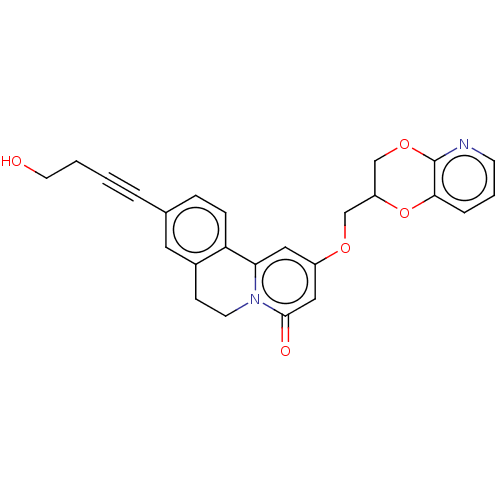
(CHEMBL4090321)Show SMILES OCCC#Cc1ccc-2c(CCn3c-2cc(OCC2COc4ncccc4O2)cc3=O)c1 Show InChI InChI=1S/C25H22N2O5/c28-11-2-1-4-17-6-7-21-18(12-17)8-10-27-22(21)13-19(14-24(27)29)30-15-20-16-31-25-23(32-20)5-3-9-26-25/h3,5-7,9,12-14,20,28H,2,8,10-11,15-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Antagonist activity at GPR84 (unknown origin) expressed in cell membranes after 90 mins by [35S]GTPgammaS binding assay |
J Med Chem 60: 527-553 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00935
BindingDB Entry DOI: 10.7270/Q29C70V2 |
More data for this
Ligand-Target Pair | |
G-protein coupled receptor 84
(Homo sapiens (Human)) | BDBM50245499
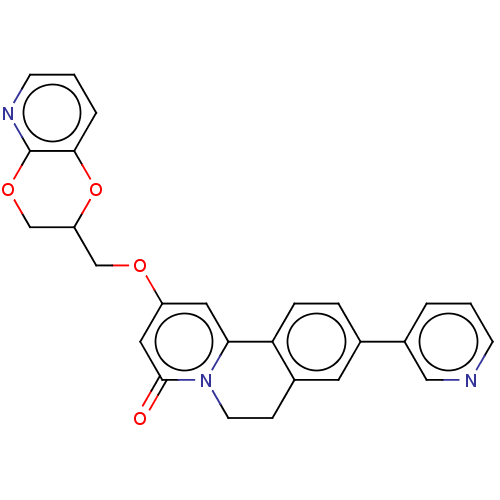
(CHEMBL4085616)Show SMILES O=c1cc(OCC2COc3ncccc3O2)cc2-c3ccc(cc3CCn12)-c1cccnc1 Show InChI InChI=1S/C26H21N3O4/c30-25-13-20(31-15-21-16-32-26-24(33-21)4-2-9-28-26)12-23-22-6-5-17(19-3-1-8-27-14-19)11-18(22)7-10-29(23)25/h1-6,8-9,11-14,21H,7,10,15-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Antagonist activity at GPR84 (unknown origin) expressed in cell membranes after 90 mins by [35S]GTPgammaS binding assay |
J Med Chem 60: 527-553 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00935
BindingDB Entry DOI: 10.7270/Q29C70V2 |
More data for this
Ligand-Target Pair | |
G-protein coupled receptor 84
(Homo sapiens (Human)) | BDBM50245494

(CHEMBL4080004)Show SMILES O=c1cc(OCC2COc3ncccc3O2)cc2-c3ccc(cc3CCn12)-c1cncnc1 Show InChI InChI=1S/C25H20N4O4/c30-24-10-19(31-13-20-14-32-25-23(33-20)2-1-6-28-25)9-22-21-4-3-16(18-11-26-15-27-12-18)8-17(21)5-7-29(22)24/h1-4,6,8-12,15,20H,5,7,13-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Antagonist activity at GPR84 (unknown origin) expressed in cell membranes after 90 mins by [35S]GTPgammaS binding assay |
J Med Chem 60: 527-553 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00935
BindingDB Entry DOI: 10.7270/Q29C70V2 |
More data for this
Ligand-Target Pair | |
G-protein coupled receptor 84
(Homo sapiens (Human)) | BDBM50245491

(CHEMBL4098028)Show SMILES Cc1noc(COc2ccc-3c(CCn4c-3cc(OCC3COc5ncccc5O3)cc4=O)c2)n1 Show InChI InChI=1S/C25H22N4O6/c1-15-27-23(35-28-15)14-32-17-4-5-20-16(9-17)6-8-29-21(20)10-18(11-24(29)30)31-12-19-13-33-25-22(34-19)3-2-7-26-25/h2-5,7,9-11,19H,6,8,12-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Antagonist activity at GPR84 (unknown origin) expressed in cell membranes after 90 mins by [35S]GTPgammaS binding assay |
J Med Chem 60: 527-553 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00935
BindingDB Entry DOI: 10.7270/Q29C70V2 |
More data for this
Ligand-Target Pair | |
G-protein coupled receptor 84
(Homo sapiens (Human)) | BDBM50245482
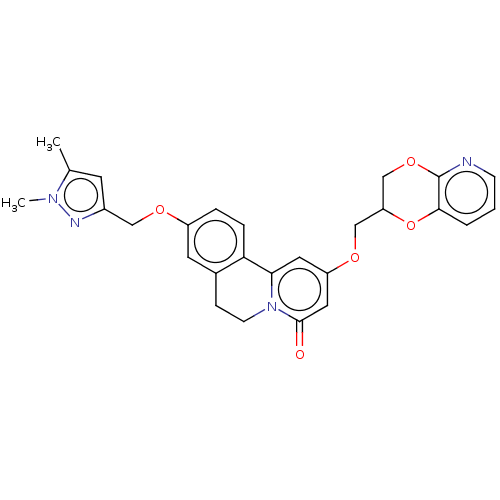
(CHEMBL4069275)Show SMILES Cc1cc(COc2ccc-3c(CCn4c-3cc(OCC3COc5ncccc5O3)cc4=O)c2)nn1C Show InChI InChI=1S/C27H26N4O5/c1-17-10-19(29-30(17)2)14-33-20-5-6-23-18(11-20)7-9-31-24(23)12-21(13-26(31)32)34-15-22-16-35-27-25(36-22)4-3-8-28-27/h3-6,8,10-13,22H,7,9,14-16H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Antagonist activity at GPR84 (unknown origin) expressed in cell membranes after 90 mins by [35S]GTPgammaS binding assay |
J Med Chem 60: 527-553 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00935
BindingDB Entry DOI: 10.7270/Q29C70V2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data