

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341578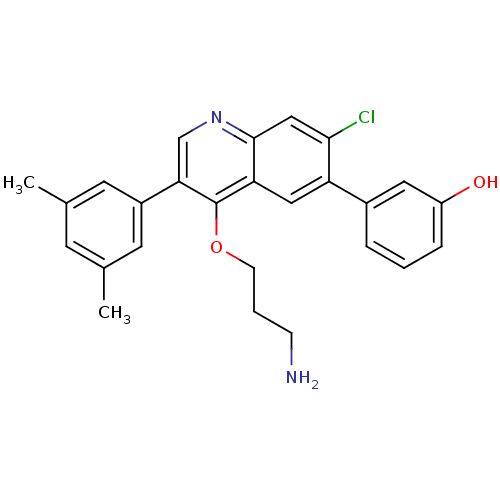 (3-[4-(3-Aminopropoxy)-7-chloro-3-(3,5-dimethylphen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341575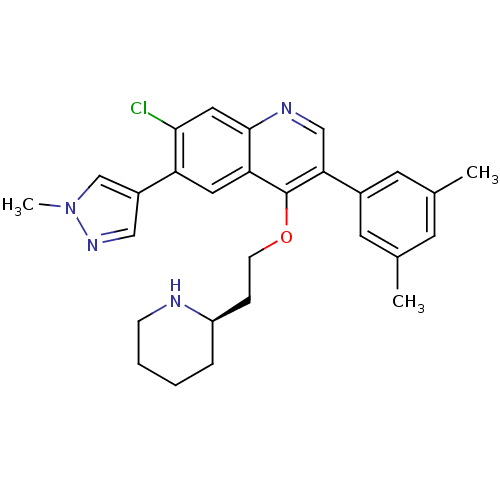 (7-Chloro-3-(3,5-dimethylphenyl)-6-(1-methyl-1H-pyr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341572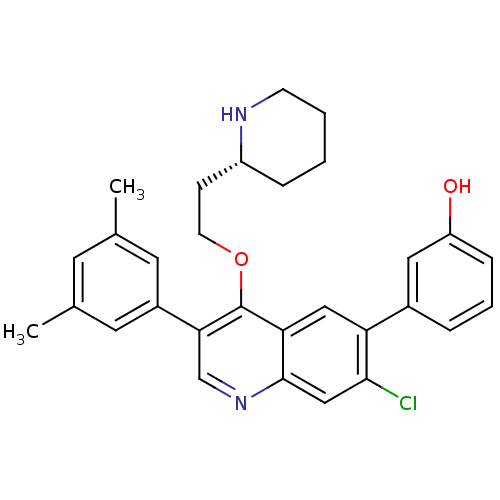 (3-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341574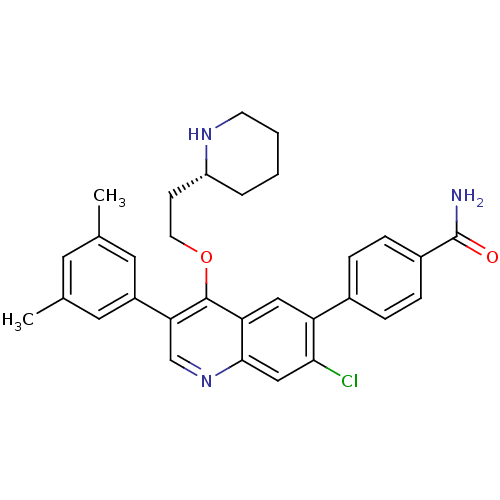 (3-{7-Chloro-3-(3,5-dimethylphenyl)-4-[2-(piperidin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341562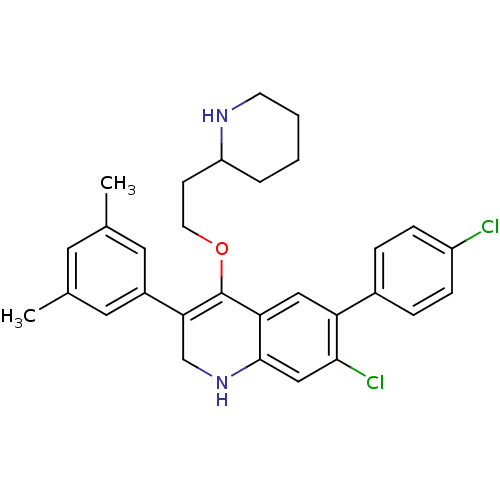 (7-chloro-6-(4-chlorophenyl)-3-(3,5-dimethylphenyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341577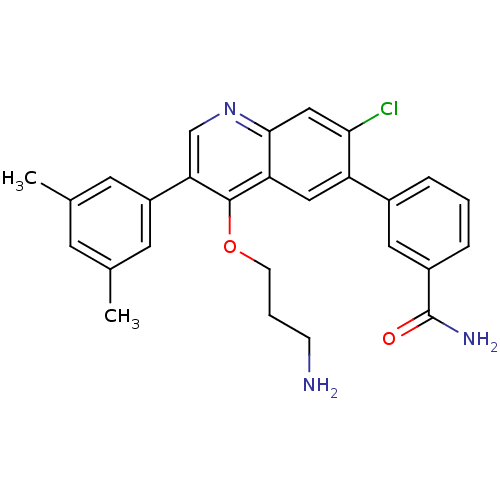 (3-[4-(3-Aminopropoxy)-7-chloro-3-(3,5-dimethylphen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341571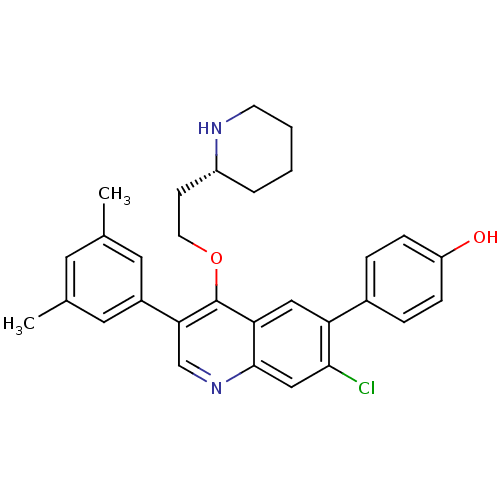 (4-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341573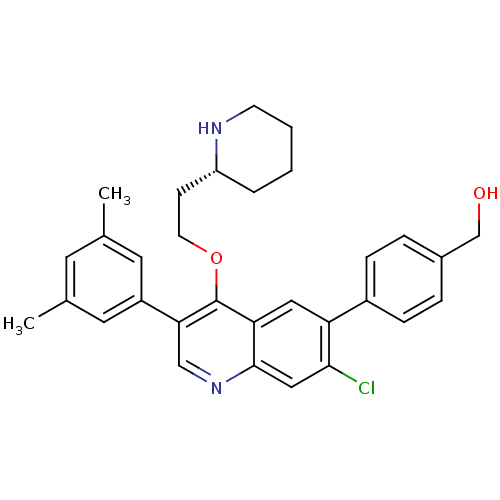 (CHEMBL1766098 | {4-[7-Chloro-3-(3,5-dimethylphenyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341570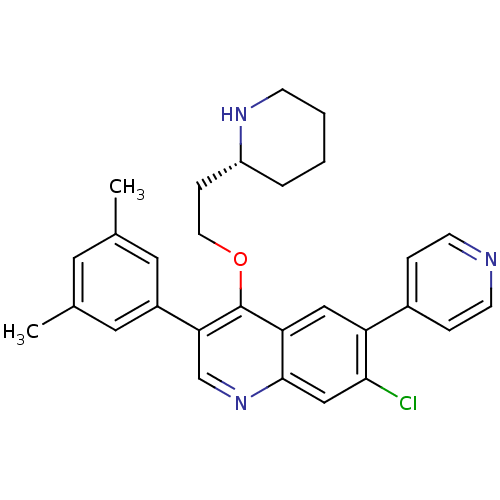 (7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-piperid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341569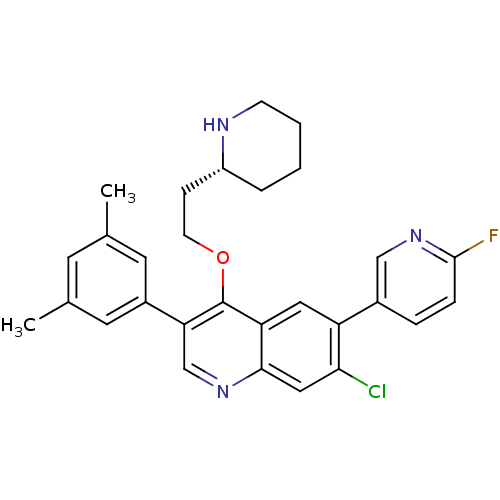 (7-Chloro-3-(3,5-dimethylphenyl)-6-(6-fluoropyridin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341568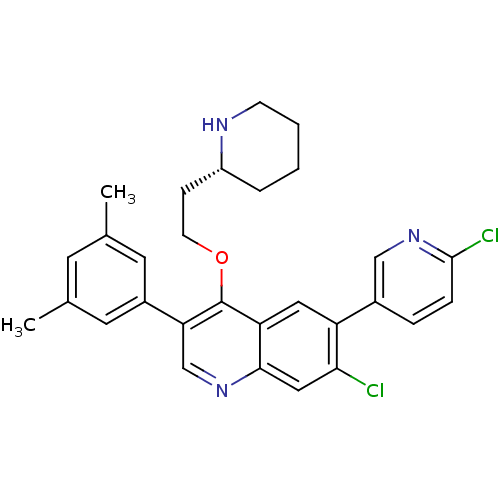 (7-Chloro-6-(6-chloropyridin-3-yl)-3-(3,5-dimethylp...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341580 (CHEMBL1766105 | [(4-{2-[(2R)-Piperidin-2-yl]ethoxy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341579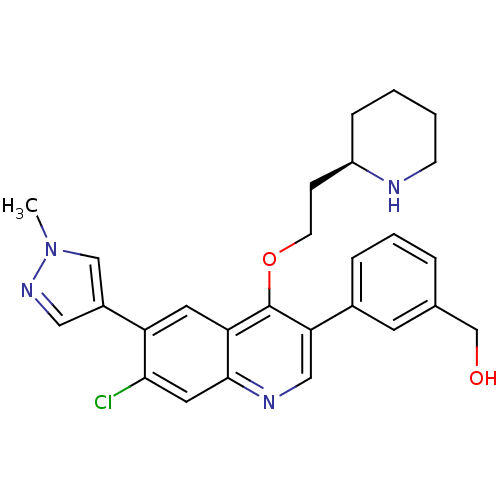 (CHEMBL1766104 | {3-[7-Chloro-6-(1-methyl-1H-pyrazo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341564 (7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-piperid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186291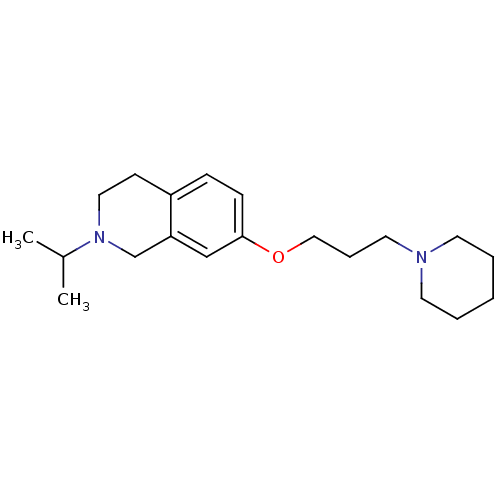 (2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341563 (7-chloro-3-(3,5-dimethylphenyl)-6-phenyl-4-(2-(pip...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186278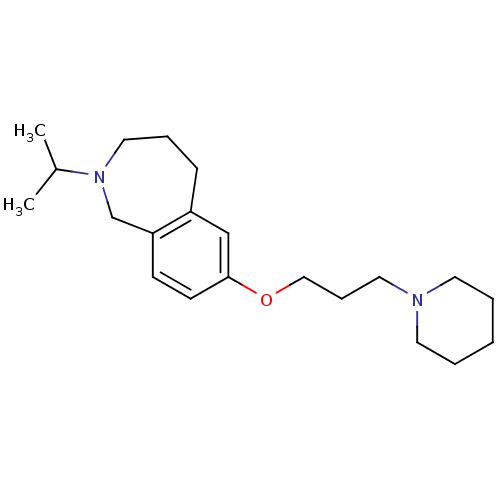 (2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186270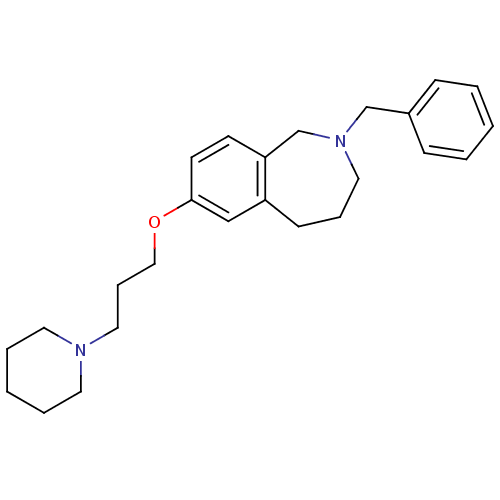 (2-benzyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156774 (CHEMBL3792888) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186290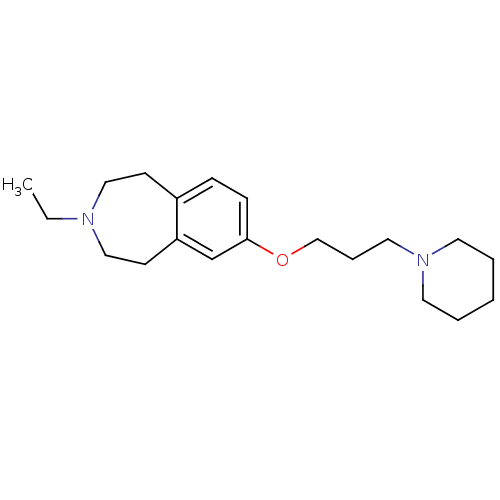 (3-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341565 (5-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50257034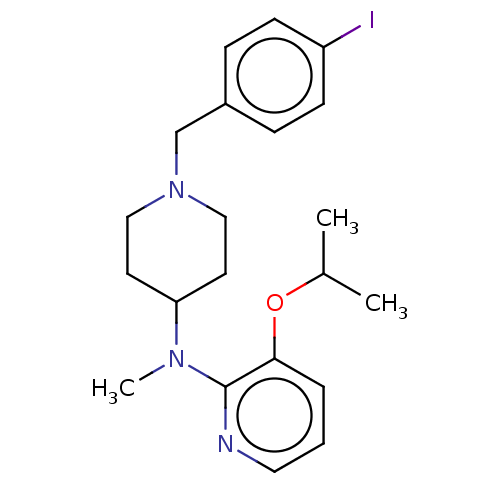 (CHEMBL4096543) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy, University of Nebraska Medical Center , Omaha, Nebraska 68198-6125, United States. Curated by ChEMBL | Assay Description Inhibition of human dopamine D4 receptor | J Med Chem 60: 7233-7243 (2017) Article DOI: 10.1021/acs.jmedchem.7b00151 BindingDB Entry DOI: 10.7270/Q2P84FBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186309 (2-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186269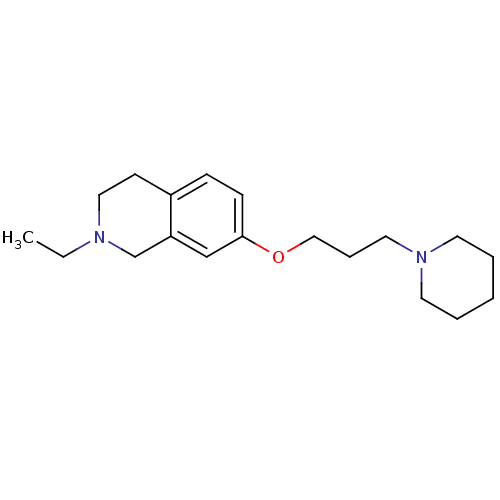 (2-ethyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186292 (2-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156799 (CHEMBL3793004) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156788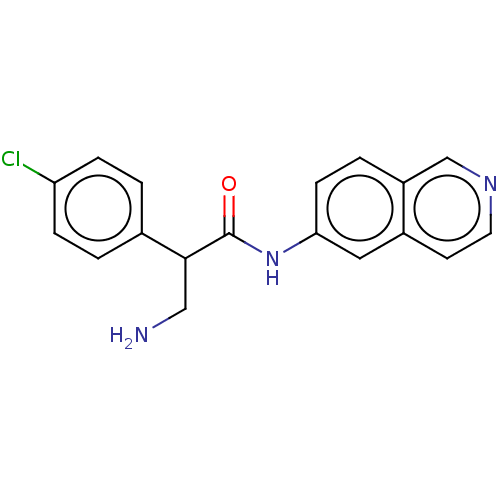 (CHEMBL3794104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156784 (CHEMBL3792673) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | Mol Pharmacol 39: 346-51 (1991) BindingDB Entry DOI: 10.7270/Q2222S8S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM85093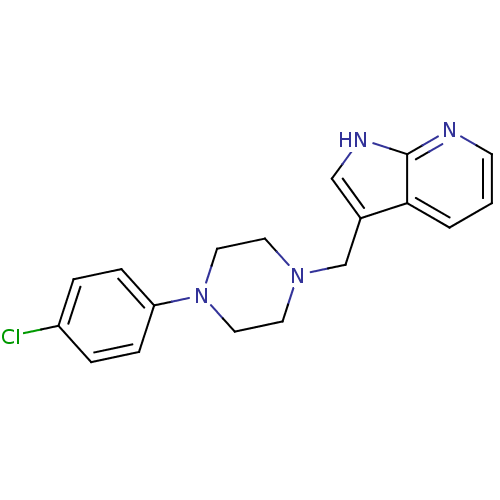 (CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy, University of Nebraska Medical Center , Omaha, Nebraska 68198-6125, United States. Curated by ChEMBL | Assay Description Inhibition of human dopamine D4 receptor | J Med Chem 60: 7233-7243 (2017) Article DOI: 10.1021/acs.jmedchem.7b00151 BindingDB Entry DOI: 10.7270/Q2P84FBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50186292 (2-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186316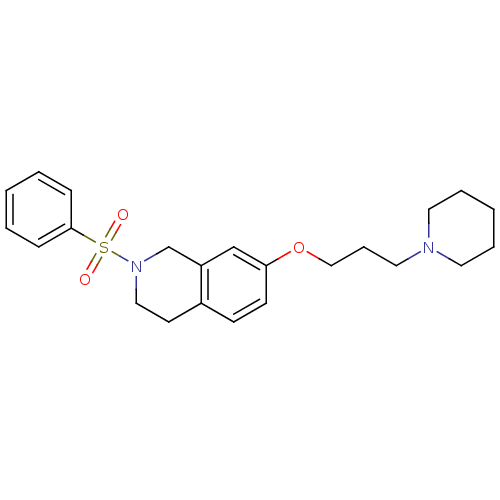 (2-(phenylsulfonyl)-7-(3-(piperidin-1-yl)propoxy)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186306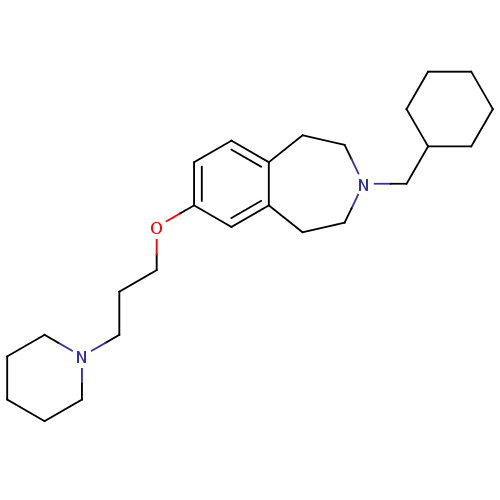 (3-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186283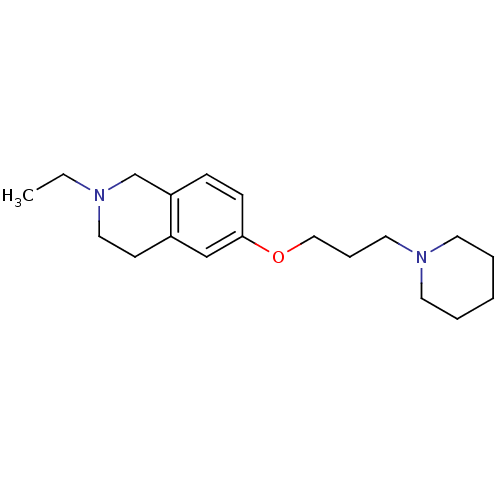 (2-ethyl-6-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (RAT) | BDBM50403547 (ATROPEN | ATROPINE) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Displacement of [3H]NMS from rat muscarinic M4 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 885-90 (2008) Article DOI: 10.1016/j.bmcl.2007.12.051 BindingDB Entry DOI: 10.7270/Q2DZ095Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50186309 (2-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50124595 (5-Allyl-10-chloro-2,2,4-trimethyl-2,5-dihydro-1H-6...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human glucocorticoid receptor (GR) was determined using [3H]-Dexamethasone as radioligand in SF-1 cells | J Med Chem 46: 1016-30 (2003) Article DOI: 10.1021/jm020335m BindingDB Entry DOI: 10.7270/Q2MP52NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186293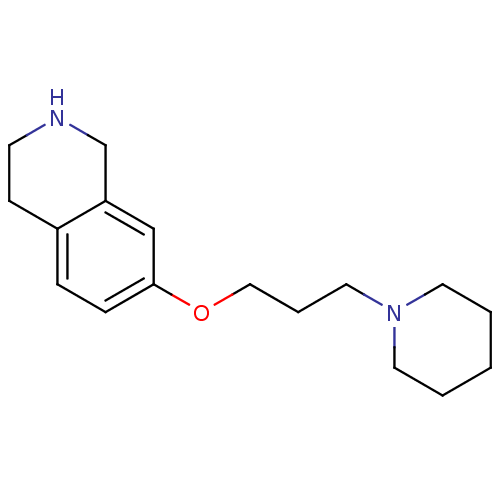 (7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetrahydrois...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341566 (5-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156782 (CHEMBL3792663) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50240552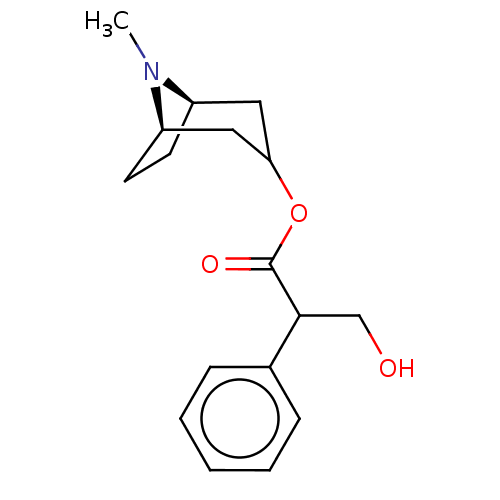 (CHEMBL195) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human recombinant muscarinic M4 receptor expressed in CHO cell membranes | J Med Chem 34: 1879-84 (1991) Article DOI: 10.1016/j.bmcl.2017.05.042 BindingDB Entry DOI: 10.7270/Q29G5KR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50186269 (2-ethyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341567 (7-Chloro-3-(3,5-dimethylphenyl)-6-(6-methoxypyridi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156683 (CHEMBL3793788) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156732 (CHEMBL3793497) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186311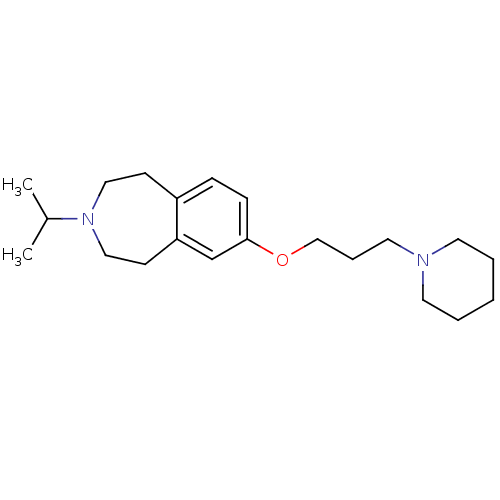 (3-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156687 (CHEMBL3793029) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156787 (CHEMBL3793404) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50148579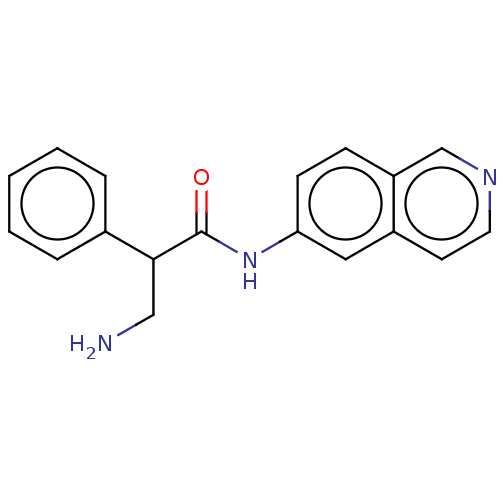 (CHEMBL3770836) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50186270 (2-benzyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 16000 total ) | Next | Last >> |