 Found 290 hits with Last Name = 'lindeberg' and Initial = 'g'
Found 290 hits with Last Name = 'lindeberg' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor B
(RAT) | BDBM50370684
(CHEMBL1791349)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C49H71N13O10/c1-6-29(4)41(46(69)58-36(24-32-25-53-27-55-32)47(70)62-21-11-15-38(62)44(67)59-37(48(71)72)23-30-12-8-7-9-13-30)61-43(66)35(22-31-16-18-33(63)19-17-31)57-45(68)40(28(2)3)60-42(65)34(56-39(64)26-52-5)14-10-20-54-49(50)51/h7-9,12-13,16-19,25,27-29,34-38,40-41,52,63H,6,10-11,14-15,20-24,26H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,67)(H,60,65)(H,61,66)(H,71,72)(H4,50,51,54)/t29-,34+,35+,36+,37+,38+,40+,41+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II receptor, type 1 in rat liver membrane using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 2 hr at ... |
J Med Chem 48: 6620-31 (2005)
Article DOI: 10.1021/jm050280z
BindingDB Entry DOI: 10.7270/Q2HX1DG6 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370684

(CHEMBL1791349)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C49H71N13O10/c1-6-29(4)41(46(69)58-36(24-32-25-53-27-55-32)47(70)62-21-11-15-38(62)44(67)59-37(48(71)72)23-30-12-8-7-9-13-30)61-43(66)35(22-31-16-18-33(63)19-17-31)57-45(68)40(28(2)3)60-42(65)34(56-39(64)26-52-5)14-10-20-54-49(50)51/h7-9,12-13,16-19,25,27-29,34-38,40-41,52,63H,6,10-11,14-15,20-24,26H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,67)(H,60,65)(H,61,66)(H,71,72)(H4,50,51,54)/t29-,34+,35+,36+,37+,38+,40+,41+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II receptor, type 2 in pig uterus myometrium using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 1.5 h... |
J Med Chem 48: 6620-31 (2005)
Article DOI: 10.1021/jm050280z
BindingDB Entry DOI: 10.7270/Q2HX1DG6 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50472361
(CHEMBL406349)Show SMILES N[C@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H]1CCSSCC[C@@H](NC(=O)[C@@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@H](Cc1ccccc1)C(O)=O |wU:59.63,46.47,9.8,31.31,wD:20.19,27.44,63.66,1.0,(-2.49,-6.28,;-2.47,-4.62,;-3.84,-3.93,;-5.11,-4.77,;-5.13,-6.21,;-6.53,-4.07,;-1.11,-3.93,;-1.11,-2.41,;.19,-4.74,;1.56,-4.01,;1.59,-2.47,;2.96,-1.73,;2.99,-.19,;4.35,.53,;4.39,2.07,;5.75,2.8,;3.09,2.89,;2.87,-4.81,;2.68,-3.3,;4.21,-5.54,;5.55,-4.77,;5.54,-3.23,;6.63,-2.13,;7.96,-1.36,;11.63,-.89,;13.17,-.89,;14.26,-1.99,;13.54,-3.36,;12.02,-3.43,;10.48,-3.48,;9.64,-2.18,;9.76,-4.85,;10.66,-6.02,;9.85,-7.26,;10.53,-8.56,;9.71,-9.78,;8.24,-9.71,;7.43,-10.95,;7.57,-8.41,;8.36,-7.17,;8.22,-4.77,;6.89,-5.54,;6.89,-7.08,;14.37,-4.67,;14.36,-6.19,;15.85,-4.25,;17.23,-4.78,;17.41,-6.19,;16.4,-7.33,;15.13,-6.82,;14.1,-7.96,;14.75,-9.19,;16.19,-8.82,;18.23,-3.64,;17.74,-2.18,;19.74,-3.95,;19.74,-2.41,;21.22,-1.94,;22.12,-3.13,;21.21,-4.39,;21.7,-5.86,;20.68,-7.01,;23.2,-6.16,;24.74,-6.17,;25.76,-4.98,;24.97,-3.64,;23.43,-3.64,;22.64,-2.29,;23.43,-.94,;24.97,-.94,;25.76,-2.29,;25.25,-7.63,;24.37,-8.87,;26.67,-7.65,)| Show InChI InChI=1S/C47H63N13O12S2/c48-30(23-38(62)63)39(64)54-31(8-4-16-52-47(49)50)40(65)55-32-14-18-73-74-19-15-33(56-43(68)34(57-41(32)66)20-27-10-12-29(61)13-11-27)42(67)58-35(22-28-24-51-25-53-28)45(70)60-17-5-9-37(60)44(69)59-36(46(71)72)21-26-6-2-1-3-7-26/h1-3,6-7,10-13,24-25,30-37,61H,4-5,8-9,14-23,48H2,(H,51,53)(H,54,64)(H,55,65)(H,56,68)(H,57,66)(H,58,67)(H,59,69)(H,62,63)(H,71,72)(H4,49,50,52)/t30-,31+,32-,33-,34-,35-,36-,37+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor, type 1 from rat liver membranes |
J Med Chem 42: 4524-37 (1999)
Article DOI: 10.1021/jm991089q
BindingDB Entry DOI: 10.7270/Q2PN98C1 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50015662
(3-Amino-N-{1-[5-[2-[2-(1-carboxy-2-phenyl-ethylcar...)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CCSSCC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C47H63N13O12S2/c48-30(23-38(62)63)39(64)54-31(8-4-16-52-47(49)50)40(65)55-32-14-18-73-74-19-15-33(56-43(68)34(57-41(32)66)20-27-10-12-29(61)13-11-27)42(67)58-35(22-28-24-51-25-53-28)45(70)60-17-5-9-37(60)44(69)59-36(46(71)72)21-26-6-2-1-3-7-26/h1-3,6-7,10-13,24-25,30-37,61H,4-5,8-9,14-23,48H2,(H,51,53)(H,54,64)(H,55,65)(H,56,68)(H,57,66)(H,58,67)(H,59,69)(H,62,63)(H,71,72)(H4,49,50,52)/t30-,31-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
In vitro binding affinity at rat liver Angiotensin II receptor, type 1 was determined based on displacement of [125I]-Ang II |
J Med Chem 45: 1767-77 (2002)
BindingDB Entry DOI: 10.7270/Q2QV3N7W |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50142916
((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...)Show SMILES COc1ccc2c(O[C@@H]3C[C@@H]4N(C3)C(=O)[C@H](CCCCC\C=C/[C@@H]3C[C@]3(NC4=O)C(O)=O)NC(=O)OC3CCCC3)cc(nc2c1)-c1csc(NC(C)C)n1 |r,c:22| Show InChI InChI=1S/C40H50N6O8S/c1-23(2)41-38-43-32(22-55-38)31-19-34(28-16-15-26(52-3)17-30(28)42-31)53-27-18-33-35(47)45-40(37(49)50)20-24(40)11-7-5-4-6-8-14-29(36(48)46(33)21-27)44-39(51)54-25-12-9-10-13-25/h7,11,15-17,19,22-25,27,29,33H,4-6,8-10,12-14,18,20-21H2,1-3H3,(H,41,43)(H,44,51)(H,45,47)(H,49,50)/b11-7-/t24-,27-,29+,33+,40-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of HCV 1a NS3 protease |
Bioorg Med Chem 15: 1448-74 (2007)
Article DOI: 10.1016/j.bmc.2006.11.003
BindingDB Entry DOI: 10.7270/Q22B8ZVH |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370575
(CHEMBL1791308)Show SMILES CC[C@@H](C)[C@H](N1Cc2cccc(NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)c2N[C@@H](Cc2ccc(O)cc2)C1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:68.73,15.15,55.57,41.42,30.30,2.2,wD:4.4,19.26,72.76,(20.86,-11.16,;20.86,-9.62,;19.5,-8.85,;18.13,-9.62,;19.5,-7.31,;18.13,-6.53,;17.29,-7.83,;15.7,-8,;15.29,-9.47,;13.78,-9.86,;12.67,-8.77,;13.1,-7.3,;11.74,-6.61,;10.41,-7.27,;10.41,-8.62,;9.06,-6.59,;7.72,-7.27,;6.37,-6.59,;6.4,-5.24,;5.03,-7.24,;5.03,-8.61,;6.22,-9.28,;6.2,-10.63,;7.41,-11.31,;7.4,-12.65,;6.2,-13.32,;8.58,-13.35,;3.69,-6.58,;2.35,-7.24,;2.35,-8.58,;.99,-6.58,;-.35,-7.21,;1.02,-5.24,;-.33,-4.54,;-1.69,-5.21,;-.32,-3.2,;9.07,-5.22,;10.45,-4.54,;7.71,-4.51,;14.61,-6.9,;14.8,-5.36,;16.14,-4.57,;15.99,-3.04,;16.77,-1.69,;15.97,-.38,;16.74,.97,;18.32,.97,;19.09,2.32,;19.13,-.34,;18.35,-1.69,;17.63,-5.09,;18.76,-3.99,;20.86,-6.53,;20.86,-5,;22.25,-7.3,;23.67,-6.69,;23.73,-5.14,;24.52,-3.79,;26.07,-3.63,;26.4,-2.11,;25.03,-1.35,;23.84,-2.38,;25,-7.41,;24.92,-8.77,;26.39,-6.67,;25.88,-5.19,;27.18,-4.28,;28.45,-5.19,;27.97,-6.67,;29.35,-7.43,;29.34,-8.98,;30.71,-6.67,;32.02,-7.41,;31.94,-8.77,;33.27,-9.48,;34.67,-8.88,;35.98,-9.63,;35.9,-10.95,;34.48,-11.61,;33.19,-10.87,;33.44,-6.8,;34.77,-7.53,;33.52,-5.43,)| Show InChI InChI=1S/C57H76N14O12/c1-5-32(4)48(53(79)67-42(26-36-28-61-30-63-36)54(80)70-23-11-17-44(70)51(77)68-43(56(82)83)25-33-12-7-6-8-13-33)71-29-35-14-9-15-39(47(35)64-41(55(71)81)24-34-18-20-37(72)21-19-34)65-52(78)46(31(2)3)69-50(76)40(16-10-22-62-57(59)60)66-49(75)38(58)27-45(73)74/h6-9,12-15,18-21,28,30-32,38,40-44,46,48,64,72H,5,10-11,16-17,22-27,29,58H2,1-4H3,(H,61,63)(H,65,78)(H,66,75)(H,67,79)(H,68,77)(H,69,76)(H,73,74)(H,82,83)(H4,59,60,62)/t32-,38+,40+,41+,42+,43+,44+,46+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370575

(CHEMBL1791308)Show SMILES CC[C@@H](C)[C@H](N1Cc2cccc(NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)c2N[C@@H](Cc2ccc(O)cc2)C1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:68.73,15.15,55.57,41.42,30.30,2.2,wD:4.4,19.26,72.76,(20.86,-11.16,;20.86,-9.62,;19.5,-8.85,;18.13,-9.62,;19.5,-7.31,;18.13,-6.53,;17.29,-7.83,;15.7,-8,;15.29,-9.47,;13.78,-9.86,;12.67,-8.77,;13.1,-7.3,;11.74,-6.61,;10.41,-7.27,;10.41,-8.62,;9.06,-6.59,;7.72,-7.27,;6.37,-6.59,;6.4,-5.24,;5.03,-7.24,;5.03,-8.61,;6.22,-9.28,;6.2,-10.63,;7.41,-11.31,;7.4,-12.65,;6.2,-13.32,;8.58,-13.35,;3.69,-6.58,;2.35,-7.24,;2.35,-8.58,;.99,-6.58,;-.35,-7.21,;1.02,-5.24,;-.33,-4.54,;-1.69,-5.21,;-.32,-3.2,;9.07,-5.22,;10.45,-4.54,;7.71,-4.51,;14.61,-6.9,;14.8,-5.36,;16.14,-4.57,;15.99,-3.04,;16.77,-1.69,;15.97,-.38,;16.74,.97,;18.32,.97,;19.09,2.32,;19.13,-.34,;18.35,-1.69,;17.63,-5.09,;18.76,-3.99,;20.86,-6.53,;20.86,-5,;22.25,-7.3,;23.67,-6.69,;23.73,-5.14,;24.52,-3.79,;26.07,-3.63,;26.4,-2.11,;25.03,-1.35,;23.84,-2.38,;25,-7.41,;24.92,-8.77,;26.39,-6.67,;25.88,-5.19,;27.18,-4.28,;28.45,-5.19,;27.97,-6.67,;29.35,-7.43,;29.34,-8.98,;30.71,-6.67,;32.02,-7.41,;31.94,-8.77,;33.27,-9.48,;34.67,-8.88,;35.98,-9.63,;35.9,-10.95,;34.48,-11.61,;33.19,-10.87,;33.44,-6.8,;34.77,-7.53,;33.52,-5.43,)| Show InChI InChI=1S/C57H76N14O12/c1-5-32(4)48(53(79)67-42(26-36-28-61-30-63-36)54(80)70-23-11-17-44(70)51(77)68-43(56(82)83)25-33-12-7-6-8-13-33)71-29-35-14-9-15-39(47(35)64-41(55(71)81)24-34-18-20-37(72)21-19-34)65-52(78)46(31(2)3)69-50(76)40(16-10-22-62-57(59)60)66-49(75)38(58)27-45(73)74/h6-9,12-15,18-21,28,30-32,38,40-44,46,48,64,72H,5,10-11,16-17,22-27,29,58H2,1-4H3,(H,61,63)(H,65,78)(H,66,75)(H,67,79)(H,68,77)(H,69,76)(H,73,74)(H,82,83)(H4,59,60,62)/t32-,38+,40+,41+,42+,43+,44+,46+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228195

(Angiotensin Ii | CHEBI:2719)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor, type 1 from rat liver membranes |
J Med Chem 42: 4524-37 (1999)
Article DOI: 10.1021/jm991089q
BindingDB Entry DOI: 10.7270/Q2PN98C1 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50139783
(Angiotensin II analogue | CHEMBL404996)Show SMILES CCSC[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CS)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C47H65N13O12S2/c1-2-74-24-36(43(68)56-33(20-28-22-51-25-53-28)45(70)60-17-7-11-37(60)44(69)57-34(46(71)72)19-26-8-4-3-5-9-26)59-41(66)32(18-27-12-14-29(61)15-13-27)55-42(67)35(23-73)58-40(65)31(10-6-16-52-47(49)50)54-39(64)30(48)21-38(62)63/h3-5,8-9,12-15,22,25,30-37,61,73H,2,6-7,10-11,16-21,23-24,48H2,1H3,(H,51,53)(H,54,64)(H,55,67)(H,56,68)(H,57,69)(H,58,65)(H,59,66)(H,62,63)(H,71,72)(H4,49,50,52)/t30-,31-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor type 2 using [125I]- Ang II in pig uterus myometrium |
J Med Chem 47: 859-70 (2004)
Article DOI: 10.1021/jm030921v
BindingDB Entry DOI: 10.7270/Q2736RNQ |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370686
(CHEMBL1791350)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](N)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C46H68N12O11/c1-5-26(4)36(56-43(66)37(48)57-41(64)35(25(2)3)55-39(62)30(18-12-20-51-46(49)50)52-38(61)29(47)24-34(59)60)42(65)53-31(22-27-14-8-6-9-15-27)44(67)58-21-13-19-33(58)40(63)54-32(45(68)69)23-28-16-10-7-11-17-28/h6-11,14-17,25-26,29-33,35-37H,5,12-13,18-24,47-48H2,1-4H3,(H,52,61)(H,53,65)(H,54,63)(H,55,62)(H,56,66)(H,57,64)(H,59,60)(H,68,69)(H4,49,50,51)/t26-,29+,30+,31+,32+,33+,35+,36+,37-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II receptor, type 2 in pig uterus myometrium using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 1.5 h... |
J Med Chem 48: 6620-31 (2005)
Article DOI: 10.1021/jm050280z
BindingDB Entry DOI: 10.7270/Q2HX1DG6 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370375

(CHEMBL268815)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#7])cc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O Show InChI InChI=1S/C53H74N12O12/c1-5-30(4)44(50(74)61-39(26-32-15-19-34(54)20-16-32)51(75)65-24-10-14-41(65)48(72)62-40(52(76)77)27-31-11-7-6-8-12-31)64-47(71)38(25-33-17-21-35(66)22-18-33)60-49(73)43(29(2)3)63-46(70)37(13-9-23-58-53(56)57)59-45(69)36(55)28-42(67)68/h6-8,11-12,15-22,29-30,36-41,43-44,66H,5,9-10,13-14,23-28,54-55H2,1-4H3,(H,59,69)(H,60,73)(H,61,74)(H,62,72)(H,63,70)(H,64,71)(H,67,68)(H,76,77)(H4,56,57,58)/t30-,36+,37+,38+,39+,40+,41+,43+,44+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ang2 from AT2 receptor in pig uterus myometrium |
J Med Chem 49: 6133-7 (2006)
Article DOI: 10.1021/jm051222g
BindingDB Entry DOI: 10.7270/Q28P619Q |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50366760
(CHEMBL2369589)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C(O)=O)C(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CS)C(O)=O Show InChI InChI=1S/C36H58N6O14S/c1-6-18(4)28(33(50)40-23(13-20-10-8-7-9-11-20)29(46)41-26(16-57)36(55)56)42-32(49)22(12-17(2)3)38-30(47)24(14-21(34(51)52)35(53)54)39-31(48)25(15-27(44)45)37-19(5)43/h17-18,20-26,28,57H,6-16H2,1-5H3,(H,37,43)(H,38,47)(H,39,48)(H,40,50)(H,41,46)(H,42,49)(H,44,45)(H,51,52)(H,53,54)(H,55,56)/t18-,22-,23-,24+,25-,26-,28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibitory activity against hepatitis C virus (HCV) NS3 protease (isolated domain) |
Bioorg Med Chem Lett 11: 203-6 (2001)
BindingDB Entry DOI: 10.7270/Q26Q1XSK |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50168428
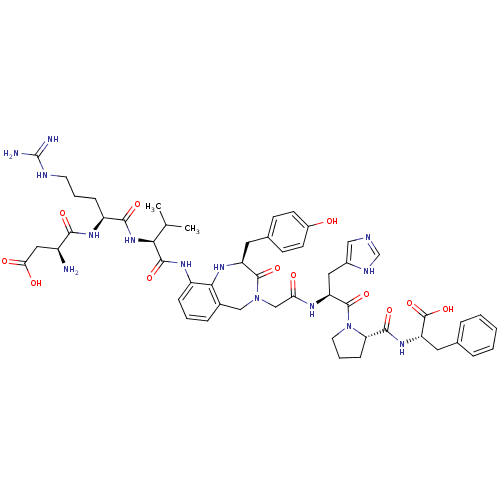
((3S)-3-amino-3-{[(1S)-1-{[(1S)-1-{[(2S)-4-({[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(=O)Nc1cccc2CN(CC(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N3CCC[C@H]3C(=O)N[C@@H](Cc3ccccc3)C(O)=O)C(=O)[C@H](Cc3ccc(O)cc3)Nc12 Show InChI InChI=1S/C53H68N14O12/c1-29(2)44(65-47(73)37(13-7-19-58-53(55)56)63-46(72)35(54)24-43(70)71)49(75)62-36-12-6-11-32-26-66(50(76)38(61-45(32)36)21-31-15-17-34(68)18-16-31)27-42(69)60-39(23-33-25-57-28-59-33)51(77)67-20-8-14-41(67)48(74)64-40(52(78)79)22-30-9-4-3-5-10-30/h3-6,9-12,15-18,25,28-29,35,37-41,44,61,68H,7-8,13-14,19-24,26-27,54H2,1-2H3,(H,57,59)(H,60,69)(H,62,75)(H,63,72)(H,64,74)(H,65,73)(H,70,71)(H,78,79)(H4,55,56,58)/t35-,37-,38-,39-,40-,41-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370375

(CHEMBL268815)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#7])cc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O Show InChI InChI=1S/C53H74N12O12/c1-5-30(4)44(50(74)61-39(26-32-15-19-34(54)20-16-32)51(75)65-24-10-14-41(65)48(72)62-40(52(76)77)27-31-11-7-6-8-12-31)64-47(71)38(25-33-17-21-35(66)22-18-33)60-49(73)43(29(2)3)63-46(70)37(13-9-23-58-53(56)57)59-45(69)36(55)28-42(67)68/h6-8,11-12,15-22,29-30,36-41,43-44,66H,5,9-10,13-14,23-28,54-55H2,1-4H3,(H,59,69)(H,60,73)(H,61,74)(H,62,72)(H,63,70)(H,64,71)(H,67,68)(H,76,77)(H4,56,57,58)/t30-,36+,37+,38+,39+,40+,41+,43+,44+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50208102
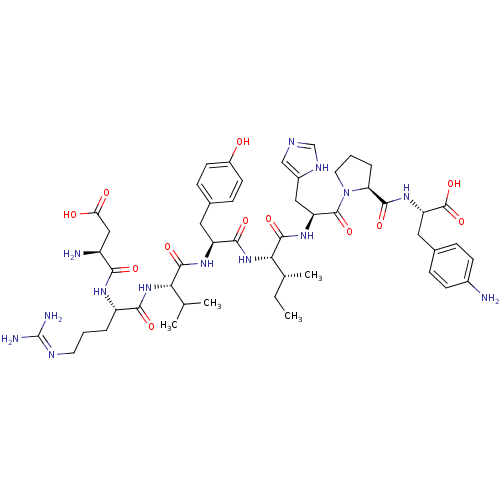
(CHEMBL424755 | [4-NH2-Phe6] Ang II)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(N)cc1)C(O)=O |wU:8.17,24.32,60.63,47.47,2.2,wD:4.4,20.21,64.66,35.36,(17.7,-20.36,;17.7,-18.82,;16.36,-18.05,;15.03,-18.83,;16.36,-16.51,;15.03,-15.75,;13.7,-16.52,;13.7,-18.06,;12.37,-15.76,;12.36,-14.22,;13.7,-13.44,;15.03,-14.21,;16.36,-13.44,;16.35,-11.9,;17.69,-11.13,;15.01,-11.13,;13.68,-11.91,;11.04,-16.53,;9.71,-15.77,;9.7,-14.23,;8.38,-16.54,;7.05,-15.77,;5.72,-16.54,;5.72,-18.08,;4.39,-15.78,;4.38,-14.24,;3.05,-13.47,;3.04,-11.93,;1.71,-11.17,;1.71,-9.63,;.37,-8.86,;3.04,-8.85,;3.06,-16.55,;1.73,-15.79,;1.72,-14.25,;.4,-16.56,;-.94,-15.79,;.4,-18.1,;-.93,-18.87,;-.93,-20.41,;-2.27,-18.1,;8.38,-18.08,;7.05,-18.85,;9.72,-18.84,;17.69,-15.74,;17.68,-14.2,;19.02,-16.5,;20.35,-15.73,;20.34,-14.19,;21.39,-13.06,;21.08,-11.55,;22.42,-10.8,;23.55,-11.84,;22.91,-13.24,;21.68,-16.5,;21.68,-18.04,;23.01,-15.72,;23.17,-14.19,;24.67,-13.86,;25.44,-15.19,;24.42,-16.34,;24.88,-17.81,;23.84,-18.94,;26.38,-18.15,;26.84,-19.62,;25.51,-20.4,;25.53,-21.94,;24.2,-22.71,;24.22,-24.25,;25.56,-25.01,;25.57,-26.55,;26.89,-24.22,;26.87,-22.68,;28.34,-19.95,;28.81,-21.42,;29.39,-18.82,)| Show InChI InChI=1S/C50H72N14O12/c1-5-27(4)41(47(73)60-36(22-31-24-55-25-57-31)48(74)64-19-7-9-38(64)45(71)61-37(49(75)76)21-28-10-14-30(51)15-11-28)63-44(70)35(20-29-12-16-32(65)17-13-29)59-46(72)40(26(2)3)62-43(69)34(8-6-18-56-50(53)54)58-42(68)33(52)23-39(66)67/h10-17,24-27,33-38,40-41,65H,5-9,18-23,51-52H2,1-4H3,(H,55,57)(H,58,68)(H,59,72)(H,60,73)(H,61,71)(H,62,69)(H,63,70)(H,66,67)(H,75,76)(H4,53,54,56)/t27-,33+,34+,35+,36+,37+,38+,40+,41+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ang2 from AT2 receptor in pig uterus myometrial membrane |
J Med Chem 50: 1711-5 (2007)
Article DOI: 10.1021/jm0613469
BindingDB Entry DOI: 10.7270/Q2NK3FTN |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50156176
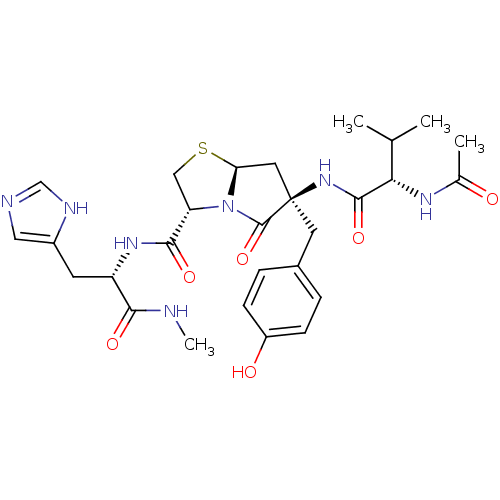
((2S,5R,7aR)-6-[2-((S)-Acetylamino)-3-methyl-butyry...)Show SMILES CNC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CS[C@H]2C[C@@](Cc3ccc(O)cc3)(NC(=O)[C@@H](NC(C)=O)C(C)C)C(=O)N12 Show InChI InChI=1S/C28H37N7O6S/c1-15(2)23(32-16(3)36)26(40)34-28(10-17-5-7-19(37)8-6-17)11-22-35(27(28)41)21(13-42-22)25(39)33-20(24(38)29-4)9-18-12-30-14-31-18/h5-8,12,14-15,20-23,37H,9-11,13H2,1-4H3,(H,29,38)(H,30,31)(H,32,36)(H,33,39)(H,34,40)/t20-,21-,22-,23-,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from pig uterus myometrium angiotensin II type 2 (AT2) receptor |
J Med Chem 47: 6009-19 (2004)
Article DOI: 10.1021/jm049651m
BindingDB Entry DOI: 10.7270/Q2668DZ3 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370375

(CHEMBL268815)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#7])cc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O Show InChI InChI=1S/C53H74N12O12/c1-5-30(4)44(50(74)61-39(26-32-15-19-34(54)20-16-32)51(75)65-24-10-14-41(65)48(72)62-40(52(76)77)27-31-11-7-6-8-12-31)64-47(71)38(25-33-17-21-35(66)22-18-33)60-49(73)43(29(2)3)63-46(70)37(13-9-23-58-53(56)57)59-45(69)36(55)28-42(67)68/h6-8,11-12,15-22,29-30,36-41,43-44,66H,5,9-10,13-14,23-28,54-55H2,1-4H3,(H,59,69)(H,60,73)(H,61,74)(H,62,72)(H,63,70)(H,64,71)(H,67,68)(H,76,77)(H4,56,57,58)/t30-,36+,37+,38+,39+,40+,41+,43+,44+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from pig uterus myometrium angiotensin II type 2 (AT2) receptor |
J Med Chem 47: 6009-19 (2004)
Article DOI: 10.1021/jm049651m
BindingDB Entry DOI: 10.7270/Q2668DZ3 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50188489
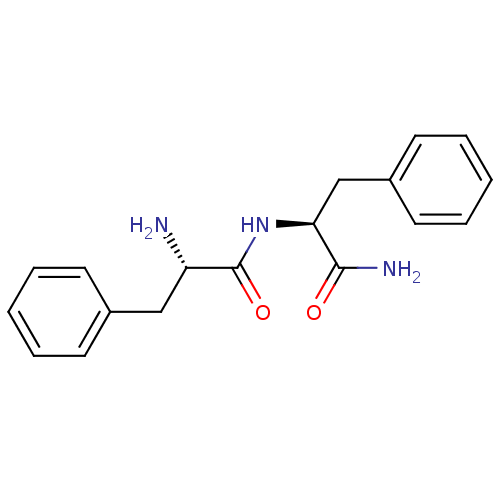
((S)-2-Amino-N-((S)-1-carbamoyl-2-phenyl-ethyl)-3-p...)Show SMILES N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C18H21N3O2/c19-15(11-13-7-3-1-4-8-13)18(23)21-16(17(20)22)12-14-9-5-2-6-10-14/h1-10,15-16H,11-12,19H2,(H2,20,22)(H,21,23)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membrane |
J Med Chem 53: 2383-9 (2010)
Article DOI: 10.1021/jm901352b
BindingDB Entry DOI: 10.7270/Q2542NQK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50308381
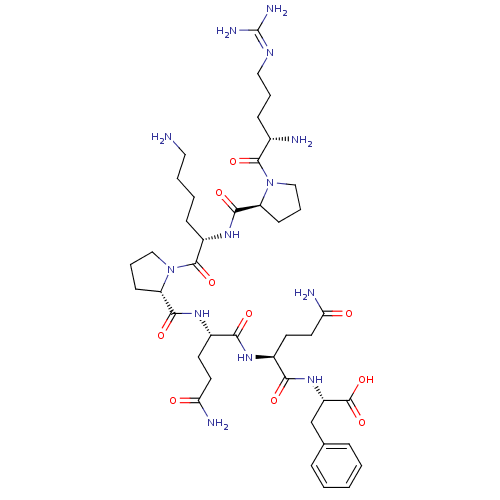
(CHEMBL589979 | H-Arg-Pro-Lys-Pro-Gln-Gln-Phe-OH)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C41H65N13O10/c42-19-5-4-12-28(51-37(60)30-13-7-21-53(30)38(61)25(43)11-6-20-48-41(46)47)39(62)54-22-8-14-31(54)36(59)50-27(16-18-33(45)56)34(57)49-26(15-17-32(44)55)35(58)52-29(40(63)64)23-24-9-2-1-3-10-24/h1-3,9-10,25-31H,4-8,11-23,42-43H2,(H2,44,55)(H2,45,56)(H,49,57)(H,50,59)(H,51,60)(H,52,58)(H,63,64)(H4,46,47,48)/t25-,26-,27-,28-,29-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membrane |
J Med Chem 53: 2383-9 (2010)
Article DOI: 10.1021/jm901352b
BindingDB Entry DOI: 10.7270/Q2542NQK |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50112098
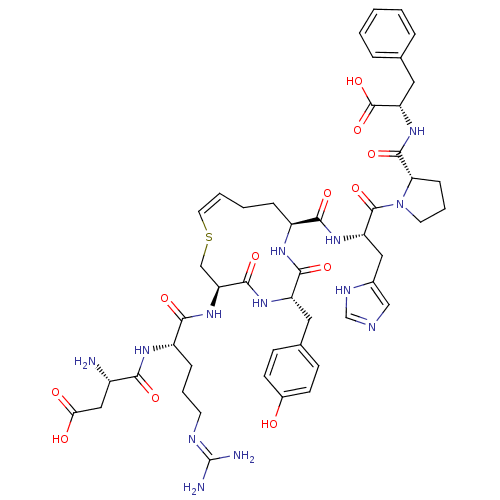
(CHEMBL412045 | analog of Angiotensin II with cis v...)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CS\C=C/CC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |c:23| Show InChI InChI=1S/C48H63N13O12S/c49-31(23-39(63)64)40(65)55-33(11-6-17-53-48(50)51)42(67)60-37-25-74-19-5-4-10-32(56-43(68)34(57-44(37)69)20-28-13-15-30(62)16-14-28)41(66)58-35(22-29-24-52-26-54-29)46(71)61-18-7-12-38(61)45(70)59-36(47(72)73)21-27-8-2-1-3-9-27/h1-3,5,8-9,13-16,19,24,26,31-38,62H,4,6-7,10-12,17-18,20-23,25,49H2,(H,52,54)(H,55,65)(H,56,68)(H,57,69)(H,58,66)(H,59,70)(H,60,67)(H,63,64)(H,72,73)(H4,50,51,53)/b19-5-/t31-,32-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
In vitro binding affinity at rat liver Angiotensin II receptor, type 1 was determined based on displacement of [125I]-Ang II |
J Med Chem 45: 1767-77 (2002)
BindingDB Entry DOI: 10.7270/Q2QV3N7W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50112097
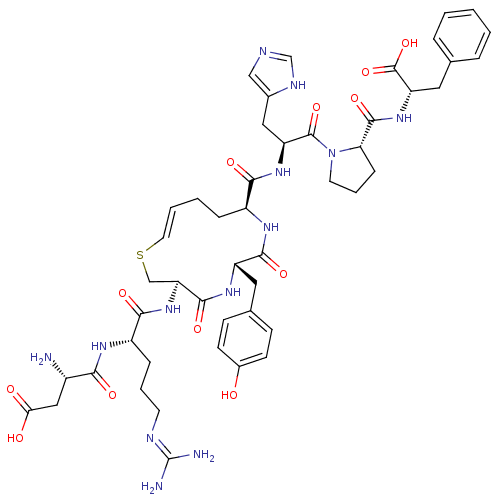
(CHEMBL266450 | analog of Angiotensin II with trans...)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CS\C=C\CC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |t:23| Show InChI InChI=1S/C48H63N13O12S/c49-31(23-39(63)64)40(65)55-33(11-6-17-53-48(50)51)42(67)60-37-25-74-19-5-4-10-32(56-43(68)34(57-44(37)69)20-28-13-15-30(62)16-14-28)41(66)58-35(22-29-24-52-26-54-29)46(71)61-18-7-12-38(61)45(70)59-36(47(72)73)21-27-8-2-1-3-9-27/h1-3,5,8-9,13-16,19,24,26,31-38,62H,4,6-7,10-12,17-18,20-23,25,49H2,(H,52,54)(H,55,65)(H,56,68)(H,57,69)(H,58,66)(H,59,70)(H,60,67)(H,63,64)(H,72,73)(H4,50,51,53)/b19-5+/t31-,32-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
In vitro binding affinity at rat liver Angiotensin II receptor, type 1 was determined based on displacement of [125I]-Ang II |
J Med Chem 45: 1767-77 (2002)
BindingDB Entry DOI: 10.7270/Q2QV3N7W |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50195697
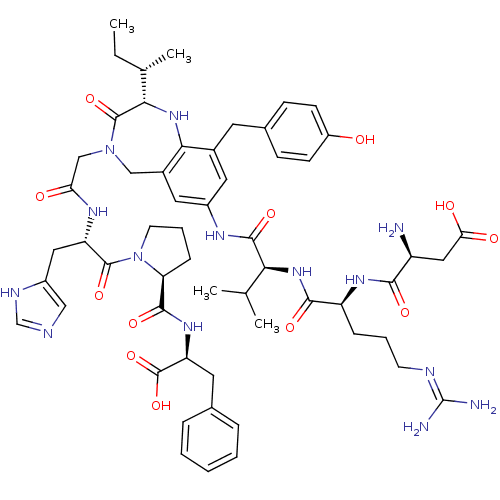
((3S)-3-amino-3-{[(1S)-1-{[(1S)-1-{[(2S)-2-[(2S)-bu...)Show SMILES CC[C@H](C)[C@@H]1Nc2c(Cc3ccc(O)cc3)cc(NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)cc2CN(CC(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](Cc2ccccc2)C(O)=O)C1=O |wU:25.33,70.73,4.3,2.2,wD:53.54,36.37,21.22,66.70,(14.02,-9.64,;14.01,-11.18,;12.68,-11.94,;11.35,-11.17,;12.67,-13.48,;11.16,-13.15,;9.94,-14.11,;8.6,-13.34,;8.6,-11.8,;7.26,-11.03,;7.27,-9.49,;5.93,-8.73,;4.6,-9.5,;3.26,-8.73,;4.61,-11.05,;5.95,-11.81,;7.28,-14.11,;7.28,-15.65,;5.96,-16.41,;4.62,-15.64,;4.62,-14.1,;3.28,-16.41,;1.95,-15.64,;.62,-16.41,;.62,-17.95,;-.72,-15.64,;-.72,-14.1,;-2.05,-13.33,;-2.05,-11.79,;-3.38,-11.02,;-3.38,-9.48,;-4.71,-8.71,;-2.05,-8.71,;-2.05,-16.41,;-3.38,-15.64,;-3.38,-14.1,;-4.71,-16.41,;-6.05,-15.64,;-4.71,-17.95,;-6.05,-18.72,;-7.39,-17.95,;-6.05,-20.26,;3.28,-17.95,;1.95,-18.72,;4.62,-18.72,;8.61,-16.42,;9.94,-15.65,;11.15,-16.63,;12.67,-16.28,;13.62,-17.48,;15.16,-17.51,;15.91,-18.86,;15.96,-16.19,;17.5,-16.21,;18.65,-17.24,;18.55,-18.78,;19.77,-19.74,;19.16,-21.18,;17.62,-21.06,;17.26,-19.56,;18.25,-14.87,;17.46,-13.55,;19.79,-14.86,;20.14,-16.33,;21.66,-16.52,;22.26,-15.08,;21.07,-14.07,;21.07,-12.53,;19.73,-11.76,;22.4,-11.75,;22.43,-10.22,;21.11,-9.42,;21.13,-7.87,;22.48,-7.13,;22.51,-5.6,;21.19,-4.8,;19.85,-5.53,;19.81,-7.08,;23.77,-9.47,;23.8,-7.93,;25.09,-10.26,;13.34,-14.88,;14.88,-14.88,)| Show InChI InChI=1S/C57H76N14O12/c1-5-32(4)48-55(81)70(29-45(73)65-42(25-38-27-61-30-63-38)54(80)71-20-10-14-44(71)52(78)67-43(56(82)83)22-33-11-7-6-8-12-33)28-36-24-37(23-35(49(36)68-48)21-34-15-17-39(72)18-16-34)64-53(79)47(31(2)3)69-51(77)41(13-9-19-62-57(59)60)66-50(76)40(58)26-46(74)75/h6-8,11-12,15-18,23-24,27,30-32,40-44,47-48,68,72H,5,9-10,13-14,19-22,25-26,28-29,58H2,1-4H3,(H,61,63)(H,64,79)(H,65,73)(H,66,76)(H,67,78)(H,69,77)(H,74,75)(H,82,83)(H4,59,60,62)/t32-,40-,41-,42-,43-,44-,47-,48-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ang2 from AT2 receptor in pig uterus myometrium |
J Med Chem 49: 6133-7 (2006)
Article DOI: 10.1021/jm051222g
BindingDB Entry DOI: 10.7270/Q28P619Q |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50346448

(2-(2-(((2S,6S,13S,E)-13-Amino-2-(4-hydroxybenzyl)-...)Show SMILES N[C@H]1CC\C=C\CC[C@H](NC(=O)C[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)NCc1ccccc1CC(O)=O |r,t:4| Show InChI InChI=1S/C29H36N4O6/c30-24-9-3-1-2-4-10-25(29(39)31-18-21-8-6-5-7-20(21)16-27(36)37)33-26(35)17-22(32-28(24)38)15-19-11-13-23(34)14-12-19/h1-2,5-8,11-14,22,24-25,34H,3-4,9-10,15-18,30H2,(H,31,39)(H,32,38)(H,33,35)(H,36,37)/b2-1+/t22-,24-,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... |
J Med Chem 54: 3779-92 (2011)
Article DOI: 10.1021/jm200036n
BindingDB Entry DOI: 10.7270/Q2348KPZ |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50175584

((S)-3-Amino-N-((S)-1-{1-[(S)-3-[2-[2-((S)-(S)-1-ca...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCc1cc(Cc2ccc(O)cc2)cc(c1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C50H64N12O11/c1-28(2)42(61-45(68)37(10-6-16-55-50(52)53)58-44(67)36(51)24-41(64)65)47(70)56-25-32-19-31(18-30-12-14-35(63)15-13-30)20-33(21-32)43(66)59-38(23-34-26-54-27-57-34)48(71)62-17-7-11-40(62)46(69)60-39(49(72)73)22-29-8-4-3-5-9-29/h3-5,8-9,12-15,19-21,26-28,36-40,42,63H,6-7,10-11,16-18,22-25,51H2,1-2H3,(H,54,57)(H,56,70)(H,58,67)(H,59,66)(H,60,69)(H,61,68)(H,64,65)(H,72,73)(H4,52,53,55)/t36-,37-,38-,39-,40-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.85 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II receptor, type 2 in pig uterus myometrium using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 1.5 h... |
J Med Chem 48: 6620-31 (2005)
Article DOI: 10.1021/jm050280z
BindingDB Entry DOI: 10.7270/Q2HX1DG6 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370375

(CHEMBL268815)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#7])cc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O Show InChI InChI=1S/C53H74N12O12/c1-5-30(4)44(50(74)61-39(26-32-15-19-34(54)20-16-32)51(75)65-24-10-14-41(65)48(72)62-40(52(76)77)27-31-11-7-6-8-12-31)64-47(71)38(25-33-17-21-35(66)22-18-33)60-49(73)43(29(2)3)63-46(70)37(13-9-23-58-53(56)57)59-45(69)36(55)28-42(67)68/h6-8,11-12,15-22,29-30,36-41,43-44,66H,5,9-10,13-14,23-28,54-55H2,1-4H3,(H,59,69)(H,60,73)(H,61,74)(H,62,72)(H,63,70)(H,64,71)(H,67,68)(H,76,77)(H4,56,57,58)/t30-,36+,37+,38+,39+,40+,41+,43+,44+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor type 2 using [125I]- Ang II in pig uterus myometrium |
J Med Chem 47: 859-70 (2004)
Article DOI: 10.1021/jm030921v
BindingDB Entry DOI: 10.7270/Q2736RNQ |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50156178
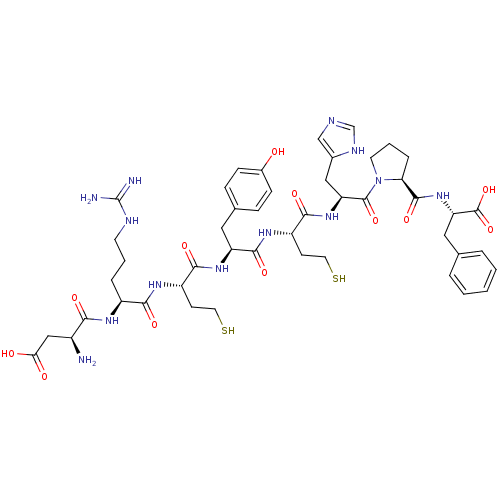
(CHEMBL216331 | c[Hcy3,5]Ang II)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCS)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCS)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C47H65N13O12S2/c48-30(23-38(62)63)39(64)54-31(8-4-16-52-47(49)50)40(65)55-32(14-18-73)41(66)57-34(20-27-10-12-29(61)13-11-27)43(68)56-33(15-19-74)42(67)58-35(22-28-24-51-25-53-28)45(70)60-17-5-9-37(60)44(69)59-36(46(71)72)21-26-6-2-1-3-7-26/h1-3,6-7,10-13,24-25,30-37,61,73-74H,4-5,8-9,14-23,48H2,(H,51,53)(H,54,64)(H,55,65)(H,56,68)(H,57,66)(H,58,67)(H,59,69)(H,62,63)(H,71,72)(H4,49,50,52)/t30-,31-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from pig uterus myometrium angiotensin II type 2 (AT2) receptor |
J Med Chem 47: 6009-19 (2004)
Article DOI: 10.1021/jm049651m
BindingDB Entry DOI: 10.7270/Q2668DZ3 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370576

(CHEMBL1791309)Show SMILES CC[C@@H](C)[C@H](N1Cc2cccc(NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)c2N[C@@H](Cc2ccc(O)cc2)C1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:61.66,48.50,15.22,34.35,2.2,wD:4.4,65.69,26.26,(17.04,-11.58,;17.04,-10.04,;15.66,-9.27,;14.31,-10.04,;15.66,-7.73,;14.31,-6.96,;13.45,-8.27,;11.88,-8.43,;11.49,-9.89,;9.96,-10.28,;8.85,-9.2,;9.27,-7.73,;7.91,-6.94,;6.57,-7.6,;6.59,-8.95,;5.22,-6.94,;5.22,-5.59,;3.89,-4.92,;3.91,-3.59,;2.54,-2.91,;2.56,-1.57,;1.23,-.89,;3.92,-.92,;3.91,-7.62,;2.54,-6.94,;2.54,-5.61,;1.2,-7.62,;-.15,-6.96,;1.2,-8.95,;-.12,-9.64,;-1.47,-8.98,;-.11,-10.97,;10.79,-7.33,;10.97,-5.81,;12.32,-5.01,;12.16,-3.47,;12.94,-2.15,;14.52,-2.12,;15.29,-.81,;14.5,.52,;15.27,1.86,;12.91,.49,;12.14,-.84,;13.8,-5.53,;14.93,-4.46,;17.04,-6.96,;17.04,-5.43,;18.42,-7.73,;19.82,-7.11,;19.89,-5.56,;20.67,-4.25,;22.22,-4.06,;22.54,-2.56,;21.18,-1.8,;20.01,-2.83,;21.14,-7.82,;21.05,-9.2,;22.54,-7.1,;22.04,-5.64,;23.33,-4.73,;24.61,-5.64,;24.12,-7.1,;25.48,-7.86,;25.48,-9.4,;26.86,-7.11,;28.17,-7.85,;28.07,-9.22,;29.42,-9.91,;29.32,-11.29,;30.62,-12.02,;32.03,-11.39,;32.13,-10.05,;30.8,-9.29,;29.59,-7.23,;30.9,-7.96,;29.66,-5.86,)| Show InChI InChI=1S/C52H67N13O11/c1-3-29(2)44(48(72)62-39(24-33-26-56-28-58-33)49(73)64-21-9-15-41(64)47(71)63-40(51(75)76)23-30-10-5-4-6-11-30)65-27-32-12-7-13-36(43(32)59-38(50(65)74)22-31-16-18-34(66)19-17-31)60-46(70)37(14-8-20-57-52(54)55)61-45(69)35(53)25-42(67)68/h4-7,10-13,16-19,26,28-29,35,37-41,44,59,66H,3,8-9,14-15,20-25,27,53H2,1-2H3,(H,56,58)(H,60,70)(H,61,69)(H,62,72)(H,63,71)(H,67,68)(H,75,76)(H4,54,55,57)/t29-,35+,37+,38+,39+,40+,41+,44+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370576

(CHEMBL1791309)Show SMILES CC[C@@H](C)[C@H](N1Cc2cccc(NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)c2N[C@@H](Cc2ccc(O)cc2)C1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:61.66,48.50,15.22,34.35,2.2,wD:4.4,65.69,26.26,(17.04,-11.58,;17.04,-10.04,;15.66,-9.27,;14.31,-10.04,;15.66,-7.73,;14.31,-6.96,;13.45,-8.27,;11.88,-8.43,;11.49,-9.89,;9.96,-10.28,;8.85,-9.2,;9.27,-7.73,;7.91,-6.94,;6.57,-7.6,;6.59,-8.95,;5.22,-6.94,;5.22,-5.59,;3.89,-4.92,;3.91,-3.59,;2.54,-2.91,;2.56,-1.57,;1.23,-.89,;3.92,-.92,;3.91,-7.62,;2.54,-6.94,;2.54,-5.61,;1.2,-7.62,;-.15,-6.96,;1.2,-8.95,;-.12,-9.64,;-1.47,-8.98,;-.11,-10.97,;10.79,-7.33,;10.97,-5.81,;12.32,-5.01,;12.16,-3.47,;12.94,-2.15,;14.52,-2.12,;15.29,-.81,;14.5,.52,;15.27,1.86,;12.91,.49,;12.14,-.84,;13.8,-5.53,;14.93,-4.46,;17.04,-6.96,;17.04,-5.43,;18.42,-7.73,;19.82,-7.11,;19.89,-5.56,;20.67,-4.25,;22.22,-4.06,;22.54,-2.56,;21.18,-1.8,;20.01,-2.83,;21.14,-7.82,;21.05,-9.2,;22.54,-7.1,;22.04,-5.64,;23.33,-4.73,;24.61,-5.64,;24.12,-7.1,;25.48,-7.86,;25.48,-9.4,;26.86,-7.11,;28.17,-7.85,;28.07,-9.22,;29.42,-9.91,;29.32,-11.29,;30.62,-12.02,;32.03,-11.39,;32.13,-10.05,;30.8,-9.29,;29.59,-7.23,;30.9,-7.96,;29.66,-5.86,)| Show InChI InChI=1S/C52H67N13O11/c1-3-29(2)44(48(72)62-39(24-33-26-56-28-58-33)49(73)64-21-9-15-41(64)47(71)63-40(51(75)76)23-30-10-5-4-6-11-30)65-27-32-12-7-13-36(43(32)59-38(50(65)74)22-31-16-18-34(66)19-17-31)60-46(70)37(14-8-20-57-52(54)55)61-45(69)35(53)25-42(67)68/h4-7,10-13,16-19,26,28-29,35,37-41,44,59,66H,3,8-9,14-15,20-25,27,53H2,1-2H3,(H,56,58)(H,60,70)(H,61,69)(H,62,72)(H,63,71)(H,67,68)(H,75,76)(H4,54,55,57)/t29-,35+,37+,38+,39+,40+,41+,44+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50168424
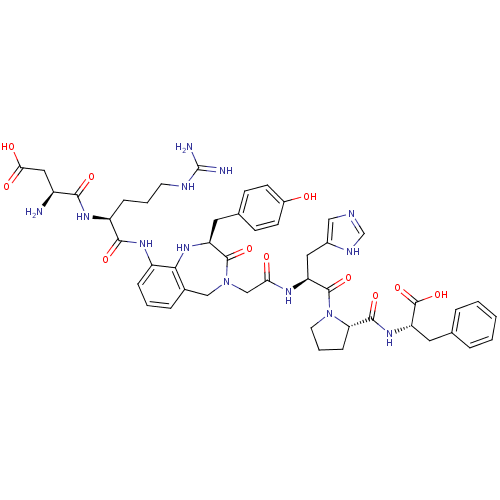
((3S)-3-amino-3-{[(1S)-1-{[(2S)-4-({[(2S)-1-[(2S)-2...)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)Nc1cccc2CN(CC(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N3CCC[C@H]3C(=O)N[C@@H](Cc3ccccc3)C(O)=O)C(=O)[C@H](Cc3ccc(O)cc3)Nc12 Show InChI InChI=1S/C48H59N13O11/c49-32(22-40(64)65)42(66)58-34(11-5-17-53-48(50)51)43(67)57-33-10-4-9-29-24-60(45(69)35(56-41(29)33)19-28-13-15-31(62)16-14-28)25-39(63)55-36(21-30-23-52-26-54-30)46(70)61-18-6-12-38(61)44(68)59-37(47(71)72)20-27-7-2-1-3-8-27/h1-4,7-10,13-16,23,26,32,34-38,56,62H,5-6,11-12,17-22,24-25,49H2,(H,52,54)(H,55,63)(H,57,67)(H,58,66)(H,59,68)(H,64,65)(H,71,72)(H4,50,51,53)/t32-,34-,35-,36-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50139787
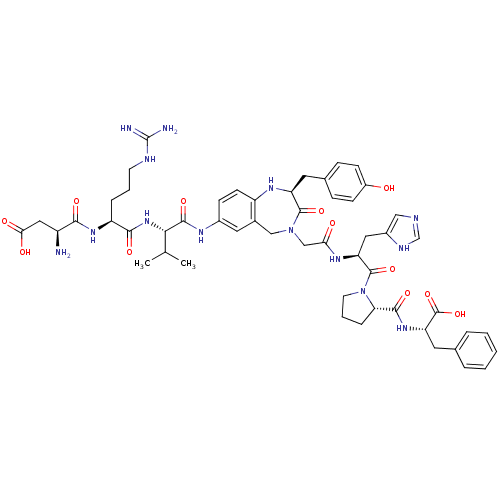
((3S)-3-amino-3-{[(1S)-1-{[(1S)-1-{[(2S)-4-({[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(=O)Nc1ccc2N[C@@H](Cc3ccc(O)cc3)C(=O)N(CC(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N3CCC[C@H]3C(=O)N[C@@H](Cc3ccccc3)C(O)=O)Cc2c1 Show InChI InChI=1S/C53H68N14O12/c1-29(2)45(65-47(73)38(10-6-18-58-53(55)56)63-46(72)36(54)24-44(70)71)49(75)60-33-14-17-37-32(22-33)26-66(50(76)39(61-37)20-31-12-15-35(68)16-13-31)27-43(69)62-40(23-34-25-57-28-59-34)51(77)67-19-7-11-42(67)48(74)64-41(52(78)79)21-30-8-4-3-5-9-30/h3-5,8-9,12-17,22,25,28-29,36,38-42,45,61,68H,6-7,10-11,18-21,23-24,26-27,54H2,1-2H3,(H,57,59)(H,60,75)(H,62,69)(H,63,72)(H,64,74)(H,65,73)(H,70,71)(H,78,79)(H4,55,56,58)/t36-,38-,39-,40-,41-,42-,45-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50139787

((3S)-3-amino-3-{[(1S)-1-{[(1S)-1-{[(2S)-4-({[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(=O)Nc1ccc2N[C@@H](Cc3ccc(O)cc3)C(=O)N(CC(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N3CCC[C@H]3C(=O)N[C@@H](Cc3ccccc3)C(O)=O)Cc2c1 Show InChI InChI=1S/C53H68N14O12/c1-29(2)45(65-47(73)38(10-6-18-58-53(55)56)63-46(72)36(54)24-44(70)71)49(75)60-33-14-17-37-32(22-33)26-66(50(76)39(61-37)20-31-12-15-35(68)16-13-31)27-43(69)62-40(23-34-25-57-28-59-34)51(77)67-19-7-11-42(67)48(74)64-41(52(78)79)21-30-8-4-3-5-9-30/h3-5,8-9,12-17,22,25,28-29,36,38-42,45,61,68H,6-7,10-11,18-21,23-24,26-27,54H2,1-2H3,(H,57,59)(H,60,75)(H,62,69)(H,63,72)(H,64,74)(H,65,73)(H,70,71)(H,78,79)(H4,55,56,58)/t36-,38-,39-,40-,41-,42-,45-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor type 2 using [125I]- Ang II in pig uterus myometrium |
J Med Chem 47: 859-70 (2004)
Article DOI: 10.1021/jm030921v
BindingDB Entry DOI: 10.7270/Q2736RNQ |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50331042
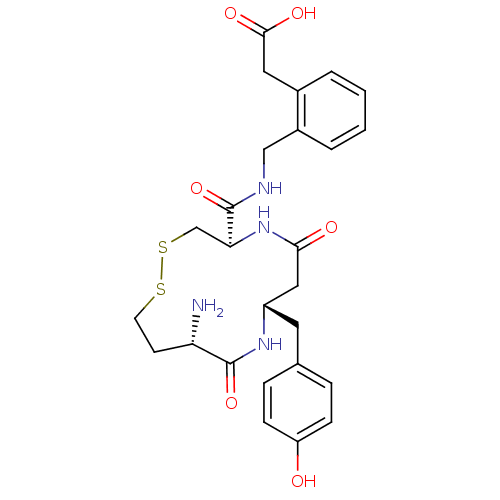
(2-(2-(((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,...)Show SMILES N[C@H]1CCSSC[C@H](NC(=O)C[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)NCc1ccccc1CC(O)=O |r| Show InChI InChI=1S/C26H32N4O6S2/c27-21-9-10-37-38-15-22(26(36)28-14-18-4-2-1-3-17(18)12-24(33)34)30-23(32)13-19(29-25(21)35)11-16-5-7-20(31)8-6-16/h1-8,19,21-22,31H,9-15,27H2,(H,28,36)(H,29,35)(H,30,32)(H,33,34)/t19-,21-,22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic activity of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 53: 8059-71 (2010)
Article DOI: 10.1021/jm100793t
BindingDB Entry DOI: 10.7270/Q23B60C6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50156177
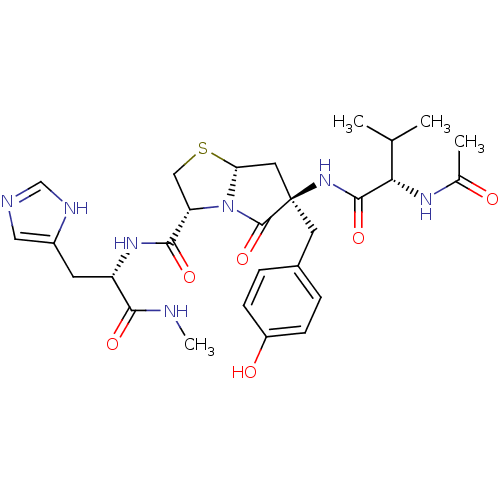
((2R,5R,7aR)-6-[2-((S)-Acetylamino)-3-methyl-butyry...)Show SMILES CNC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CS[C@@H]2C[C@@](Cc3ccc(O)cc3)(NC(=O)[C@@H](NC(C)=O)C(C)C)C(=O)N12 Show InChI InChI=1S/C28H37N7O6S/c1-15(2)23(32-16(3)36)26(40)34-28(10-17-5-7-19(37)8-6-17)11-22-35(27(28)41)21(13-42-22)25(39)33-20(24(38)29-4)9-18-12-30-14-31-18/h5-8,12,14-15,20-23,37H,9-11,13H2,1-4H3,(H,29,38)(H,30,31)(H,32,36)(H,33,39)(H,34,40)/t20-,21-,22+,23-,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from pig uterus myometrium angiotensin II type 2 (AT2) receptor |
J Med Chem 47: 6009-19 (2004)
Article DOI: 10.1021/jm049651m
BindingDB Entry DOI: 10.7270/Q2668DZ3 |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50346455

(2-(2-(((2S,5S,13S,E)-13-Amino-2-(4-hydroxybenzyl)-...)Show SMILES N[C@H]1CCCC\C=C\C[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)NCc1ccccc1CC(O)=O |r,t:6| Show InChI InChI=1S/C29H36N4O6/c30-23-10-4-2-1-3-5-11-24(28(38)31-18-21-9-7-6-8-20(21)17-26(35)36)32-29(39)25(33-27(23)37)16-19-12-14-22(34)15-13-19/h3,5-9,12-15,23-25,34H,1-2,4,10-11,16-18,30H2,(H,31,38)(H,32,39)(H,33,37)(H,35,36)/b5-3+/t23-,24-,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... |
J Med Chem 54: 3779-92 (2011)
Article DOI: 10.1021/jm200036n
BindingDB Entry DOI: 10.7270/Q2348KPZ |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50195698
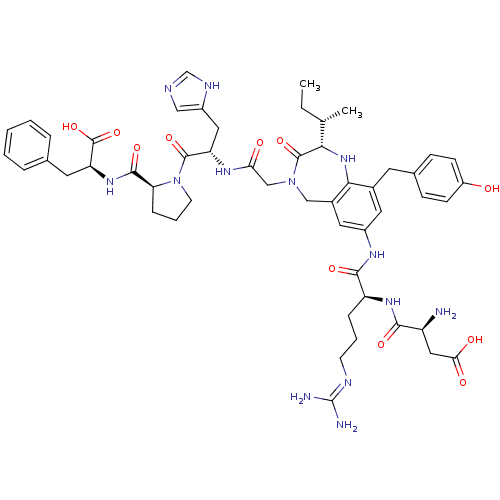
((3S)-3-amino-3-{[(1S)-1-{[(2S)-2-[(2S)-butan-2-yl]...)Show SMILES CC[C@H](C)[C@@H]1Nc2c(Cc3ccc(O)cc3)cc(NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)cc2CN(CC(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](Cc2ccccc2)C(O)=O)C1=O |wU:32.33,4.3,2.2,63.66,wD:21.29,46.47,59.63,(14.77,-5.6,;13.95,-6.91,;12.41,-6.86,;11.69,-5.5,;11.6,-8.16,;10.09,-7.83,;8.87,-8.79,;7.53,-8.02,;7.53,-6.48,;6.19,-5.71,;6.2,-4.17,;4.85,-3.41,;3.53,-4.18,;2.19,-3.41,;3.54,-5.73,;4.87,-6.49,;6.21,-8.79,;6.21,-10.32,;4.87,-11.09,;3.54,-10.32,;3.54,-8.78,;2.2,-11.09,;2.2,-12.63,;3.54,-13.4,;3.54,-14.94,;4.87,-15.71,;4.87,-17.25,;6.21,-18.02,;3.54,-18.02,;.87,-10.32,;-.46,-11.09,;-.46,-12.63,;-1.8,-10.32,;-3.13,-11.09,;-1.8,-8.78,;-3.13,-8.01,;-4.46,-8.78,;-3.13,-6.47,;7.54,-11.1,;8.87,-10.33,;10.08,-11.31,;11.6,-10.96,;12.55,-12.16,;14.09,-12.11,;14.92,-13.39,;14.81,-10.72,;16.34,-10.65,;17.53,-11.64,;17.47,-13.18,;18.76,-14.05,;18.26,-15.53,;16.72,-15.53,;16.25,-14.07,;17.09,-9.31,;16.31,-8,;18.62,-9.3,;19.15,-10.73,;20.69,-10.73,;21.12,-9.23,;19.81,-8.37,;19.05,-7.02,;17.51,-7,;19.83,-5.69,;19.11,-4.35,;17.56,-4.3,;16.83,-2.94,;17.63,-1.63,;16.91,-.28,;15.37,-.23,;14.55,-1.54,;15.29,-2.89,;19.9,-3.03,;19.17,-1.68,;21.45,-3.07,;12.27,-9.56,;13.81,-9.56,)| Show InChI InChI=1S/C52H67N13O11/c1-3-29(2)44-50(74)64(27-42(67)60-39(23-35-25-56-28-58-35)49(73)65-18-8-12-41(65)48(72)62-40(51(75)76)20-30-9-5-4-6-10-30)26-33-22-34(21-32(45(33)63-44)19-31-13-15-36(66)16-14-31)59-47(71)38(11-7-17-57-52(54)55)61-46(70)37(53)24-43(68)69/h4-6,9-10,13-16,21-22,25,28-29,37-41,44,63,66H,3,7-8,11-12,17-20,23-24,26-27,53H2,1-2H3,(H,56,58)(H,59,71)(H,60,67)(H,61,70)(H,62,72)(H,68,69)(H,75,76)(H4,54,55,57)/t29-,37-,38-,39-,40-,41-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ang2 from AT2 receptor in pig uterus myometrium |
J Med Chem 49: 6133-7 (2006)
Article DOI: 10.1021/jm051222g
BindingDB Entry DOI: 10.7270/Q28P619Q |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50331046
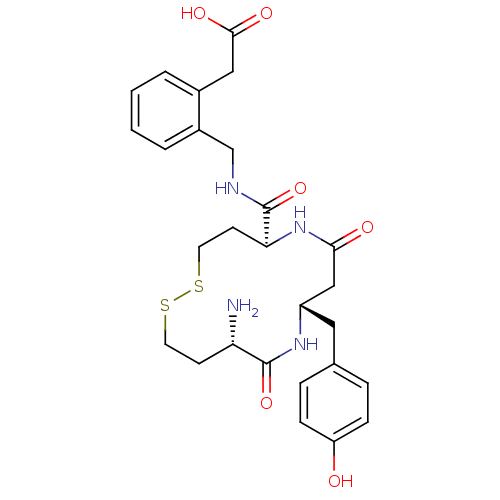
(2-(2-(((5S,9S,12S)-12-amino-9-(4-hydroxybenzyl)-7,...)Show SMILES N[C@H]1CCSSCC[C@H](NC(=O)C[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)NCc1ccccc1CC(O)=O |r| Show InChI InChI=1S/C27H34N4O6S2/c28-22-9-11-38-39-12-10-23(27(37)29-16-19-4-2-1-3-18(19)14-25(34)35)31-24(33)15-20(30-26(22)36)13-17-5-7-21(32)8-6-17/h1-8,20,22-23,32H,9-16,28H2,(H,29,37)(H,30,36)(H,31,33)(H,34,35)/t20-,22-,23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic activity of human recombinant IRAP expressed in HEK293 cells |
J Med Chem 53: 8059-71 (2010)
Article DOI: 10.1021/jm100793t
BindingDB Entry DOI: 10.7270/Q23B60C6 |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM85550

(Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C40H54N8O8/c1-5-24(4)34(47-35(50)29(44-37(52)33(41)23(2)3)18-26-13-15-28(49)16-14-26)38(53)45-30(20-27-21-42-22-43-27)39(54)48-17-9-12-32(48)36(51)46-31(40(55)56)19-25-10-7-6-8-11-25/h6-8,10-11,13-16,21-24,29-34,49H,5,9,12,17-20,41H2,1-4H3,(H,42,43)(H,44,52)(H,45,53)(H,46,51)(H,47,50)(H,55,56)/t24-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]Angiotensin 4 from human recombinant IRAP expressed in CHOK1 cells |
Bioorg Med Chem 16: 6924-35 (2008)
Article DOI: 10.1016/j.bmc.2008.05.046
BindingDB Entry DOI: 10.7270/Q2B857XC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membrane |
J Med Chem 53: 2383-9 (2010)
Article DOI: 10.1021/jm901352b
BindingDB Entry DOI: 10.7270/Q2542NQK |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50331035
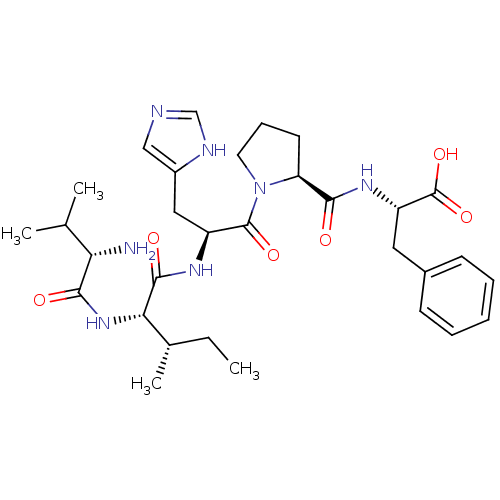
((S)-2-((S)-1-((S)-2-((2S,3S)-2-((S)-2-amino-3-meth...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C31H45N7O6/c1-5-19(4)26(37-28(40)25(32)18(2)3)29(41)35-22(15-21-16-33-17-34-21)30(42)38-13-9-12-24(38)27(39)36-23(31(43)44)14-20-10-7-6-8-11-20/h6-8,10-11,16-19,22-26H,5,9,12-15,32H2,1-4H3,(H,33,34)(H,35,41)(H,36,39)(H,37,40)(H,43,44)/t19-,22-,23-,24-,25-,26-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]AL-11 from human IRAP expressed in CHO-K1 cells after 30 mins by liquid scintillation counting in presence of 30 mM EDTA/600 uM 1... |
J Med Chem 53: 8059-71 (2010)
Article DOI: 10.1021/jm100793t
BindingDB Entry DOI: 10.7270/Q23B60C6 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50168423

((3S)-3-amino-3-{[(1S)-1-{[(1S)-1-{[(2S)-4-({[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)CC(O)=O)C(=O)Nc1cccc2CN(CC(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N3CCC[C@H]3C(=O)N[C@@H](Cc3ccccc3)C(O)=O)C(=O)[C@H](Cc3ccc(O)cc3)Nc12 Show InChI InChI=1S/C50H61N11O12/c1-27(2)42(59-44(66)28(3)54-45(67)34(51)22-41(64)65)47(69)57-35-12-7-11-31-24-60(48(70)36(56-43(31)35)19-30-14-16-33(62)17-15-30)25-40(63)55-37(21-32-23-52-26-53-32)49(71)61-18-8-13-39(61)46(68)58-38(50(72)73)20-29-9-5-4-6-10-29/h4-7,9-12,14-17,23,26-28,34,36-39,42,56,62H,8,13,18-22,24-25,51H2,1-3H3,(H,52,53)(H,54,67)(H,55,63)(H,57,69)(H,58,68)(H,59,66)(H,64,65)(H,72,73)/t28-,34-,36-,37-,38-,39-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50209313

((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C26H33N5O5/c1-16(24(34)30-21(23(28)33)15-17-6-3-2-4-7-17)29-25(35)22-8-5-13-31(22)26(36)20(27)14-18-9-11-19(32)12-10-18/h2-4,6-7,9-12,16,20-22,32H,5,8,13-15,27H2,1H3,(H2,28,33)(H,29,35)(H,30,34)/t16-,20-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membrane |
J Med Chem 53: 2383-9 (2010)
Article DOI: 10.1021/jm901352b
BindingDB Entry DOI: 10.7270/Q2542NQK |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50175586
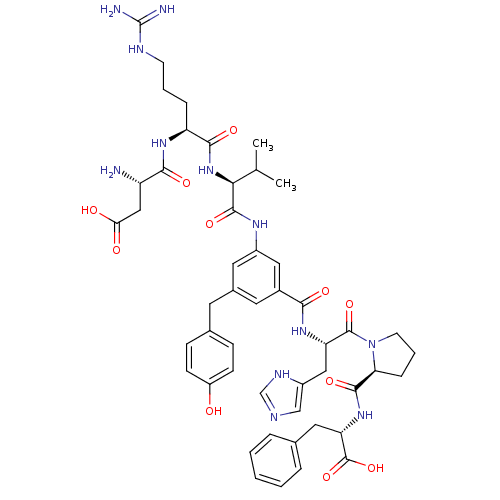
((S)-3-Amino-N-((S)-1-{(S)-1-[(S)-3-[2-[2-((S)-(S)-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(=O)Nc1cc(Cc2ccc(O)cc2)cc(c1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C49H62N12O11/c1-27(2)41(60-44(67)36(10-6-16-54-49(51)52)57-43(66)35(50)24-40(63)64)46(69)56-32-20-30(18-29-12-14-34(62)15-13-29)19-31(22-32)42(65)58-37(23-33-25-53-26-55-33)47(70)61-17-7-11-39(61)45(68)59-38(48(71)72)21-28-8-4-3-5-9-28/h3-5,8-9,12-15,19-20,22,25-27,35-39,41,62H,6-7,10-11,16-18,21,23-24,50H2,1-2H3,(H,53,55)(H,56,69)(H,57,66)(H,58,65)(H,59,68)(H,60,67)(H,63,64)(H,71,72)(H4,51,52,54)/t35-,36-,37-,38-,39-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II receptor, type 2 in pig uterus myometrium using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 1.5 h... |
J Med Chem 48: 6620-31 (2005)
Article DOI: 10.1021/jm050280z
BindingDB Entry DOI: 10.7270/Q2HX1DG6 |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50331046

(2-(2-(((5S,9S,12S)-12-amino-9-(4-hydroxybenzyl)-7,...)Show SMILES N[C@H]1CCSSCC[C@H](NC(=O)C[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)NCc1ccccc1CC(O)=O |r| Show InChI InChI=1S/C27H34N4O6S2/c28-22-9-11-38-39-12-10-23(27(37)29-16-19-4-2-1-3-18(19)14-25(34)35)31-24(33)15-20(30-26(22)36)13-17-5-7-21(32)8-6-17/h1-8,20,22-23,32H,9-16,28H2,(H,29,37)(H,30,36)(H,31,33)(H,34,35)/t20-,22-,23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]AL-11 from human IRAP expressed in CHO-K1 cells after 30 mins by liquid scintillation counting in presence of 30 mM EDTA/600 uM 1... |
J Med Chem 53: 8059-71 (2010)
Article DOI: 10.1021/jm100793t
BindingDB Entry DOI: 10.7270/Q23B60C6 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50175585

((S)-3-Amino-N-{(S)-1-[3-[(S)-2-[2-((S)-(S)-1-carbo...)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCc1cc(Cc2ccc(O)cc2)cc(c1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C45H55N11O10/c46-33(22-38(58)59)40(61)53-34(8-4-14-50-45(47)48)41(62)51-23-29-17-28(16-27-10-12-32(57)13-11-27)18-30(19-29)39(60)54-35(21-31-24-49-25-52-31)43(64)56-15-5-9-37(56)42(63)55-36(44(65)66)20-26-6-2-1-3-7-26/h1-3,6-7,10-13,17-19,24-25,33-37,57H,4-5,8-9,14-16,20-23,46H2,(H,49,52)(H,51,62)(H,53,61)(H,54,60)(H,55,63)(H,58,59)(H,65,66)(H4,47,48,50)/t33-,34-,35-,36-,37-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II receptor, type 2 in pig uterus myometrium using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 1.5 h... |
J Med Chem 48: 6620-31 (2005)
Article DOI: 10.1021/jm050280z
BindingDB Entry DOI: 10.7270/Q2HX1DG6 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50308392
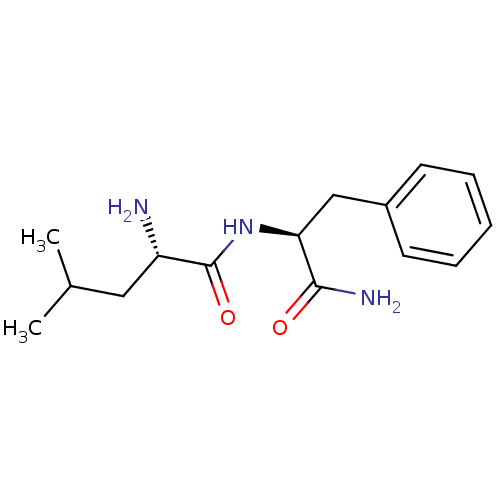
(CHEMBL590518 | H-Leu-Phe-NH2)Show SMILES CC(C)C[C@H](N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C15H23N3O2/c1-10(2)8-12(16)15(20)18-13(14(17)19)9-11-6-4-3-5-7-11/h3-7,10,12-13H,8-9,16H2,1-2H3,(H2,17,19)(H,18,20)/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membrane |
J Med Chem 53: 2383-9 (2010)
Article DOI: 10.1021/jm901352b
BindingDB Entry DOI: 10.7270/Q2542NQK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50308383
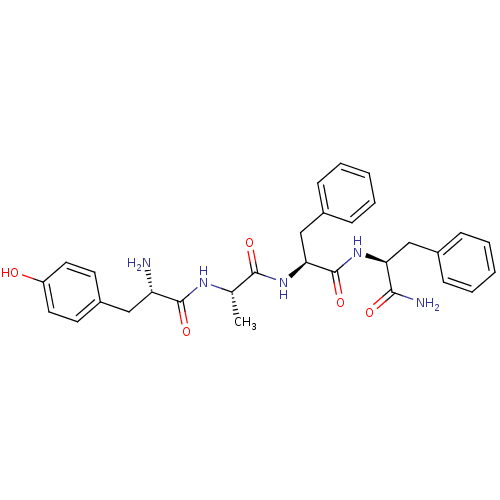
(1N-[1-[1-[1-carbamoyl-2-phenyl-(1S)-ethylcarbamoyl...)Show SMILES C[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C30H35N5O5/c1-19(33-29(39)24(31)16-22-12-14-23(36)15-13-22)28(38)35-26(18-21-10-6-3-7-11-21)30(40)34-25(27(32)37)17-20-8-4-2-5-9-20/h2-15,19,24-26,36H,16-18,31H2,1H3,(H2,32,37)(H,33,39)(H,34,40)(H,35,38)/t19-,24-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membrane |
J Med Chem 53: 2383-9 (2010)
Article DOI: 10.1021/jm901352b
BindingDB Entry DOI: 10.7270/Q2542NQK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50308385

(CHEMBL591489 | H-Pro-Phe-Phe-NH2)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C23H28N4O3/c24-21(28)19(14-16-8-3-1-4-9-16)26-23(30)20(15-17-10-5-2-6-11-17)27-22(29)18-12-7-13-25-18/h1-6,8-11,18-20,25H,7,12-15H2,(H2,24,28)(H,26,30)(H,27,29)/t18-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membrane |
J Med Chem 53: 2383-9 (2010)
Article DOI: 10.1021/jm901352b
BindingDB Entry DOI: 10.7270/Q2542NQK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50308382
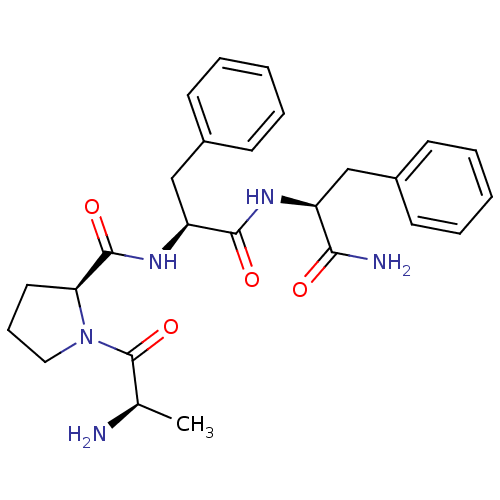
(CHEMBL589082 | H-Ala-Pro-Phe-Phe-NH2)Show SMILES C[C@@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C26H33N5O4/c1-17(27)26(35)31-14-8-13-22(31)25(34)30-21(16-19-11-6-3-7-12-19)24(33)29-20(23(28)32)15-18-9-4-2-5-10-18/h2-7,9-12,17,20-22H,8,13-16,27H2,1H3,(H2,28,32)(H,29,33)(H,30,34)/t17-,20+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membrane |
J Med Chem 53: 2383-9 (2010)
Article DOI: 10.1021/jm901352b
BindingDB Entry DOI: 10.7270/Q2542NQK |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50366760

(CHEMBL2369589)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C(O)=O)C(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CS)C(O)=O Show InChI InChI=1S/C36H58N6O14S/c1-6-18(4)28(33(50)40-23(13-20-10-8-7-9-11-20)29(46)41-26(16-57)36(55)56)42-32(49)22(12-17(2)3)38-30(47)24(14-21(34(51)52)35(53)54)39-31(48)25(15-27(44)45)37-19(5)43/h17-18,20-26,28,57H,6-16H2,1-5H3,(H,37,43)(H,38,47)(H,39,48)(H,40,50)(H,41,46)(H,42,49)(H,44,45)(H,51,52)(H,53,54)(H,55,56)/t18-,22-,23-,24+,25-,26-,28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) |
Bioorg Med Chem Lett 11: 203-6 (2001)
BindingDB Entry DOI: 10.7270/Q26Q1XSK |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50208099
((2S,3R)-2-(3-((1H-imidazol-1-yl)methyl)benzamido)-...)Show SMILES CC[C@@H](C)[C@H](NC(=O)c1cccc(Cn2ccnc2)c1)C(O)=O Show InChI InChI=1S/C17H21N3O3/c1-3-12(2)15(17(22)23)19-16(21)14-6-4-5-13(9-14)10-20-8-7-18-11-20/h4-9,11-12,15H,3,10H2,1-2H3,(H,19,21)(H,22,23)/t12-,15+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ang2 from AT2 receptor in pig uterus myometrial membrane |
J Med Chem 50: 1711-5 (2007)
Article DOI: 10.1021/jm0613469
BindingDB Entry DOI: 10.7270/Q2NK3FTN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


















































