

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092989 (1-[1-(4-Benzenesulfonyl-phenyl)-ethyl]-4-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D1 in rat striatal tissue by [3H]-SCH- 23390 displacement. | Bioorg Med Chem Lett 2: 399-402 (1992) Article DOI: 10.1016/S0960-894X(00)80155-9 BindingDB Entry DOI: 10.7270/Q2C53KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222885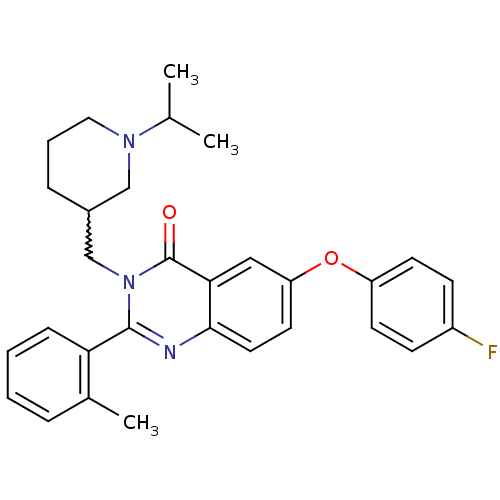 (6-(4-fluorophenoxy)-3-[(1-isopropylpiperidin-3-yl)...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092984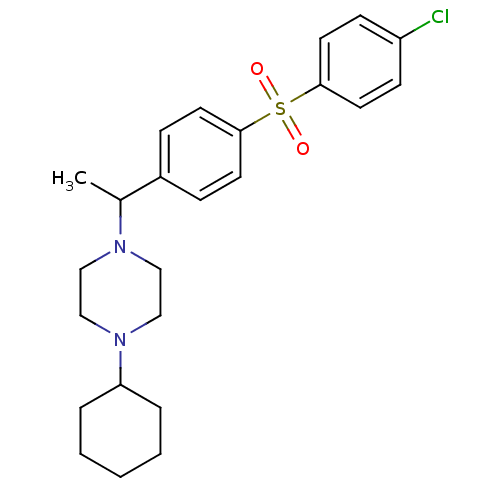 (1-{1-[4-(4-Chloro-benzenesulfonyl)-phenyl]-ethyl}-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092996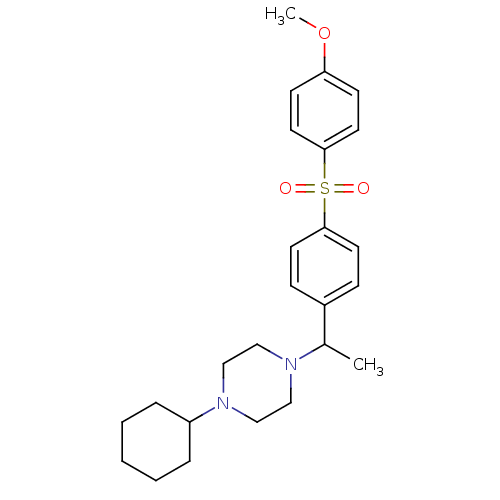 (1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfonyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092995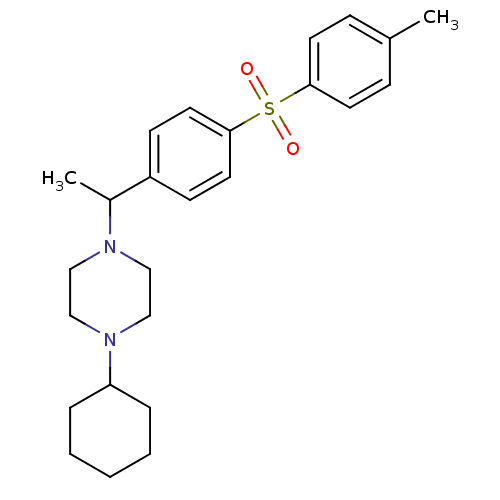 (1-Cyclohexyl-4-{1-[4-(toluene-4-sulfonyl)-phenyl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222867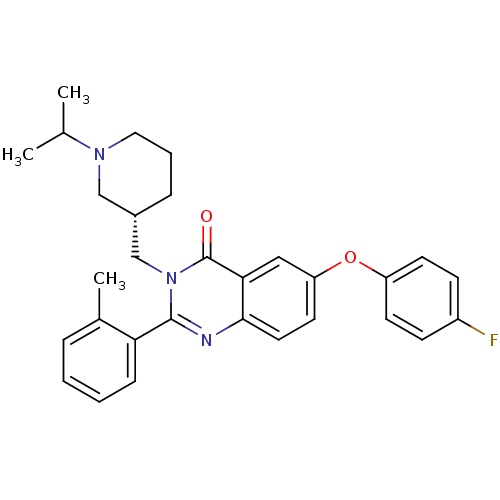 ((S)-6-(4-fluorophenoxy)-3-((1-isopropylpiperidin-3...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222867 ((S)-6-(4-fluorophenoxy)-3-((1-isopropylpiperidin-3...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222875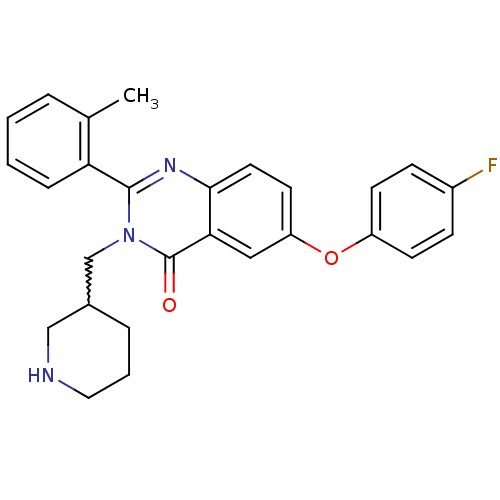 (6-(4-fluorophenoxy)-2-(2-methylphenyl)-3-(piperidi...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092319 (1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfinyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50092989 (1-[1-(4-Benzenesulfonyl-phenyl)-ethyl]-4-cyclohexy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M1 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222859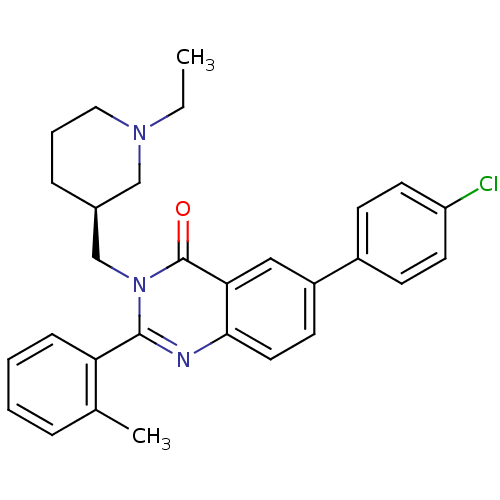 (6-(4-chlorophenyl)-3-{[(3S)-1-ethylpiperidin-3-yl]...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222873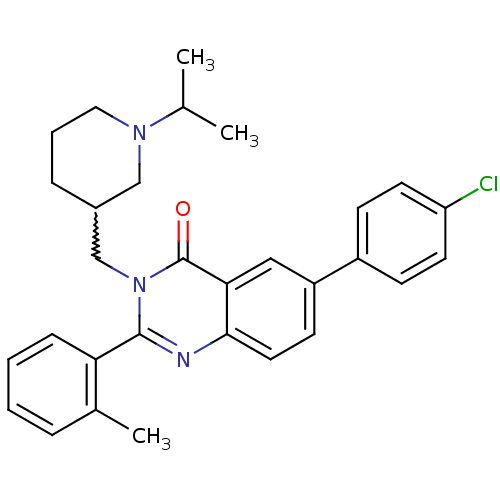 (6-(4-chlorophenyl)-3-[(1-isopropylpiperidin-3-yl)m...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222884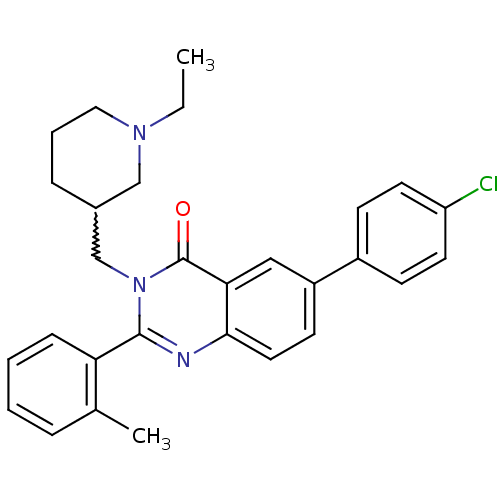 (6-(4-chlorophenyl)-3-[(-1-ethylpiperidin-3-yl)meth...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50044287 (CHEMBL3356900) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50280440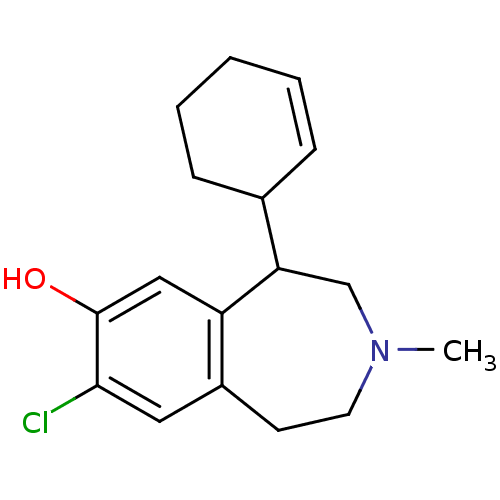 (8-Chloro-5-cyclohex-2-enyl-3-methyl-2,3,4,5-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D1 in rat striatal tissue by [3H]-SCH- 23390 displacement. | Bioorg Med Chem Lett 2: 399-402 (1992) Article DOI: 10.1016/S0960-894X(00)80155-9 BindingDB Entry DOI: 10.7270/Q2C53KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50280435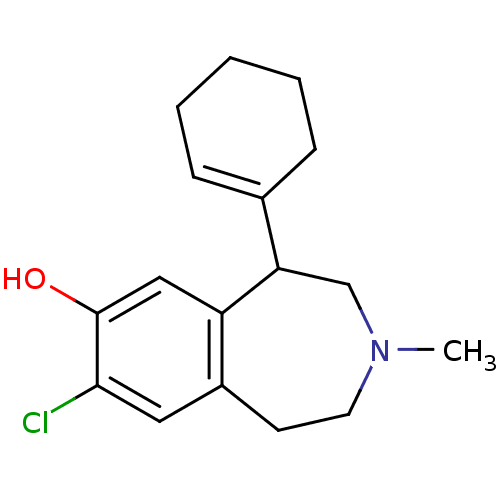 (8-Chloro-5-cyclohex-1-enyl-3-methyl-2,3,4,5-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D1 in rat striatal tissue by [3H]-SCH- 23390 displacement. | Bioorg Med Chem Lett 2: 399-402 (1992) Article DOI: 10.1016/S0960-894X(00)80155-9 BindingDB Entry DOI: 10.7270/Q2C53KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222863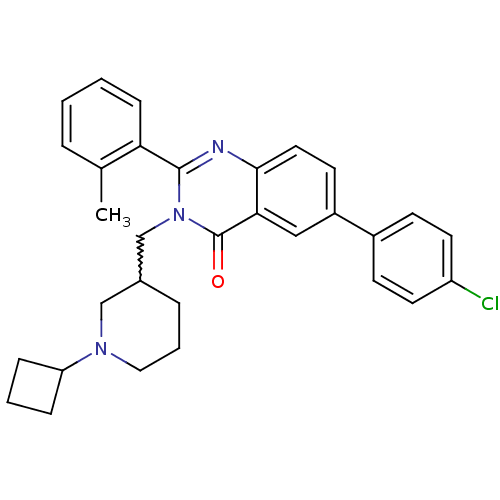 (6-(4-chlorophenyl)-3-[(1-cyclobutylpiperidin-3-yl)...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50451110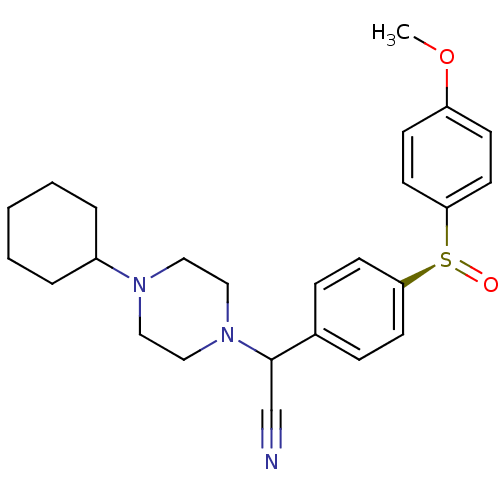 (CHEMBL2111540) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274571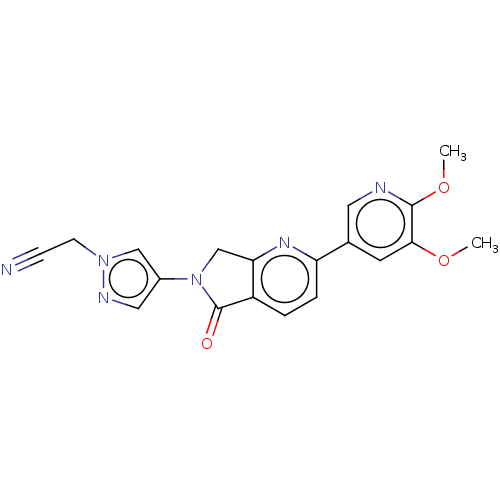 (CHEMBL4127784) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50280434 (8-Chloro-3-methyl-5-propyl-2,3,4,5-tetrahydro-1H-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D1 in rat striatal tissue by [3H]-SCH- 23390 displacement. | Bioorg Med Chem Lett 2: 399-402 (1992) Article DOI: 10.1016/S0960-894X(00)80155-9 BindingDB Entry DOI: 10.7270/Q2C53KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50280443 (8-Chloro-5-cyclohepta-2,4,6-trienyl-3-methyl-2,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D1 in rat striatal tissue by [3H]-SCH- 23390 displacement. | Bioorg Med Chem Lett 2: 399-402 (1992) Article DOI: 10.1016/S0960-894X(00)80155-9 BindingDB Entry DOI: 10.7270/Q2C53KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50451110 (CHEMBL2111540) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50451110 (CHEMBL2111540) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274538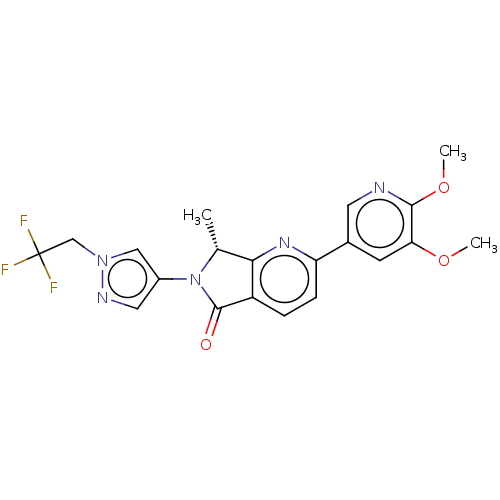 (CHEMBL4126773) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222879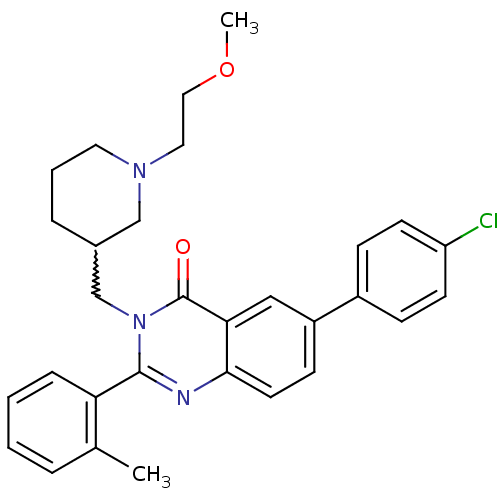 (6-(4-chlorophenyl)-3-{[1-(2-methoxyethyl)piperidin...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274537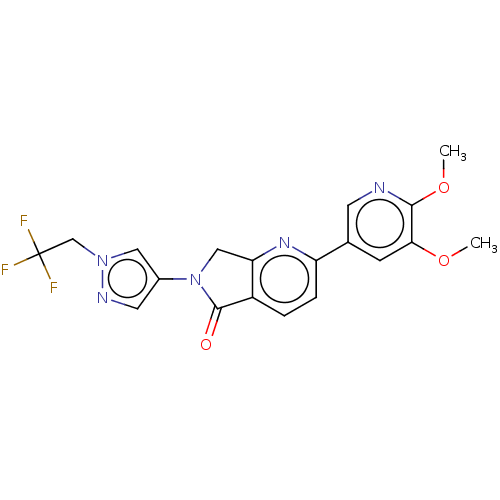 (CHEMBL4129974) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222858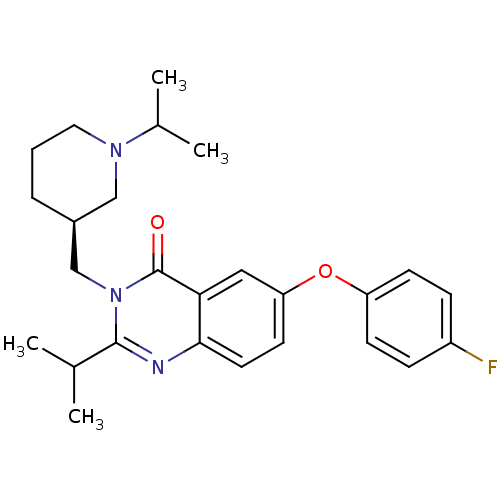 (6-(4-fluorophenoxy)-2-isopropyl-3-[(1-isopropylpip...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092993 (1-Cyclohexyl-4-{2,2,2-trifluoro-1-[4-(4-methoxy-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50280441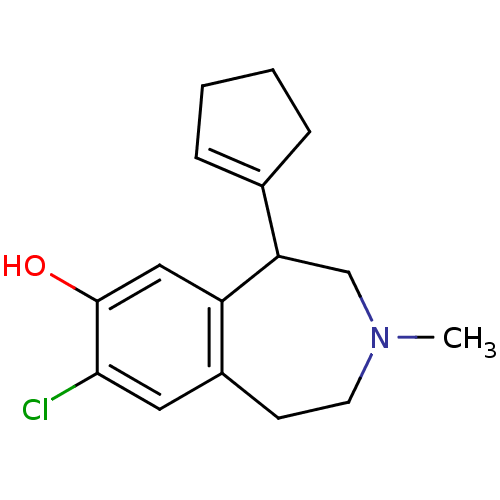 (8-Chloro-5-cyclopent-1-enyl-3-methyl-2,3,4,5-tetra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D1 in rat striatal tissue by [3H]-SCH- 23390 displacement. | Bioorg Med Chem Lett 2: 399-402 (1992) Article DOI: 10.1016/S0960-894X(00)80155-9 BindingDB Entry DOI: 10.7270/Q2C53KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50280439 (5-Allyl-8-chloro-3-methyl-2,3,4,5-tetrahydro-1H-be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D1 in rat striatal tissue by [3H]-SCH- 23390 displacement. | Bioorg Med Chem Lett 2: 399-402 (1992) Article DOI: 10.1016/S0960-894X(00)80155-9 BindingDB Entry DOI: 10.7270/Q2C53KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274572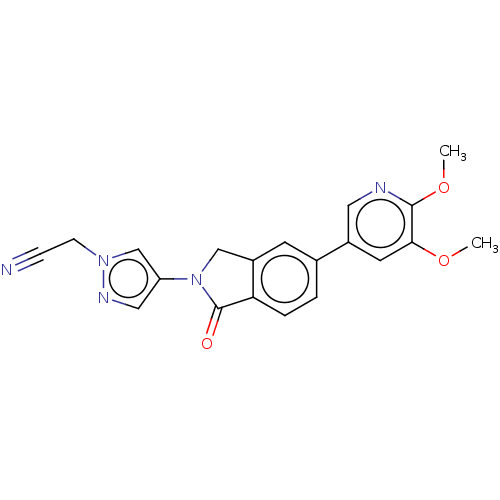 (CHEMBL4129180) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50092319 (1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfinyl)-p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M1 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50092996 (1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfonyl)-p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M1 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274541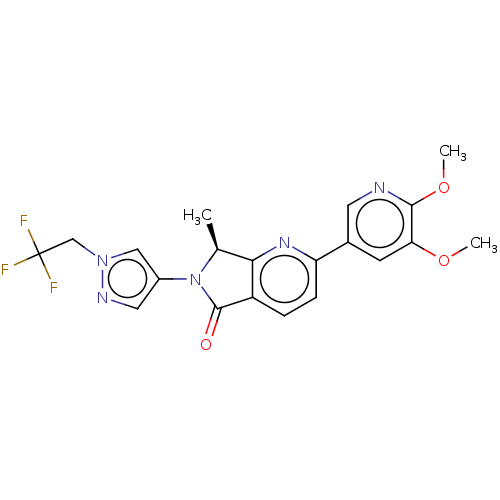 (CHEMBL4130036) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222862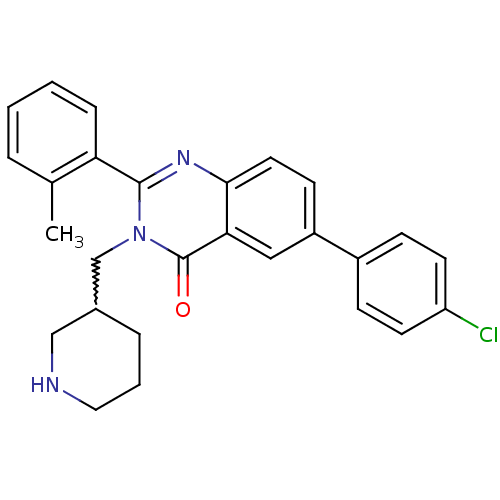 (6-(4-chlorophenyl)-2-(2-methylphenyl)-3-(piperidin...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50280438 (8-Chloro-5-cycloheptyl-3-methyl-2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D1 in rat striatal tissue by [3H]-SCH- 23390 displacement. | Bioorg Med Chem Lett 2: 399-402 (1992) Article DOI: 10.1016/S0960-894X(00)80155-9 BindingDB Entry DOI: 10.7270/Q2C53KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50092984 (1-{1-[4-(4-Chloro-benzenesulfonyl)-phenyl]-ethyl}-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M1 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274559 (CHEMBL4126707) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092317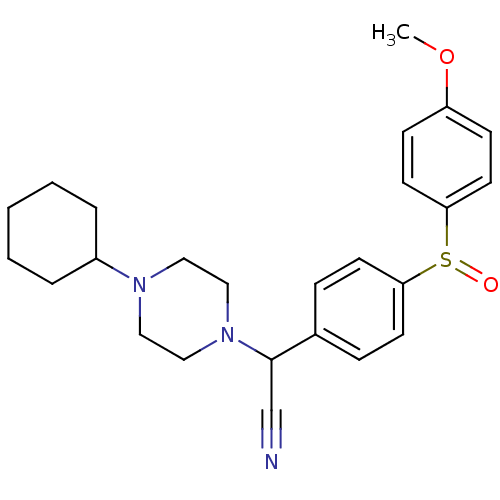 ((4-Cyclohexyl-piperazin-1-yl)-[4-(4-methoxy-benzen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50280431 (8-Chloro-3-methyl-5-prop-2-ynyl-2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D1 in rat striatal tissue by [3H]-SCH- 23390 displacement. | Bioorg Med Chem Lett 2: 399-402 (1992) Article DOI: 10.1016/S0960-894X(00)80155-9 BindingDB Entry DOI: 10.7270/Q2C53KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274542 (CHEMBL4127853) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274543 (CHEMBL4129251) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50451110 (CHEMBL2111540) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50280430 (8-Chloro-5-cyclohexyl-3-methyl-2,3,4,5-tetrahydro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D1 in rat striatal tissue by [3H]-SCH- 23390 displacement. | Bioorg Med Chem Lett 2: 399-402 (1992) Article DOI: 10.1016/S0960-894X(00)80155-9 BindingDB Entry DOI: 10.7270/Q2C53KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274540 (CHEMBL4128822) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50092995 (1-Cyclohexyl-4-{1-[4-(toluene-4-sulfonyl)-phenyl]-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M1 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274560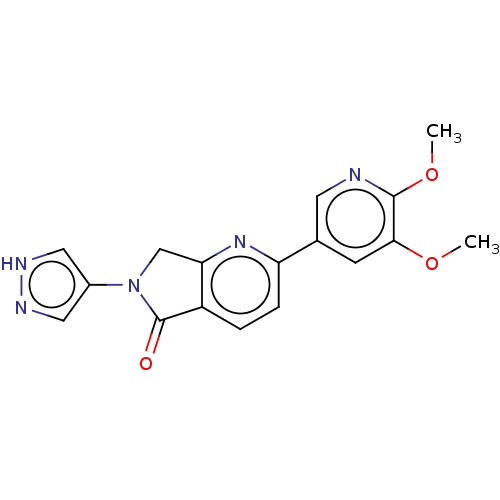 (CHEMBL4125738) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50280437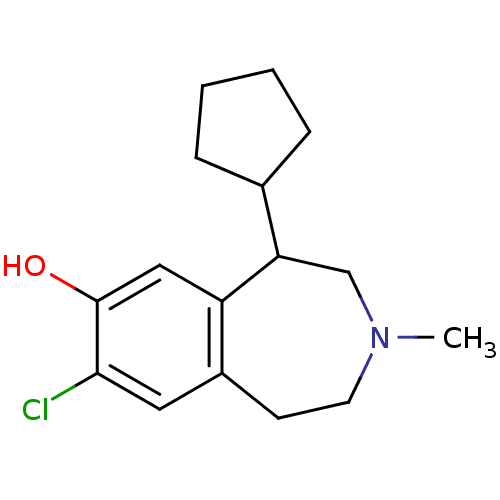 (8-Chloro-5-cyclopentyl-3-methyl-2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D1 in rat striatal tissue by [3H]-SCH- 23390 displacement. | Bioorg Med Chem Lett 2: 399-402 (1992) Article DOI: 10.1016/S0960-894X(00)80155-9 BindingDB Entry DOI: 10.7270/Q2C53KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222881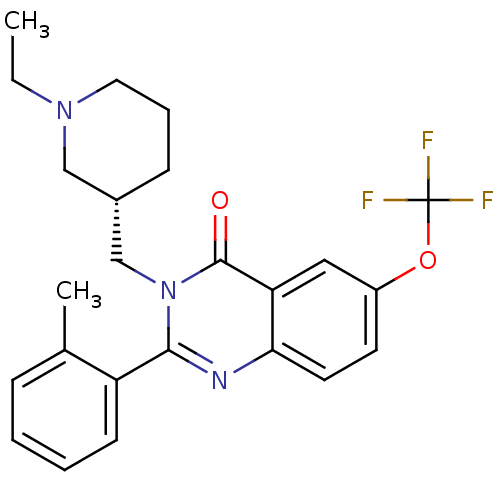 ((S)-3-((1-ethylpiperidin-3-yl)methyl)-2-o-tolyl-6-...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 535 total ) | Next | Last >> |