

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301911 (CHEMBL583269 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301902 (CHEMBL571825 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301903 (CHEMBL569120 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301914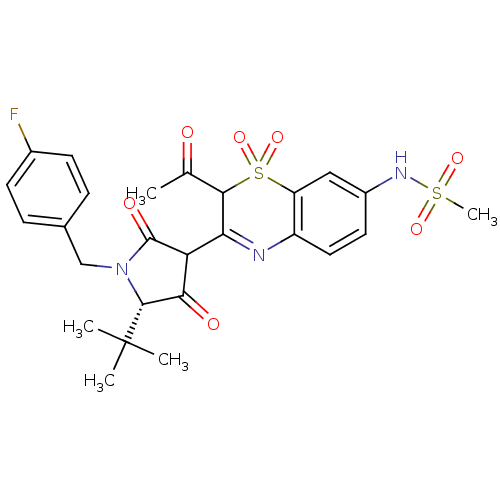 (CHEMBL570249 | N-{2-Acetyl-3-[(S)-5-tert-butyl-1-(...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301901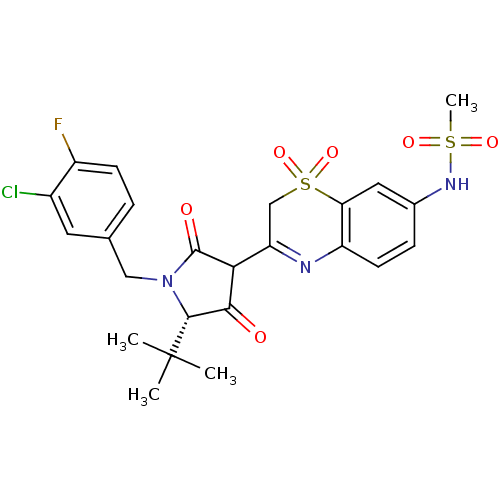 (CHEMBL570934 | N-{3-[(S)-5-tert-Butyl-1-(3-chloro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301906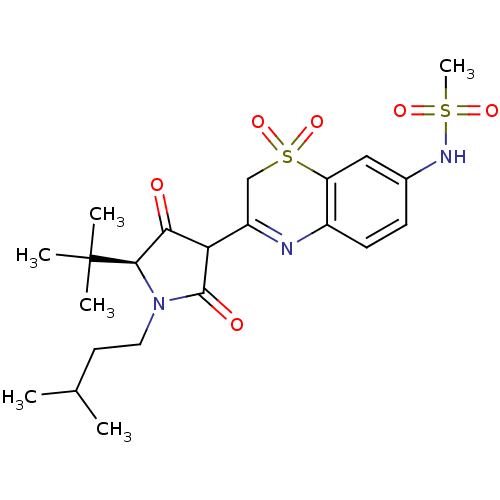 (CHEMBL571606 | N-{3-[(S)-5-tert-Butyl-4-hydroxy-1-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301913 (CHEMBL571564 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301900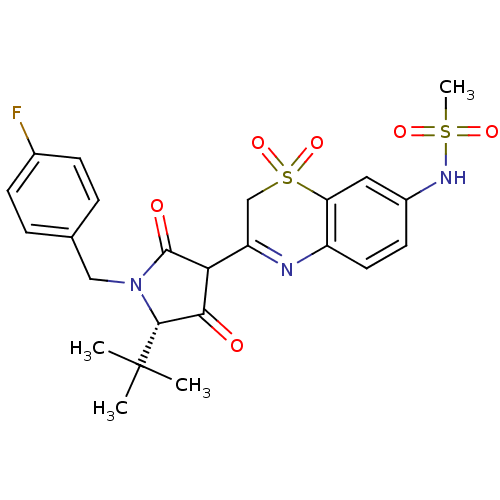 (CHEMBL568894 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301899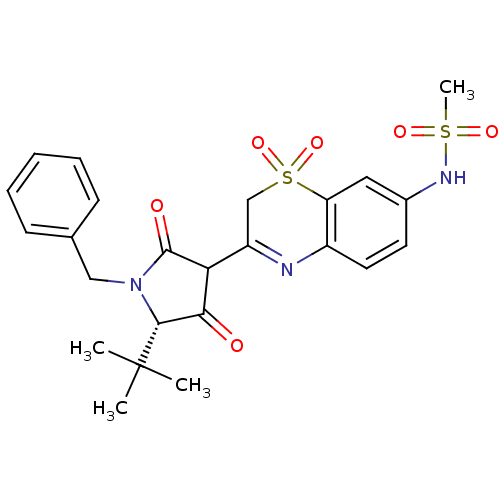 (CHEMBL585384 | N-[3-((S)-1-Benzyl-5-tert-butyl-4-h...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301907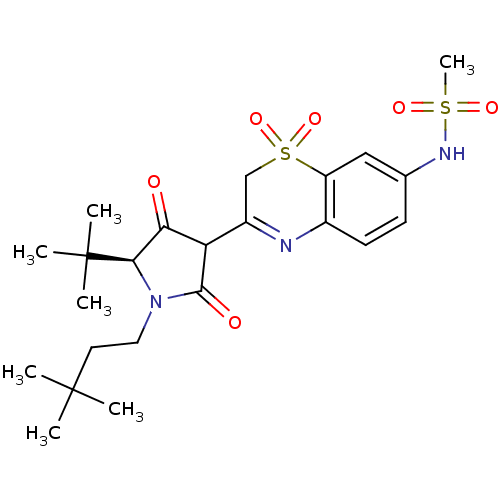 (CHEMBL572246 | N-{3-[(S)-5-tert-Butyl-1-(3,3-dimet...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301916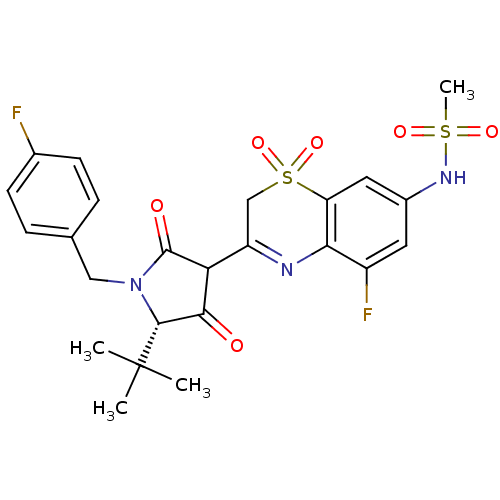 (CHEMBL585715 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301912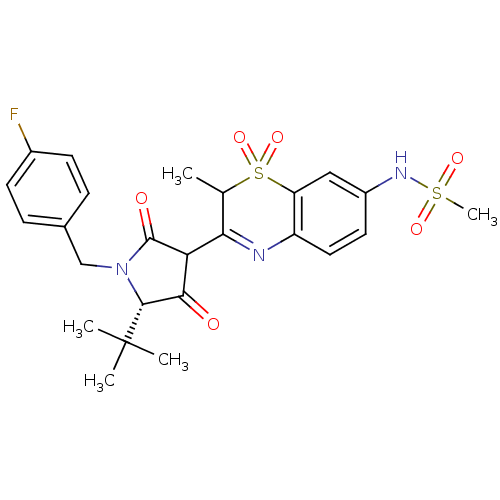 (CHEMBL584537 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301915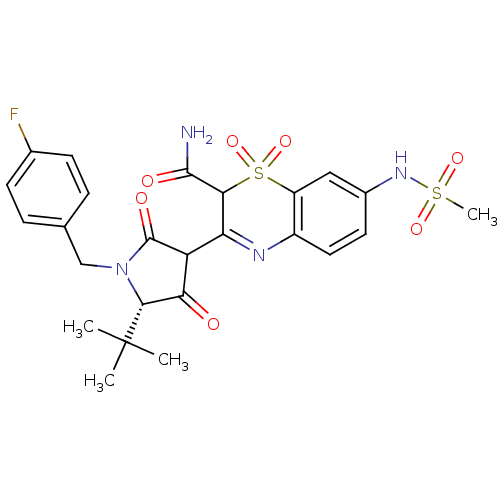 (3-[(S)-5-tert-Butyl-1-(4-fluoro-benzyl)-4-hydroxy-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301904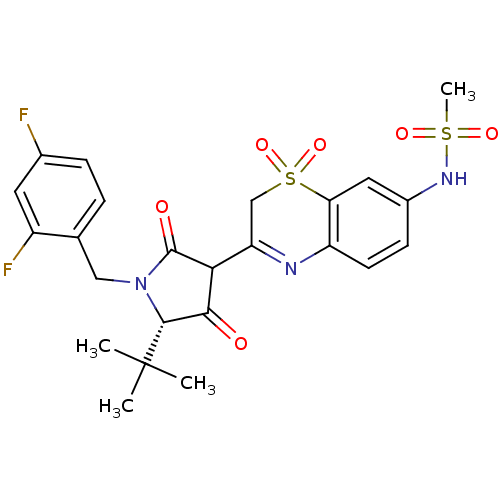 (CHEMBL569360 | N-{3-[(S)-5-tert-Butyl-1-(2,4-diflu...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301917 (CHEMBL585383 | N-{3-[(5S)-5-(1,1-dimethylpropyl)-1...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301905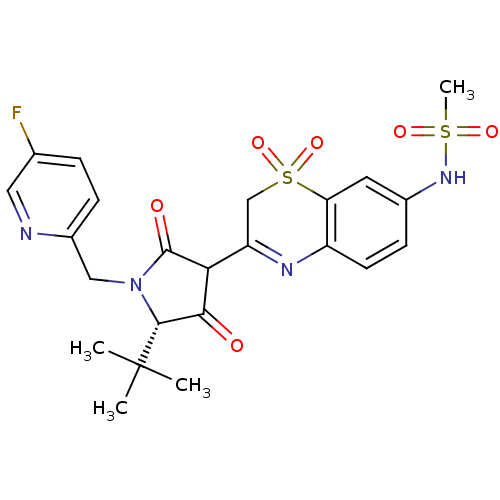 (CHEMBL569361 | N-{3-[(S)-5-tert-Butyl-1-(5-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301908 ((S)-5-tert-Butyl-1-(4-fluoro-benzyl)-3-(7-fluoro-1...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 338 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301909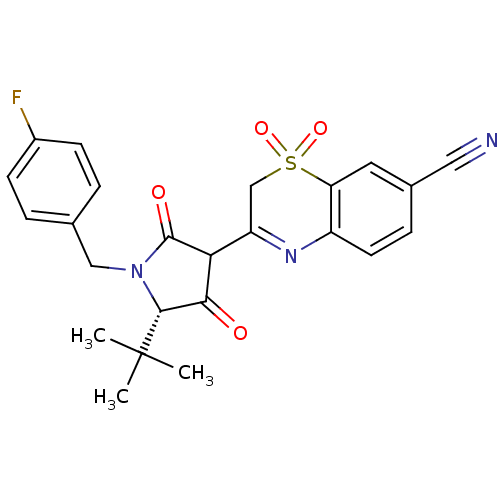 (3-[(S)-5-tert-Butyl-1-(4-fluoro-benzyl)-4-hydroxy-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301910 ((S)-5-tert-Butyl-1-(4-fluoro-benzyl)-4-hydroxy-3-(...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50441246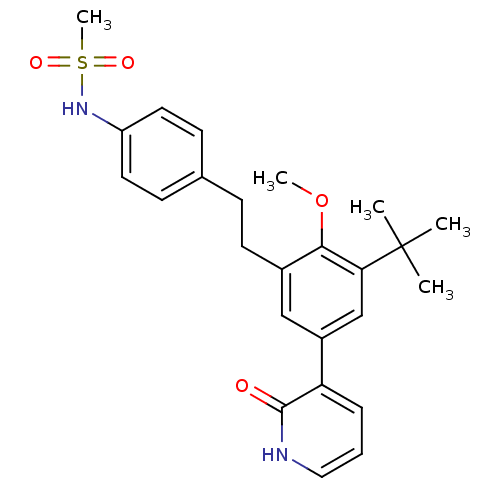 (CHEMBL2431456) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | J Med Chem 56: 8163-82 (2013) Article DOI: 10.1021/jm401266k BindingDB Entry DOI: 10.7270/Q2WQ0574 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441246 (CHEMBL2431456) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 56: 8163-82 (2013) Article DOI: 10.1021/jm401266k BindingDB Entry DOI: 10.7270/Q2WQ0574 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50441246 (CHEMBL2431456) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 56: 8163-82 (2013) Article DOI: 10.1021/jm401266k BindingDB Entry DOI: 10.7270/Q2WQ0574 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2-microglobulin (Homo sapiens (Human)) | BDBM52947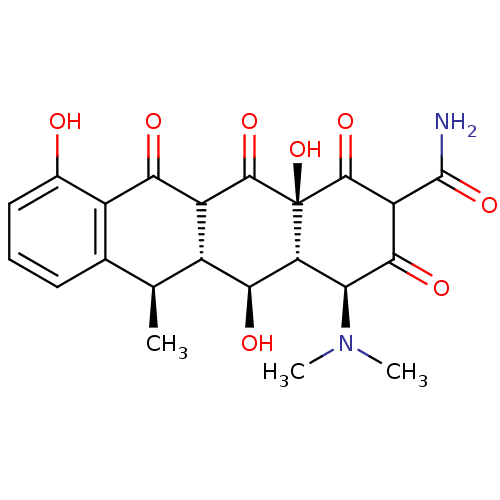 ((2Z,4S,4aR,5S,5aR,6R,12aS)-2-[amino(hydroxy)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Pavia | Assay Description Inhibition of wild type beta-m fibrillogenesis tetracycline congeners and was follow by thioflavin fluorescence assay. | J Biol Chem 286: 2121-31 (2011) Article DOI: 10.1074/jbc.M110.178376 BindingDB Entry DOI: 10.7270/Q2542M6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2-microglobulin (Homo sapiens (Human)) | BDBM92397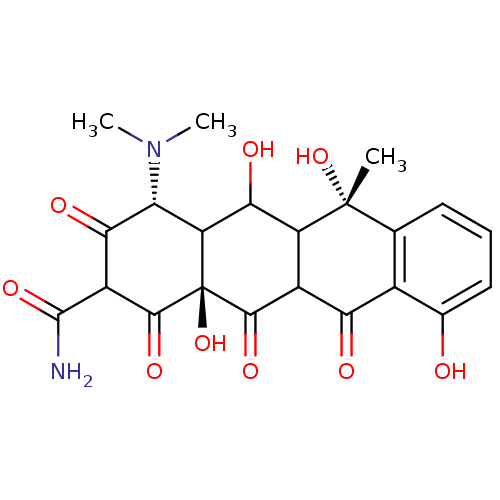 (4-epi-oxytetracycline) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Pavia | Assay Description Inhibition of wild type beta-m fibrillogenesis tetracycline congeners and was follow by thioflavin fluorescence assay. | J Biol Chem 286: 2121-31 (2011) Article DOI: 10.1074/jbc.M110.178376 BindingDB Entry DOI: 10.7270/Q2542M6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50441245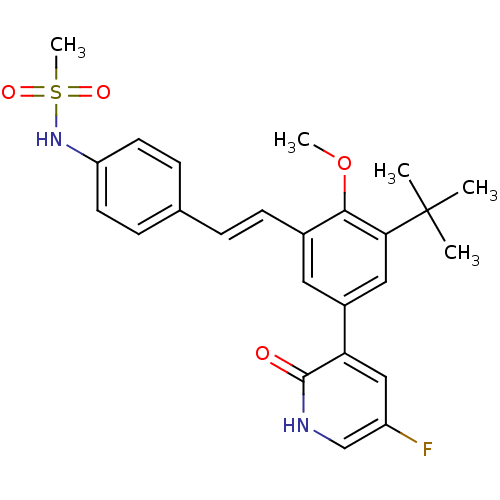 (CHEMBL2431365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | J Med Chem 56: 8163-82 (2013) Article DOI: 10.1021/jm401266k BindingDB Entry DOI: 10.7270/Q2WQ0574 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441245 (CHEMBL2431365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 56: 8163-82 (2013) Article DOI: 10.1021/jm401266k BindingDB Entry DOI: 10.7270/Q2WQ0574 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50441245 (CHEMBL2431365) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 56: 8163-82 (2013) Article DOI: 10.1021/jm401266k BindingDB Entry DOI: 10.7270/Q2WQ0574 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2-microglobulin (Homo sapiens (Human)) | BDBM37536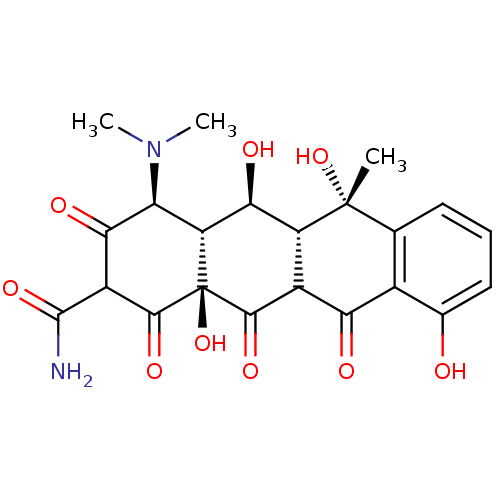 ((2E,4S,4aR,5S,5aR,6S,12aS)-2-[amino(hydroxy)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Pavia | Assay Description Inhibition of wild type beta-m fibrillogenesis tetracycline congeners and was follow by thioflavin fluorescence assay. | J Biol Chem 286: 2121-31 (2011) Article DOI: 10.1074/jbc.M110.178376 BindingDB Entry DOI: 10.7270/Q2542M6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2-microglobulin (Homo sapiens (Human)) | BDBM55038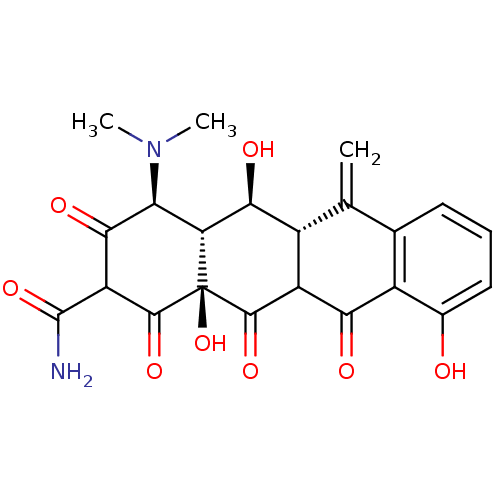 ((2E,4S,4aR,5S,5aR,12aS)-2-[amino(hydroxy)methylene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Pavia | Assay Description Inhibition of wild type beta-m fibrillogenesis tetracycline congeners and was follow by thioflavin fluorescence assay. | J Biol Chem 286: 2121-31 (2011) Article DOI: 10.1074/jbc.M110.178376 BindingDB Entry DOI: 10.7270/Q2542M6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2-microglobulin (Homo sapiens (Human)) | BDBM92395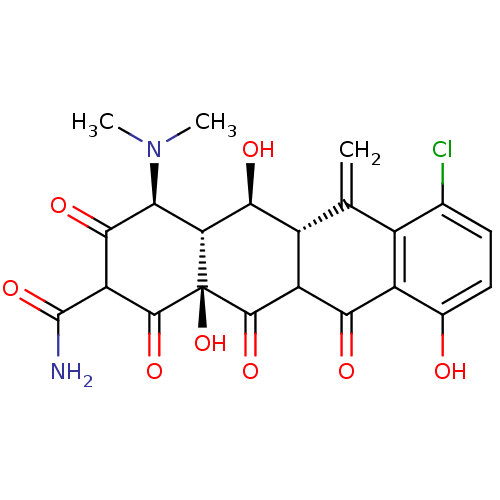 (Meclocycline) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Pavia | Assay Description Inhibition of wild type beta-m fibrillogenesis tetracycline congeners and was follow by thioflavin fluorescence assay. | J Biol Chem 286: 2121-31 (2011) Article DOI: 10.1074/jbc.M110.178376 BindingDB Entry DOI: 10.7270/Q2542M6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2-microglobulin (Homo sapiens (Human)) | BDBM92398 (Rolitetracycline) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Pavia | Assay Description Inhibition of wild type beta-m fibrillogenesis tetracycline congeners and was follow by thioflavin fluorescence assay. | J Biol Chem 286: 2121-31 (2011) Article DOI: 10.1074/jbc.M110.178376 BindingDB Entry DOI: 10.7270/Q2542M6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2-microglobulin (Homo sapiens (Human)) | BDBM92399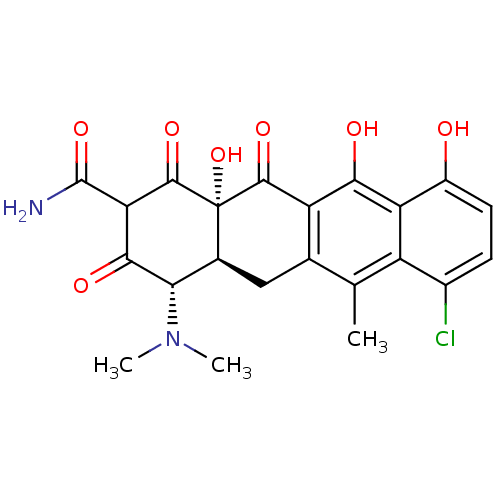 (Anhydrochlortetracycline) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Pavia | Assay Description Inhibition of wild type beta-m fibrillogenesis tetracycline congeners and was follow by thioflavin fluorescence assay. | J Biol Chem 286: 2121-31 (2011) Article DOI: 10.1074/jbc.M110.178376 BindingDB Entry DOI: 10.7270/Q2542M6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH oxidase organizer 1 (Homo sapiens) | BDBM50614675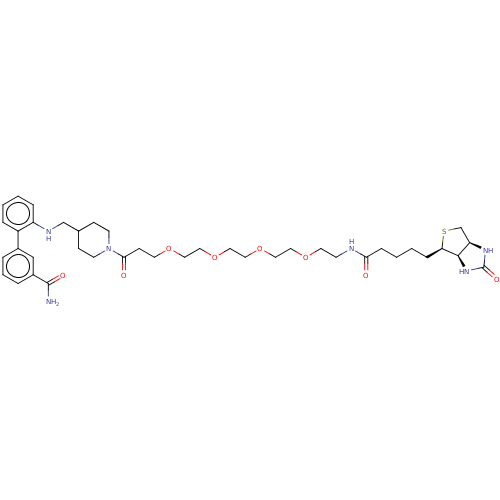 (CHEMBL5271171) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 5 (Homo sapiens (Human)) | BDBM50503349 (CHEMBL4476817) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a |
Beckman Research Institute of the City of Hope Curated by ChEMBL | Assay Description Binding affinity to human 15N-labeled STEP PTP domain (281 to 563 residues) expressed in Escherichia coli OD2 by 1H,15N TROSY NMR spectral analysis | J Med Chem 62: 306-316 (2019) Article DOI: 10.1021/acs.jmedchem.8b00857 BindingDB Entry DOI: 10.7270/Q2K35XWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 5 (Homo sapiens (Human)) | BDBM50503349 (CHEMBL4476817) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | 8.00E+5 | n/a | n/a | n/a | n/a | n/a |
Beckman Research Institute of the City of Hope Curated by ChEMBL | Assay Description Binding affinity to human STEP PTP domain (281 to 563 residues) expressed in Escherichia coli BL21(DE3) by ITC | J Med Chem 62: 306-316 (2019) Article DOI: 10.1021/acs.jmedchem.8b00857 BindingDB Entry DOI: 10.7270/Q2K35XWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||