 Found 753 hits with Last Name = 'lukacs' and Initial = 'cm'
Found 753 hits with Last Name = 'lukacs' and Initial = 'cm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100437
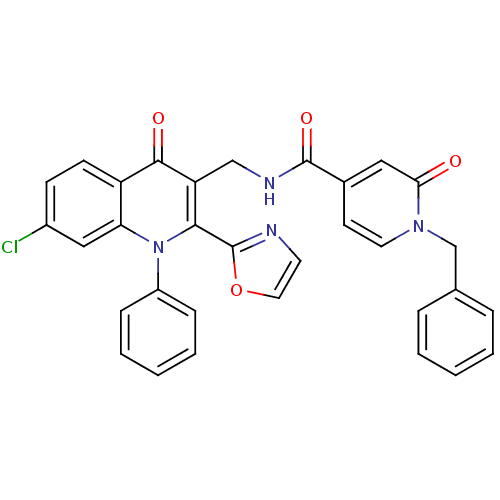
(US8501732, I-1)Show SMILES Clc1ccc2c(c1)n(-c1ccccc1)c(-c1ncco1)c(CNC(=O)c1ccn(Cc3ccccc3)c(=O)c1)c2=O Show InChI InChI=1S/C32H23ClN4O4/c33-23-11-12-25-27(18-23)37(24-9-5-2-6-10-24)29(32-34-14-16-41-32)26(30(25)39)19-35-31(40)22-13-15-36(28(38)17-22)20-21-7-3-1-4-8-21/h1-18H,19-20H2,(H,35,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100609
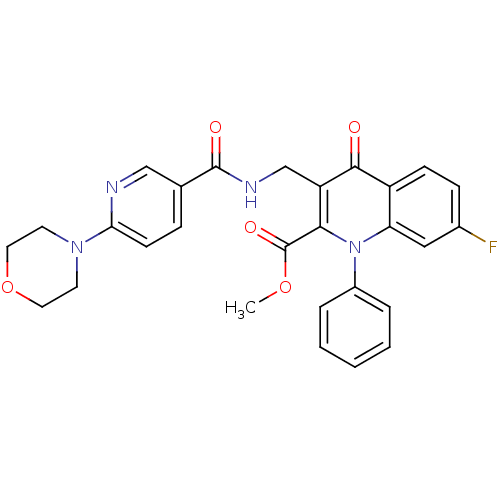
(US8501732, I-169)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(nc2)N2CCOCC2)c(=O)c2ccc(F)cc2n1-c1ccccc1 Show InChI InChI=1S/C28H25FN4O5/c1-37-28(36)25-22(17-31-27(35)18-7-10-24(30-16-18)32-11-13-38-14-12-32)26(34)21-9-8-19(29)15-23(21)33(25)20-5-3-2-4-6-20/h2-10,15-16H,11-14,17H2,1H3,(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100445
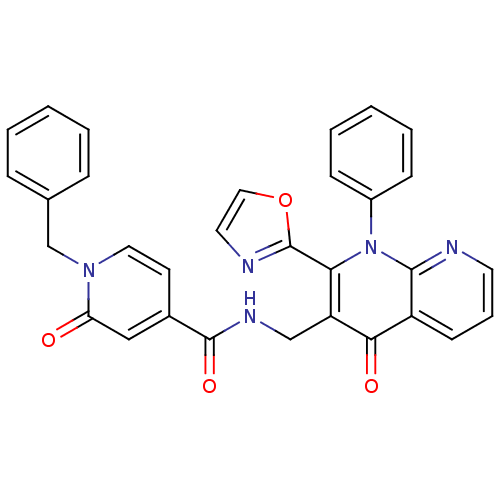
(US8501732, I-9)Show SMILES O=C(NCc1c(-c2ncco2)n(-c2ccccc2)c2ncccc2c1=O)c1ccn(Cc2ccccc2)c(=O)c1 Show InChI InChI=1S/C31H23N5O4/c37-26-18-22(13-16-35(26)20-21-8-3-1-4-9-21)30(39)34-19-25-27(31-33-15-17-40-31)36(23-10-5-2-6-11-23)29-24(28(25)38)12-7-14-32-29/h1-18H,19-20H2,(H,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100550
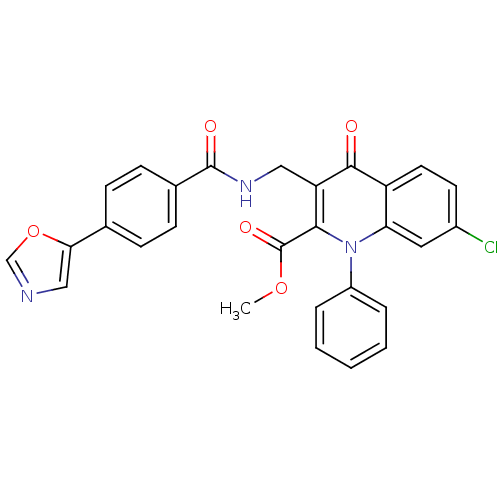
(US8501732, I-110)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(cc2)-c2cnco2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C28H20ClN3O5/c1-36-28(35)25-22(14-31-27(34)18-9-7-17(8-10-18)24-15-30-16-37-24)26(33)21-12-11-19(29)13-23(21)32(25)20-5-3-2-4-6-20/h2-13,15-16H,14H2,1H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100549
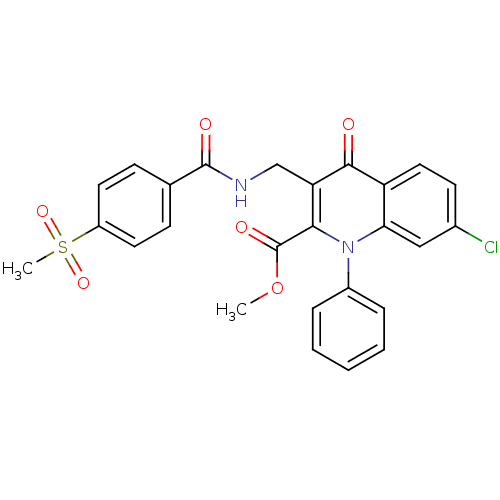
(US8501732, I-109)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(cc2)S(C)(=O)=O)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C26H21ClN2O6S/c1-35-26(32)23-21(15-28-25(31)16-8-11-19(12-9-16)36(2,33)34)24(30)20-13-10-17(27)14-22(20)29(23)18-6-4-3-5-7-18/h3-14H,15H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM537077

(US11247971, Cmpd ID 400)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(OCC3CC3(F)F)c2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489091

(2-(5- (cyclopropylmethyl)- 3-(4-fluoro-3- ((tetrah...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(OCC3CCOC3)c2)-c2nc(cs2)C(O)=O)cc1F Show InChI InChI=1S/C29H28F2N4O6S2/c30-21-5-4-19(12-25(21)41-14-18-7-8-40-13-18)27-20(9-17-3-6-26(22(31)10-17)43(32,38)39)24(11-16-1-2-16)35(34-27)29-33-23(15-42-29)28(36)37/h3-6,10,12,15-16,18H,1-2,7-9,11,13-14H2,(H,36,37)(H2,32,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100551
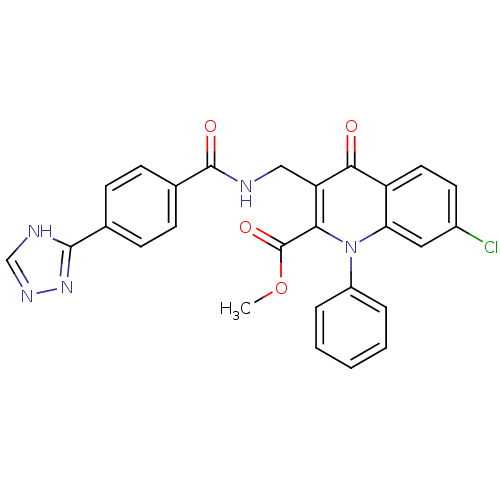
(US8501732, I-111)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(cc2)-c2nnc[nH]2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C27H20ClN5O4/c1-37-27(36)23-21(14-29-26(35)17-9-7-16(8-10-17)25-30-15-31-32-25)24(34)20-12-11-18(28)13-22(20)33(23)19-5-3-2-4-6-19/h2-13,15H,14H2,1H3,(H,29,35)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441819
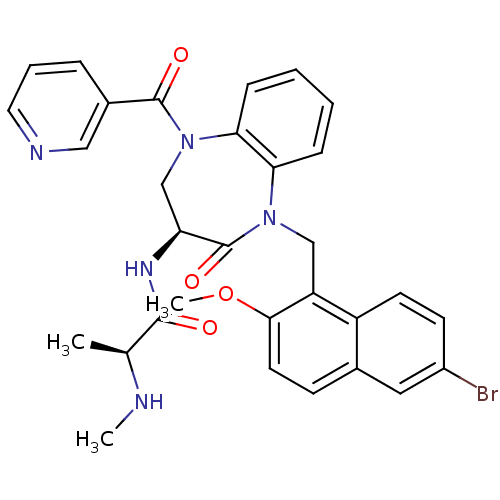
(CHEMBL2436209 | US10053431, 89b)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)c2cccnc2)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C31H30BrN5O4/c1-19(33-2)29(38)35-25-18-37(30(39)21-7-6-14-34-16-21)27-9-5-4-8-26(27)36(31(25)40)17-24-23-12-11-22(32)15-20(23)10-13-28(24)41-3/h4-16,19,25,33H,17-18H2,1-3H3,(H,35,38)/t19-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489090

(2-(5- (cyclopropylmethyl)- 3-(4-fluoro-3- ((tetrah...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(OCC3CCCO3)c2)-c2nc(cs2)C(O)=O)cc1F Show InChI InChI=1S/C29H28F2N4O6S2/c30-21-7-6-18(13-25(21)41-14-19-2-1-9-40-19)27-20(10-17-5-8-26(22(31)11-17)43(32,38)39)24(12-16-3-4-16)35(34-27)29-33-23(15-42-29)28(36)37/h5-8,11,13,15-16,19H,1-4,9-10,12,14H2,(H,36,37)(H2,32,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489160

(2-(3-(3-(tert- butylcarbamoyl)-4- fluorophenyl)-5-...)Show SMILES CC(C)(C)NC(=O)c1cc(ccc1F)-c1nn(c(CC2CC2)c1Cc1ccc(c(F)c1)S(N)(=O)=O)-c1nc(cs1)C(O)=O Show InChI InChI=1S/C29H29F2N5O5S2/c1-29(2,3)34-26(37)18-13-17(7-8-20(18)30)25-19(10-16-6-9-24(21(31)11-16)43(32,40)41)23(12-15-4-5-15)36(35-25)28-33-22(14-42-28)27(38)39/h6-9,11,13-15H,4-5,10,12H2,1-3H3,(H,34,37)(H,38,39)(H2,32,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441818

(CHEMBL2436213 | US10053431, 75d)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)C2CCOCC2)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C31H35BrN4O5/c1-19(33-2)29(37)34-25-18-36(30(38)20-12-14-41-15-13-20)27-7-5-4-6-26(27)35(31(25)39)17-24-23-10-9-22(32)16-21(23)8-11-28(24)40-3/h4-11,16,19-20,25,33H,12-15,17-18H2,1-3H3,(H,34,37)/t19-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441831
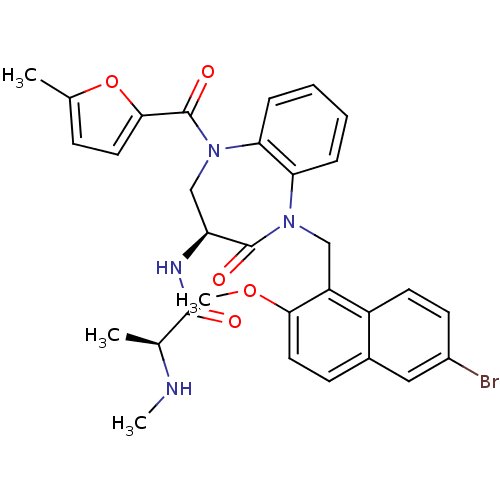
(CHEMBL2436212 | US10053431, 91c)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)c2ccc(C)o2)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C31H31BrN4O5/c1-18-9-13-28(41-18)31(39)36-17-24(34-29(37)19(2)33-3)30(38)35(25-7-5-6-8-26(25)36)16-23-22-12-11-21(32)15-20(22)10-14-27(23)40-4/h5-15,19,24,33H,16-17H2,1-4H3,(H,34,37)/t19-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441816
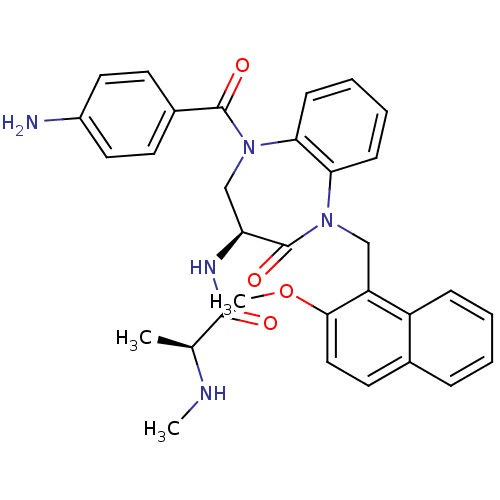
(CHEMBL2436208 | US10053431, 41)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)c2ccc(N)cc2)c2ccccc2N(Cc2c(OC)ccc3ccccc23)C1=O |r| Show InChI InChI=1S/C32H33N5O4/c1-20(34-2)30(38)35-26-19-37(31(39)22-12-15-23(33)16-13-22)28-11-7-6-10-27(28)36(32(26)40)18-25-24-9-5-4-8-21(24)14-17-29(25)41-3/h4-17,20,26,34H,18-19,33H2,1-3H3,(H,35,38)/t20-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100573
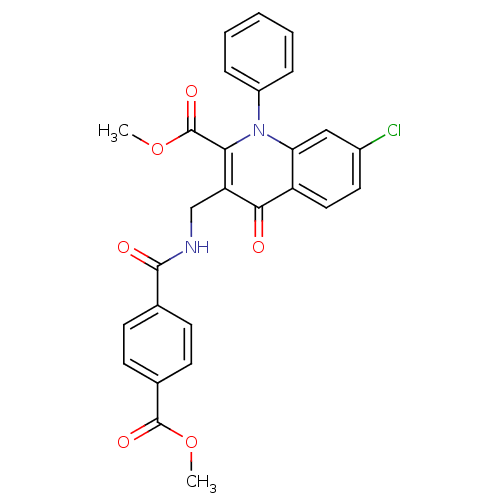
(US8501732, I-133)Show SMILES COC(=O)c1ccc(cc1)C(=O)NCc1c(C(=O)OC)n(-c2ccccc2)c2cc(Cl)ccc2c1=O Show InChI InChI=1S/C27H21ClN2O6/c1-35-26(33)17-10-8-16(9-11-17)25(32)29-15-21-23(27(34)36-2)30(19-6-4-3-5-7-19)22-14-18(28)12-13-20(22)24(21)31/h3-14H,15H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441833

(CHEMBL2436334 | US10053431, 75b)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)c2ccc(C)cc2)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C33H33BrN4O4/c1-20-9-11-22(12-10-20)32(40)38-19-27(36-31(39)21(2)35-3)33(41)37(28-7-5-6-8-29(28)38)18-26-25-15-14-24(34)17-23(25)13-16-30(26)42-4/h5-17,21,27,35H,18-19H2,1-4H3,(H,36,39)/t21-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441834
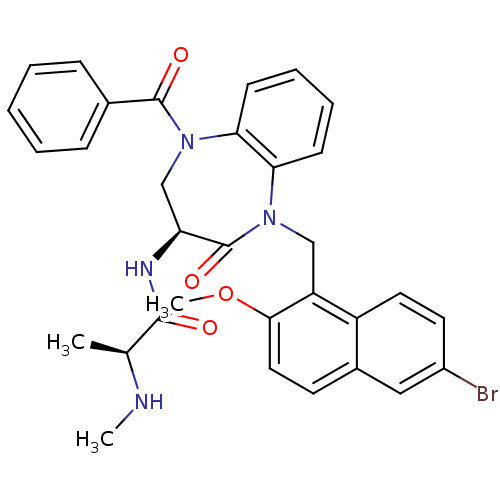
(CHEMBL2436330 | US10053431, 91a)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)c2ccccc2)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C32H31BrN4O4/c1-20(34-2)30(38)35-26-19-37(31(39)21-9-5-4-6-10-21)28-12-8-7-11-27(28)36(32(26)40)18-25-24-15-14-23(33)17-22(24)13-16-29(25)41-3/h4-17,20,26,34H,18-19H2,1-3H3,(H,35,38)/t20-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100552
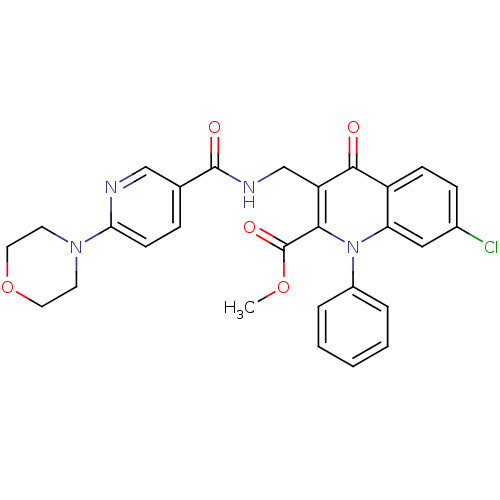
(US8501732, I-112)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(nc2)N2CCOCC2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C28H25ClN4O5/c1-37-28(36)25-22(17-31-27(35)18-7-10-24(30-16-18)32-11-13-38-14-12-32)26(34)21-9-8-19(29)15-23(21)33(25)20-5-3-2-4-6-20/h2-10,15-16H,11-14,17H2,1H3,(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441832
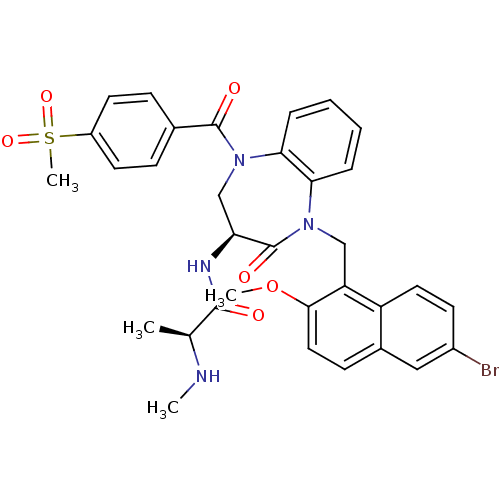
(CHEMBL2436205 | US10053431, 90d)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)c2ccc(cc2)S(C)(=O)=O)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C33H33BrN4O6S/c1-20(35-2)31(39)36-27-19-38(32(40)21-9-13-24(14-10-21)45(4,42)43)29-8-6-5-7-28(29)37(33(27)41)18-26-25-15-12-23(34)17-22(25)11-16-30(26)44-3/h5-17,20,27,35H,18-19H2,1-4H3,(H,36,39)/t20-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50569440

(CHEMBL4877988 | US11752138, Compound 152)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(NC(=O)c3ccccc3)c2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441830

(CHEMBL2436210 | US10053431, 85)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)Cc2cccnc2)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C32H32BrN5O4/c1-20(34-2)31(40)36-26-19-37(30(39)15-21-7-6-14-35-17-21)27-8-4-5-9-28(27)38(32(26)41)18-25-24-12-11-23(33)16-22(24)10-13-29(25)42-3/h4-14,16-17,20,26,34H,15,18-19H2,1-3H3,(H,36,40)/t20-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100553
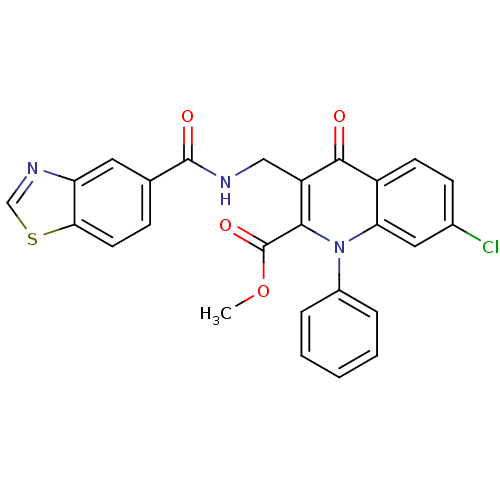
(US8501732, I-113)Show SMILES COC(=O)c1c(CNC(=O)c2ccc3scnc3c2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C26H18ClN3O4S/c1-34-26(33)23-19(13-28-25(32)15-7-10-22-20(11-15)29-14-35-22)24(31)18-9-8-16(27)12-21(18)30(23)17-5-3-2-4-6-17/h2-12,14H,13H2,1H3,(H,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100448
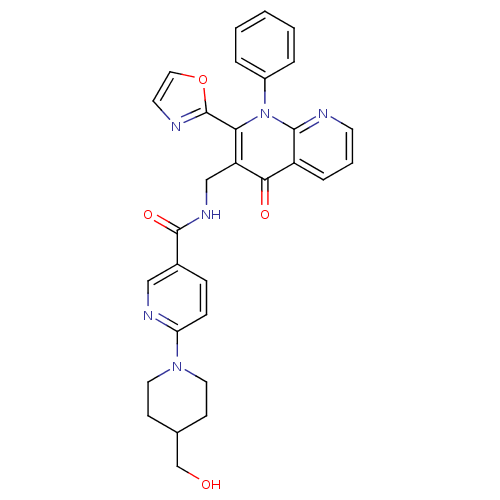
(US8501732, I-12)Show SMILES OCC1CCN(CC1)c1ccc(cn1)C(=O)NCc1c(-c2ncco2)n(-c2ccccc2)c2ncccc2c1=O Show InChI InChI=1S/C30H28N6O4/c37-19-20-10-14-35(15-11-20)25-9-8-21(17-33-25)29(39)34-18-24-26(30-32-13-16-40-30)36(22-5-2-1-3-6-22)28-23(27(24)38)7-4-12-31-28/h1-9,12-13,16-17,20,37H,10-11,14-15,18-19H2,(H,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100574
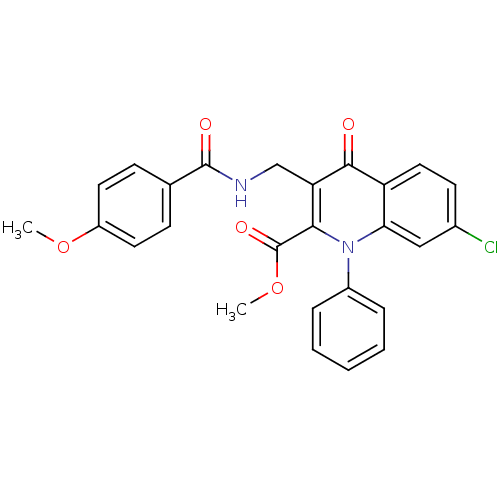
(US8501732, I-134)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(OC)cc2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C26H21ClN2O5/c1-33-19-11-8-16(9-12-19)25(31)28-15-21-23(26(32)34-2)29(18-6-4-3-5-7-18)22-14-17(27)10-13-20(22)24(21)30/h3-14H,15H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100438

(US8501732, I-2)Show SMILES Clc1ccc2c(c1)n(-c1ccccc1)c(-c1ncco1)c(CNC(=O)c1ccnc(c1)N1CCOCC1)c2=O Show InChI InChI=1S/C29H24ClN5O4/c30-20-6-7-22-24(17-20)35(21-4-2-1-3-5-21)26(29-32-10-13-39-29)23(27(22)36)18-33-28(37)19-8-9-31-25(16-19)34-11-14-38-15-12-34/h1-10,13,16-17H,11-12,14-15,18H2,(H,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100554
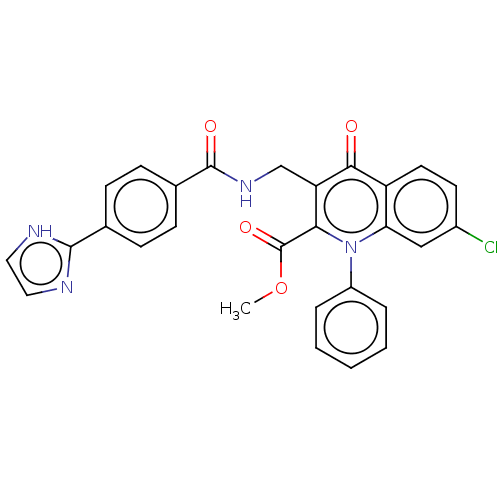
(US8501732, I-114)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(cc2)-c2ncc[nH]2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C28H21ClN4O4/c1-37-28(36)24-22(16-32-27(35)18-9-7-17(8-10-18)26-30-13-14-31-26)25(34)21-12-11-19(29)15-23(21)33(24)20-5-3-2-4-6-20/h2-15H,16H2,1H3,(H,30,31)(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489161

(2-(3-(3- (benzylcarbamoyl)-4- fluorophenyl)-5- (cy...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C(=O)NCc2ccccc2)-c2nc(cs2)C(O)=O)cc1F Show InChI InChI=1S/C32H27F2N5O5S2/c33-24-10-9-21(15-22(24)30(40)36-16-19-4-2-1-3-5-19)29-23(12-20-8-11-28(25(34)13-20)46(35,43)44)27(14-18-6-7-18)39(38-29)32-37-26(17-45-32)31(41)42/h1-5,8-11,13,15,17-18H,6-7,12,14,16H2,(H,36,40)(H,41,42)(H2,35,43,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM536955

(US11247971, Cmpd ID 278)Show SMILES COc1cc(ccc1F)-c1nn(c(CC2CC2)c1Cc1ccc(cc1)S(N)(=O)=O)-c1nc(cs1)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489092

(2-(3-(3- cydopropoxy-4- fluorophenyl)-5- (cyclopro...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(OC3CC3)c2)-c2nc(cs2)C(O)=O)cc1F Show InChI InChI=1S/C27H24F2N4O5S2/c28-19-7-4-16(12-23(19)38-17-5-6-17)25-18(9-15-3-8-24(20(29)10-15)40(30,36)37)22(11-14-1-2-14)33(32-25)27-31-21(13-39-27)26(34)35/h3-4,7-8,10,12-14,17H,1-2,5-6,9,11H2,(H,34,35)(H2,30,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50250655
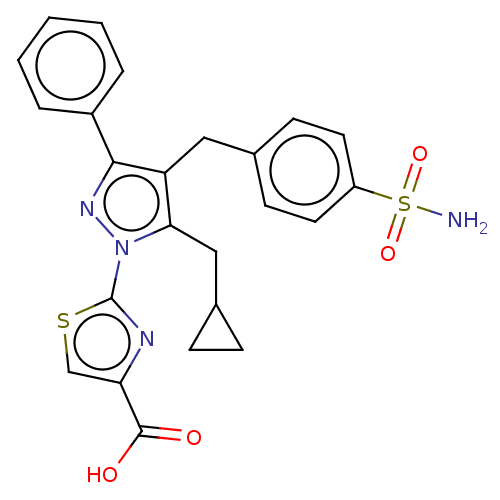
(CHEMBL4059985 | US10961200, Compound 189 | US11247...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccccc2)-c2nc(cs2)C(O)=O)cc1 Show InChI InChI=1S/C24H22N4O4S2/c25-34(31,32)18-10-8-15(9-11-18)12-19-21(13-16-6-7-16)28(24-26-20(14-33-24)23(29)30)27-22(19)17-4-2-1-3-5-17/h1-5,8-11,14,16H,6-7,12-13H2,(H,29,30)(H2,25,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes LDHB using sodium pyruvate as substrate after 5 mins in presence of NAPDH by diaphorase/resazurin based fluorescence... |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100555
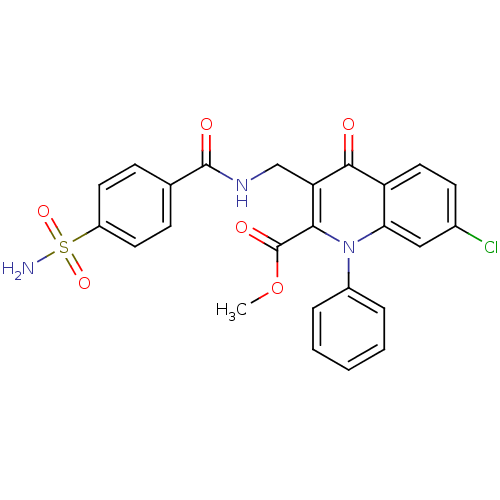
(US8501732, I-115)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(cc2)S(N)(=O)=O)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C25H20ClN3O6S/c1-35-25(32)22-20(14-28-24(31)15-7-10-18(11-8-15)36(27,33)34)23(30)19-12-9-16(26)13-21(19)29(22)17-5-3-2-4-6-17/h2-13H,14H2,1H3,(H,28,31)(H2,27,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100556
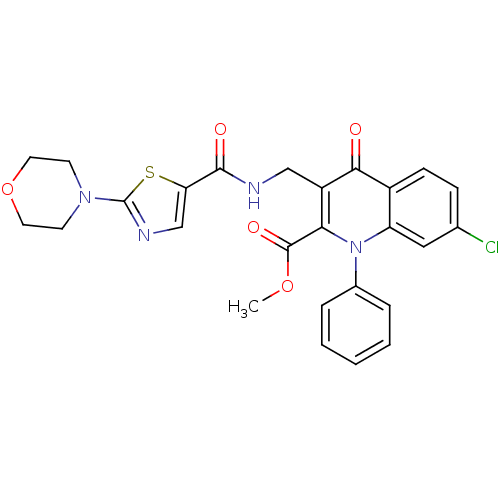
(US8501732, I-116)Show SMILES COC(=O)c1c(CNC(=O)c2cnc(s2)N2CCOCC2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C26H23ClN4O5S/c1-35-25(34)22-19(14-28-24(33)21-15-29-26(37-21)30-9-11-36-12-10-30)23(32)18-8-7-16(27)13-20(18)31(22)17-5-3-2-4-6-17/h2-8,13,15H,9-12,14H2,1H3,(H,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100575
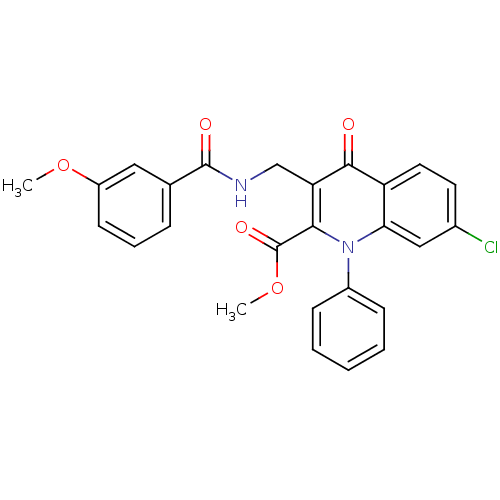
(US8501732, I-135)Show SMILES COC(=O)c1c(CNC(=O)c2cccc(OC)c2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C26H21ClN2O5/c1-33-19-10-6-7-16(13-19)25(31)28-15-21-23(26(32)34-2)29(18-8-4-3-5-9-18)22-14-17(27)11-12-20(22)24(21)30/h3-14H,15H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441829
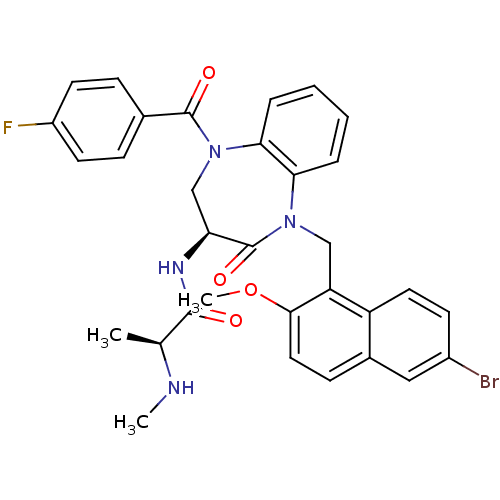
(CHEMBL2436331 | US10053431, 75a)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)c2ccc(F)cc2)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C32H30BrFN4O4/c1-19(35-2)30(39)36-26-18-38(31(40)20-8-12-23(34)13-9-20)28-7-5-4-6-27(28)37(32(26)41)17-25-24-14-11-22(33)16-21(24)10-15-29(25)42-3/h4-16,19,26,35H,17-18H2,1-3H3,(H,36,39)/t19-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50250655

(CHEMBL4059985 | US10961200, Compound 189 | US11247...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccccc2)-c2nc(cs2)C(O)=O)cc1 Show InChI InChI=1S/C24H22N4O4S2/c25-34(31,32)18-10-8-15(9-11-18)12-19-21(13-16-6-7-16)28(24-26-20(14-33-24)23(29)30)27-22(19)17-4-2-1-3-5-17/h1-5,8-11,14,16H,6-7,12-13H2,(H,29,30)(H2,25,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged LDHA in presence of NADH by SPR assay |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50250655

(CHEMBL4059985 | US10961200, Compound 189 | US11247...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccccc2)-c2nc(cs2)C(O)=O)cc1 Show InChI InChI=1S/C24H22N4O4S2/c25-34(31,32)18-10-8-15(9-11-18)12-19-21(13-16-6-7-16)28(24-26-20(14-33-24)23(29)30)27-22(19)17-4-2-1-3-5-17/h1-5,8-11,14,16H,6-7,12-13H2,(H,29,30)(H2,25,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human liver LDHA using sodium pyruvate as substrate after 5 mins in presence of NAPDH and in absence of EDTA by diaphorase/resazurin ba... |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100439
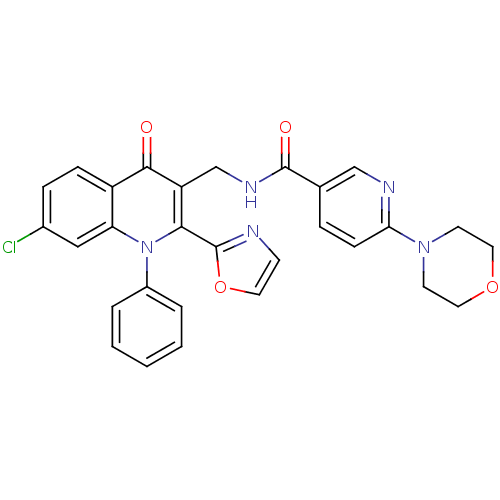
(US8501732, I-3)Show SMILES Clc1ccc2c(c1)n(-c1ccccc1)c(-c1ncco1)c(CNC(=O)c1ccc(nc1)N1CCOCC1)c2=O Show InChI InChI=1S/C29H24ClN5O4/c30-20-7-8-22-24(16-20)35(21-4-2-1-3-5-21)26(29-31-10-13-39-29)23(27(22)36)18-33-28(37)19-6-9-25(32-17-19)34-11-14-38-15-12-34/h1-10,13,16-17H,11-12,14-15,18H2,(H,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441810
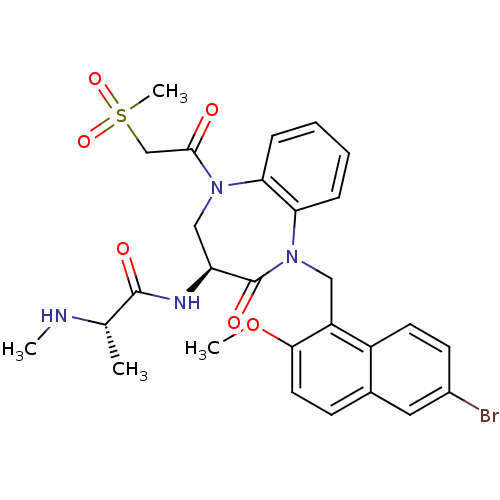
(CHEMBL2436215 | US10053431, 76b)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)CS(C)(=O)=O)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C28H31BrN4O6S/c1-17(30-2)27(35)31-22-15-32(26(34)16-40(4,37)38)23-7-5-6-8-24(23)33(28(22)36)14-21-20-11-10-19(29)13-18(20)9-12-25(21)39-3/h5-13,17,22,30H,14-16H2,1-4H3,(H,31,35)/t17-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441828
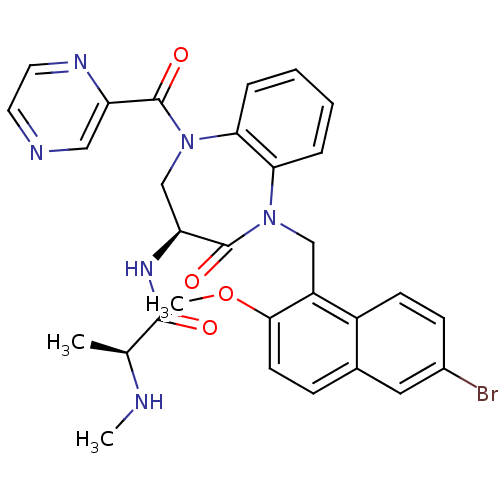
(CHEMBL2436211 | US10053431, 76c)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)c2cnccn2)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C30H29BrN6O4/c1-18(32-2)28(38)35-24-17-37(29(39)23-15-33-12-13-34-23)26-7-5-4-6-25(26)36(30(24)40)16-22-21-10-9-20(31)14-19(21)8-11-27(22)41-3/h4-15,18,24,32H,16-17H2,1-3H3,(H,35,38)/t18-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441827
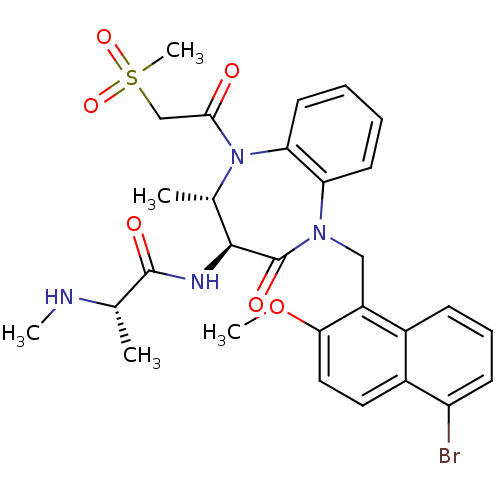
(CHEMBL2436217 | US9422331, 11)Show SMILES CN[C@@H](C)C(=O)N[C@H]1[C@H](C)N(C(=O)CS(C)(=O)=O)c2ccccc2N(Cc2c(OC)ccc3c(Br)cccc23)C1=O |r| Show InChI InChI=1S/C29H33BrN4O6S/c1-17(31-3)28(36)32-27-18(2)34(26(35)16-41(5,38)39)24-12-7-6-11-23(24)33(29(27)37)15-21-19-9-8-10-22(30)20(19)13-14-25(21)40-4/h6-14,17-18,27,31H,15-16H2,1-5H3,(H,32,36)/t17-,18-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM197160

(GNE-140 (6))Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)N[C@@](C1)(c1ccsc1)c1ccc(cc1)N1CCOCC1 |r,c:1| Show InChI InChI=1S/C25H23ClN2O3S2/c26-20-3-1-2-4-22(20)33-23-21(29)15-25(27-24(23)30,18-9-14-32-16-18)17-5-7-19(8-6-17)28-10-12-31-13-11-28/h1-9,14,16,29H,10-13,15H2,(H,27,30)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human LDHA using sodium pyruvate as substrate in presence of NAPDH |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100610
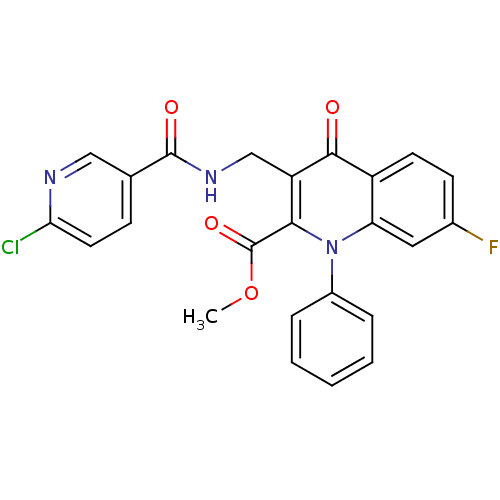
(US8501732, I-170)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(Cl)nc2)c(=O)c2ccc(F)cc2n1-c1ccccc1 Show InChI InChI=1S/C24H17ClFN3O4/c1-33-24(32)21-18(13-28-23(31)14-7-10-20(25)27-12-14)22(30)17-9-8-15(26)11-19(17)29(21)16-5-3-2-4-6-16/h2-12H,13H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100557
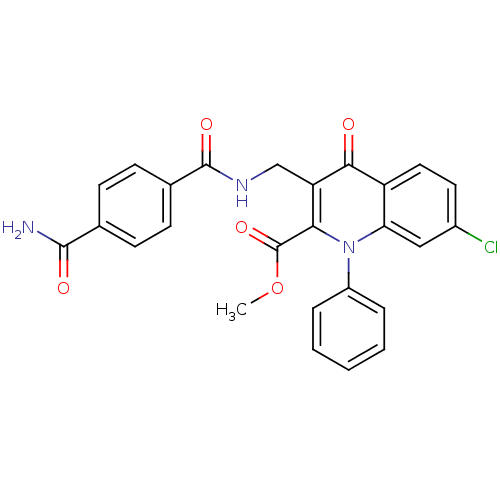
(US8501732, I-117)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(cc2)C(N)=O)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C26H20ClN3O5/c1-35-26(34)22-20(14-29-25(33)16-9-7-15(8-10-16)24(28)32)23(31)19-12-11-17(27)13-21(19)30(22)18-5-3-2-4-6-18/h2-13H,14H2,1H3,(H2,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441826
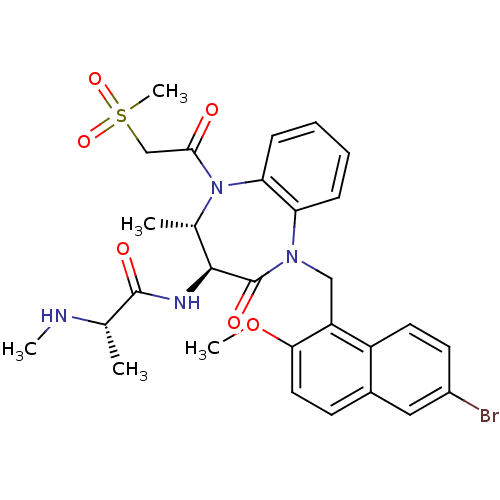
(CHEMBL2436216 | US9422331, 10)Show SMILES CN[C@@H](C)C(=O)N[C@H]1[C@H](C)N(C(=O)CS(C)(=O)=O)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C29H33BrN4O6S/c1-17(31-3)28(36)32-27-18(2)34(26(35)16-41(5,38)39)24-9-7-6-8-23(24)33(29(27)37)15-22-21-12-11-20(30)14-19(21)10-13-25(22)40-4/h6-14,17-18,27,31H,15-16H2,1-5H3,(H,32,36)/t17-,18-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441821

(CHEMBL2436223 | US9422331, 27)Show SMILES CN[C@@H](C)C(=O)N[C@H]1[C@H](C)N(C(=O)CS(C)(=O)=O)c2cc(ccc2N(Cc2c(OC)ccc3ccccc23)C1=O)C#N |r| Show InChI InChI=1S/C30H33N5O6S/c1-18(32-3)29(37)33-28-19(2)35(27(36)17-42(5,39)40)25-14-20(15-31)10-12-24(25)34(30(28)38)16-23-22-9-7-6-8-21(22)11-13-26(23)41-4/h6-14,18-19,28,32H,16-17H2,1-5H3,(H,33,37)/t18-,19-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441825
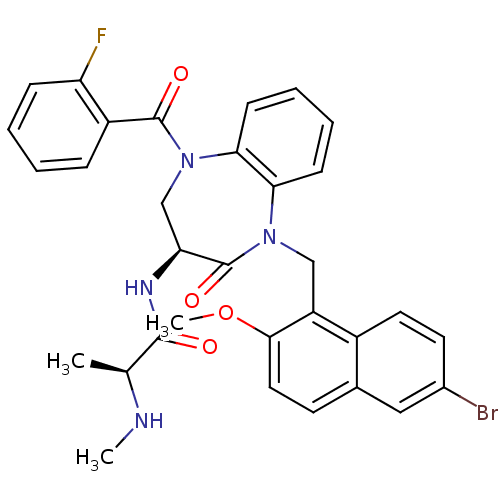
(CHEMBL2436333 | US10053431, 90a)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)c2ccccc2F)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C32H30BrFN4O4/c1-19(35-2)30(39)36-26-18-38(31(40)23-8-4-5-9-25(23)34)28-11-7-6-10-27(28)37(32(26)41)17-24-22-14-13-21(33)16-20(22)12-15-29(24)42-3/h4-16,19,26,35H,17-18H2,1-3H3,(H,36,39)/t19-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100502

(US8501732, I-62)Show SMILES CC(C)(C)OC(=O)N1CC2CC1CN2c1ccc(cn1)C(=O)NCc1cn(-c2ccccc2)c2cc(Cl)ccc2c1=O |THB:5:7:12.13:10,14:13:7.8:10| Show InChI InChI=1S/C32H32ClN5O4/c1-32(2,3)42-31(41)38-19-24-14-25(38)18-37(24)28-12-9-20(15-34-28)30(40)35-16-21-17-36(23-7-5-4-6-8-23)27-13-22(33)10-11-26(27)29(21)39/h4-13,15,17,24-25H,14,16,18-19H2,1-3H3,(H,35,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100558
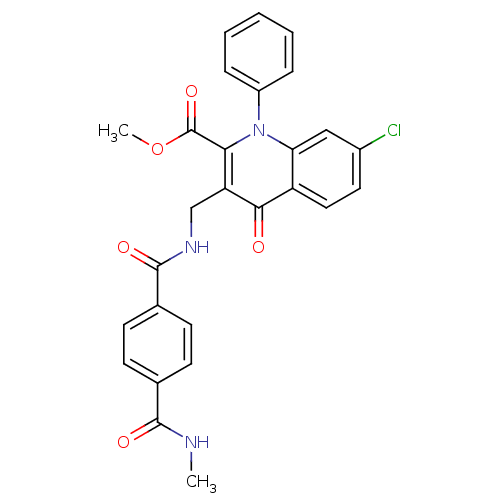
(US8501732, I-118)Show SMILES CNC(=O)c1ccc(cc1)C(=O)NCc1c(C(=O)OC)n(-c2ccccc2)c2cc(Cl)ccc2c1=O Show InChI InChI=1S/C27H22ClN3O5/c1-29-25(33)16-8-10-17(11-9-16)26(34)30-15-21-23(27(35)36-2)31(19-6-4-3-5-7-19)22-14-18(28)12-13-20(22)24(21)32/h3-14H,15H2,1-2H3,(H,29,33)(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441817

(CHEMBL2436332 | US10053431, 90b)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)c2cccc(F)c2)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C32H30BrFN4O4/c1-19(35-2)30(39)36-26-18-38(31(40)21-7-6-8-23(34)16-21)28-10-5-4-9-27(28)37(32(26)41)17-25-24-13-12-22(33)15-20(24)11-14-29(25)42-3/h4-16,19,26,35H,17-18H2,1-3H3,(H,36,39)/t19-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392983
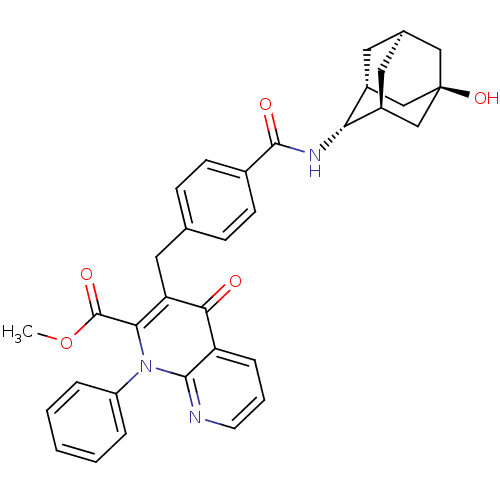
(CHEMBL2152383)Show SMILES COC(=O)c1c(Cc2ccc(cc2)C(=O)N[C@H]2[C@H]3C[C@H]4C[C@@H]2C[C@](O)(C4)C3)c(=O)c2cccnc2n1-c1ccccc1 |r,TLB:26:23:20:16.17.18,THB:18:17:22:20.19.25,18:19:16.17.26:22,15:16:22:20.19.25,24:23:20:16.17.18| Show InChI InChI=1S/C34H33N3O5/c1-42-33(40)29-27(30(38)26-8-5-13-35-31(26)37(29)25-6-3-2-4-7-25)16-20-9-11-22(12-10-20)32(39)36-28-23-14-21-15-24(28)19-34(41,17-21)18-23/h2-13,21,23-24,28,41H,14-19H2,1H3,(H,36,39)/t21-,23-,24+,28-,34- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data