

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Escherichia coli) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate by fluorescence spectrophotometric ana... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029090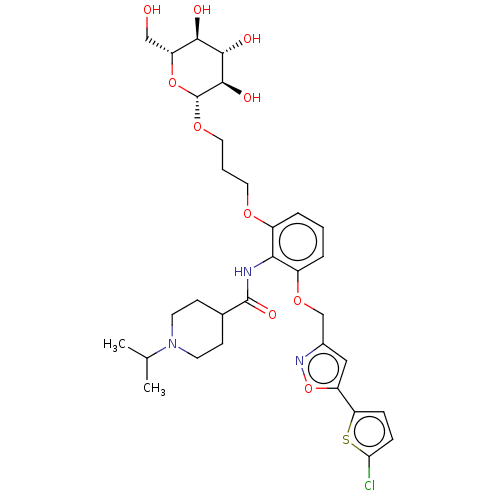 (CHEMBL3343301) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029090 (CHEMBL3343301) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Lineweaver-Burk analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029091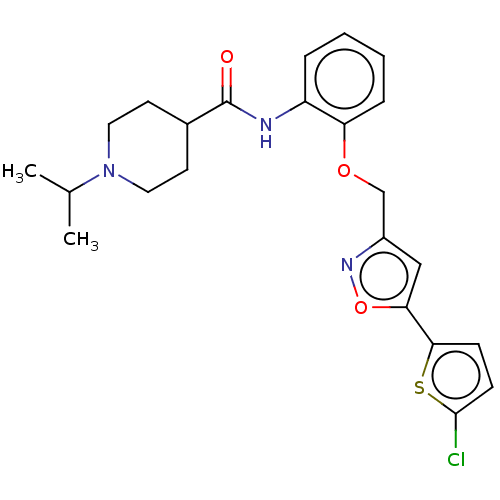 (CHEMBL3343299) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Lineweaver-Burk analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029095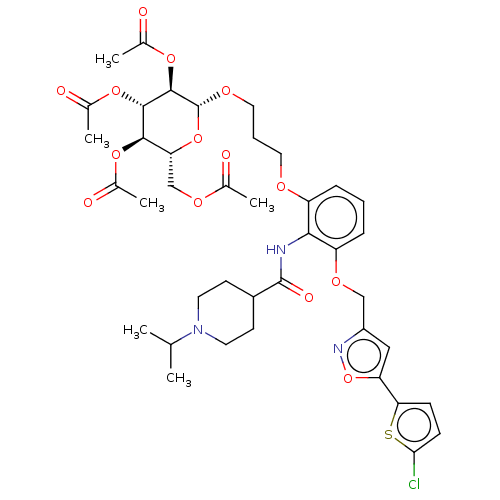 (CHEMBL3343300) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50568077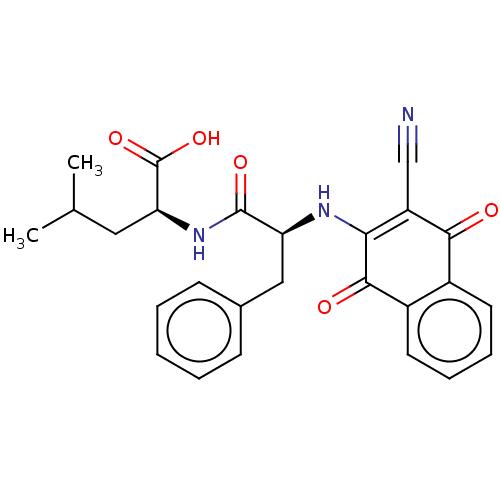 (CHEMBL4865195) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to Trypanosoma brucei rhodesain | Citation and Details Article DOI: 10.1016/j.bmc.2021.116213 BindingDB Entry DOI: 10.7270/Q2183B90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029091 (CHEMBL3343299) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029090 (CHEMBL3343301) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Lineweaver-Burk analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210930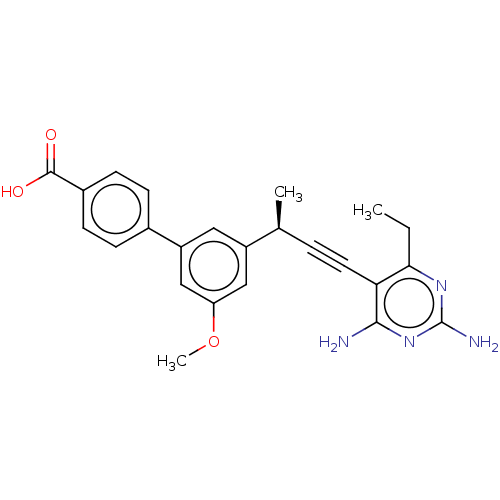 (UCP1173) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190621 (CHEMBL3827532 | US10870625, Compound 15) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50514105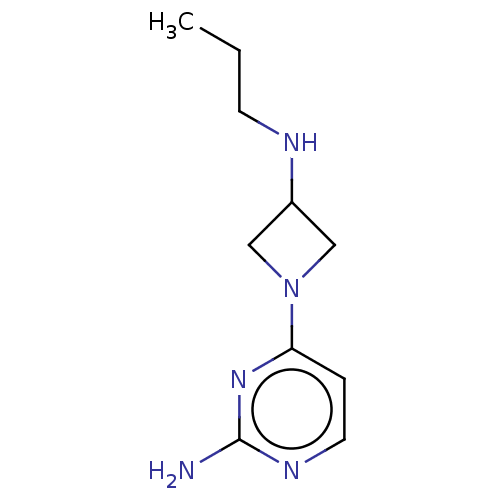 (CHEMBL4441731) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]NAMH from mouse H3R expressed in HEK293T cells incubated for 2 hrs by microbeta scintillation counting analysis | J Med Chem 62: 10848-10866 (2019) Article DOI: 10.1021/acs.jmedchem.9b01462 BindingDB Entry DOI: 10.7270/Q2M61PKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50184825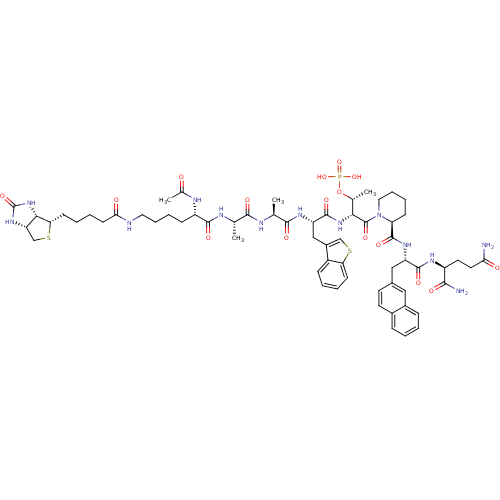 (Ac-Lys(N-epsilon-biotinoyl)-Ala-Ala-Bth-D-Thr(PO3H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of human Pin1 PPIase Activity by protease free PPIase assay | J Med Chem 49: 2147-50 (2006) Article DOI: 10.1021/jm060036n BindingDB Entry DOI: 10.7270/Q2S46RJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210930 (UCP1173) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210931 (UCP1175) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50061231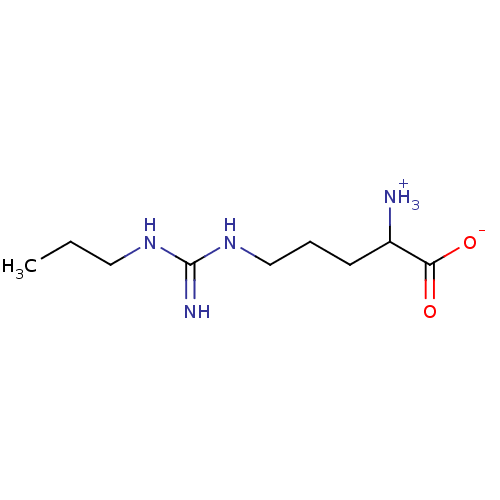 (2-Amino-5-(N'-propyl-guanidino)-pentanoic acid | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Compound was tested for binding affinity against recombinant inducible nitric oxide synthase (iNOS) from mouse | J Med Chem 40: 3869-70 (1998) Article DOI: 10.1021/jm970550g BindingDB Entry DOI: 10.7270/Q2C829ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210929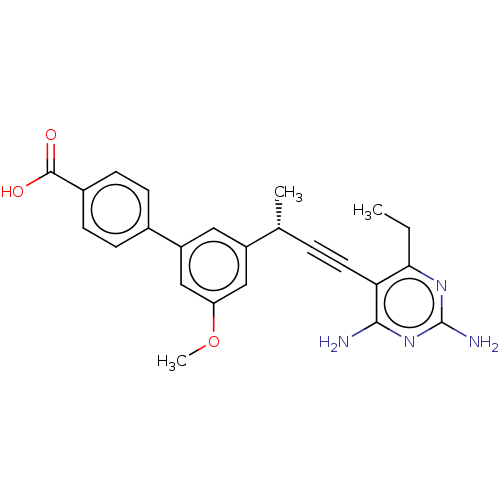 (UCP1172) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190622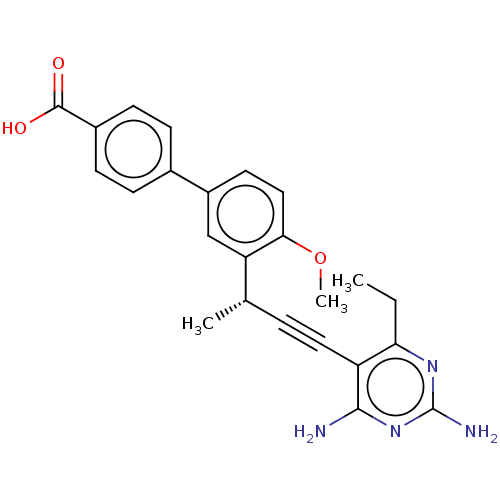 (CHEMBL3827326) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50118337 (1-(benzo[b]thiophen-3-yl)-3-(4-(3,4-dihydro-2H-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ornithine aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50121376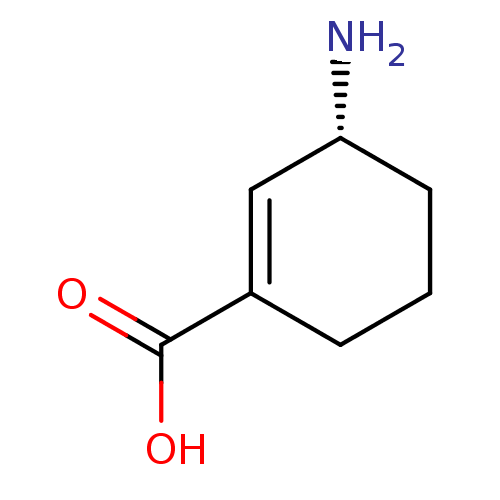 (GABACULINE) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University-Hadassah Medical Center Curated by ChEMBL | Assay Description Inhibition of human recombinant OAT using 1 mM alpha-ketoglutarate as substrate | ACS Med Chem Lett 6: 840-4 (2015) Article DOI: 10.1021/acsmedchemlett.5b00153 BindingDB Entry DOI: 10.7270/Q2TQ63B9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190619 (CHEMBL3827086 | US10870625, Compound 14) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210929 (UCP1172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50415830 (CHEMBL1095256) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50118336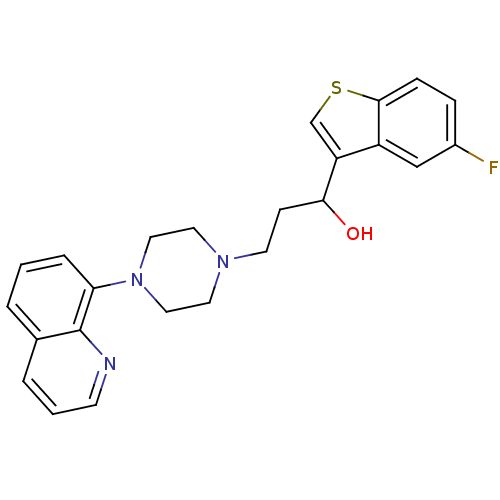 (1-(5-Fluoro-benzo[b]thiophen-3-yl)-3-(4-quinolin-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50118327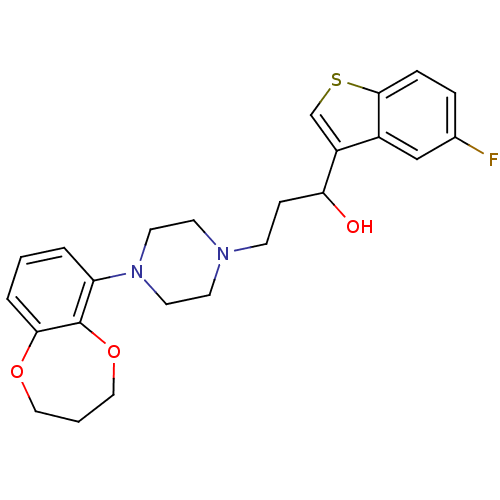 (3-(4-(3,4-dihydro-2H-benzo[b][1,4]dioxepin-6-yl)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210928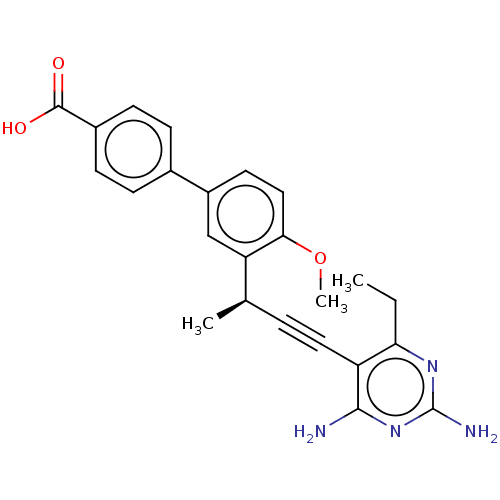 (UCP1164) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50514105 (CHEMBL4441731) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]NAMH from human H3R expressed in HEK293T cells incubated for 2 hrs by microbeta scintillation counting analysis | J Med Chem 62: 10848-10866 (2019) Article DOI: 10.1021/acs.jmedchem.9b01462 BindingDB Entry DOI: 10.7270/Q2M61PKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ornithine aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50199989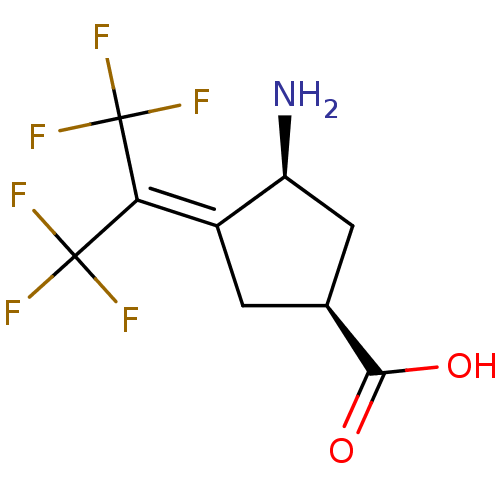 ((1S,3S)-3-amino-4-(2,2,2-trifluoro-1-trifluorometh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University-Hadassah Medical Center Curated by ChEMBL | Assay Description Inhibition of human recombinant OAT using 1 mM alpha-ketoglutarate as substrate | ACS Med Chem Lett 6: 840-4 (2015) Article DOI: 10.1021/acsmedchemlett.5b00153 BindingDB Entry DOI: 10.7270/Q2TQ63B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50190621 (CHEMBL3827532 | US10870625, Compound 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50184823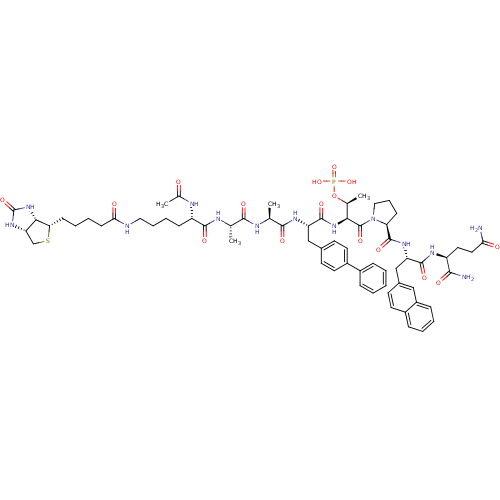 (Ac-Lys(N-epsilon-biotinoyl)-Ala-Ala-Bip-Thr(PO3H2)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of human Pin1 PPIase Activity by protease free PPIase assay | J Med Chem 49: 2147-50 (2006) Article DOI: 10.1021/jm060036n BindingDB Entry DOI: 10.7270/Q2S46RJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50448835 (CHEMBL3128246) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS overexpressed in Escherichia coli using L-arginine as substrate assessed as nitric oxide formation measured for 60... | J Med Chem 57: 686-700 (2014) Article DOI: 10.1021/jm401252e BindingDB Entry DOI: 10.7270/Q2SF2XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM202643 (US9242957, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The compounds were evaluated for in vitro inhibition against three NOS isozymes: rat nNOS, bovine eNOS and marine iNOS using known literature methods... | US Patent US9242957 (2016) BindingDB Entry DOI: 10.7270/Q24B3047 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM202644 (US9242957, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The compounds were evaluated for in vitro inhibition against three NOS isozymes: rat nNOS, bovine eNOS and marine iNOS using known literature methods... | US Patent US9242957 (2016) BindingDB Entry DOI: 10.7270/Q24B3047 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50448825 (CHEMBL3128245) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS overexpressed in Escherichia coli using L-arginine as substrate assessed as nitric oxide formation measured for 60... | J Med Chem 57: 686-700 (2014) Article DOI: 10.1021/jm401252e BindingDB Entry DOI: 10.7270/Q2SF2XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50330882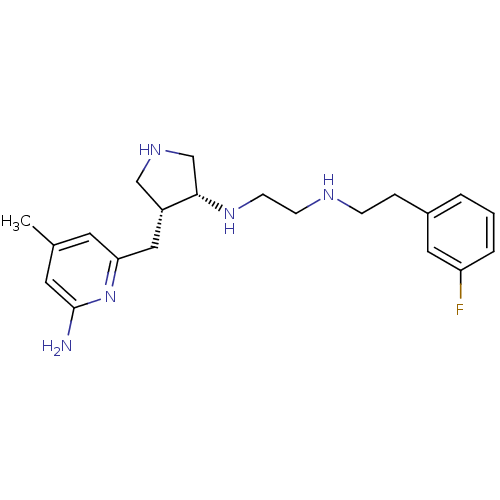 (CHEMBL1277951 | CHEMBL594682 | N1-((3R,4R)-4-((6-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS | J Med Chem 53: 7804-24 (2010) Article DOI: 10.1021/jm100947x BindingDB Entry DOI: 10.7270/Q2NC61FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102331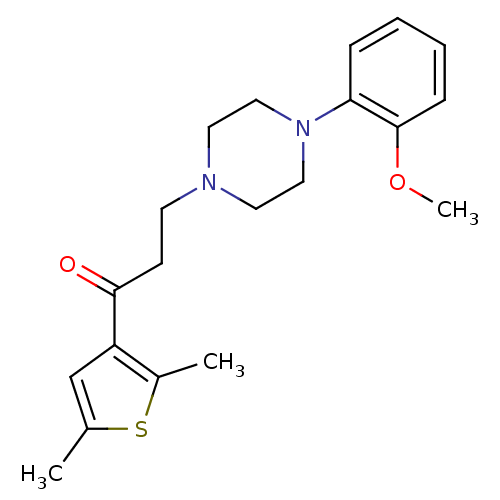 (1-(2,5-Dimethyl-thiophen-3-yl)-3-[4-(2-methoxy-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor | Eur J Med Chem 43: 364-72 (2008) Article DOI: 10.1016/j.ejmech.2007.03.036 BindingDB Entry DOI: 10.7270/Q2SQ91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50330882 (CHEMBL1277951 | CHEMBL594682 | N1-((3R,4R)-4-((6-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of nNOS | J Med Chem 54: 2039-48 (2011) Article DOI: 10.1021/jm101071n BindingDB Entry DOI: 10.7270/Q2571CB0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50190622 (CHEMBL3827326) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210931 (UCP1175) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190620 (CHEMBL3828724) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50442068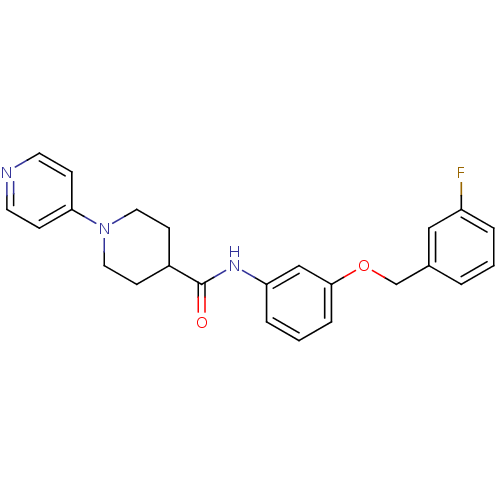 (CHEMBL2441057) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari "Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of bovine thrombin using D-Phe-Pip-Arg-p-NA as substrate by chromogenic assay | J Med Chem 56: 8696-711 (2013) Article DOI: 10.1021/jm401169a BindingDB Entry DOI: 10.7270/Q2SN0BDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50442068 (CHEMBL2441057) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari "Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of bovine thrombin using D-Phe-Pip-Arg-p-NA as substrate by chromogenic assay | J Med Chem 56: 8696-711 (2013) Article DOI: 10.1021/jm401169a BindingDB Entry DOI: 10.7270/Q2SN0BDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50311245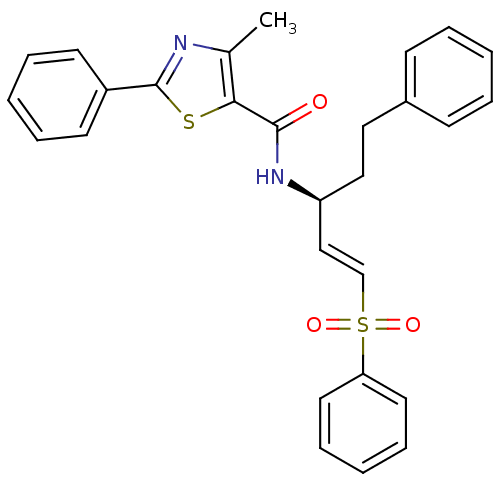 ((S)-4-methyl-2-phenyl-N-(5-phenyl-1-(phenylsulfony...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50118335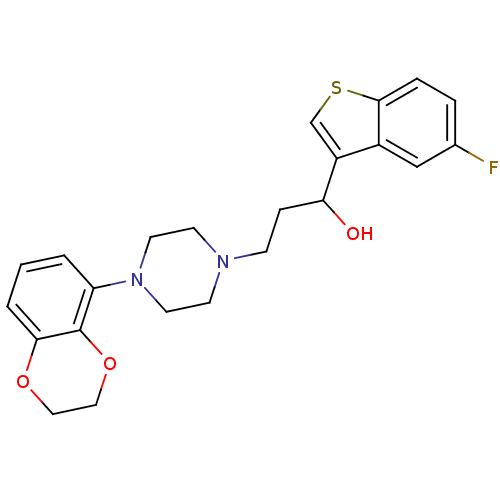 (3-(4-(2,3-dihydrobenzo[b][1,4]dioxin-5-yl)piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50514097 (CHEMBL4466700) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]NAMH from human H3R expressed in HEK293T cells incubated for 2 hrs by microbeta scintillation counting analysis | J Med Chem 62: 10848-10866 (2019) Article DOI: 10.1021/acs.jmedchem.9b01462 BindingDB Entry DOI: 10.7270/Q2M61PKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50095181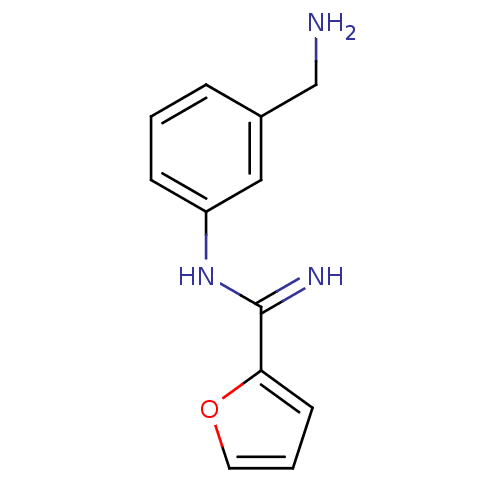 (CHEMBL96680 | N-(3-Aminomethyl-phenyl)-furan-2-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition against neuronal Nitric Oxide Synthase(nNOS) | Bioorg Med Chem Lett 10: 2771-4 (2000) BindingDB Entry DOI: 10.7270/Q23B60NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50102377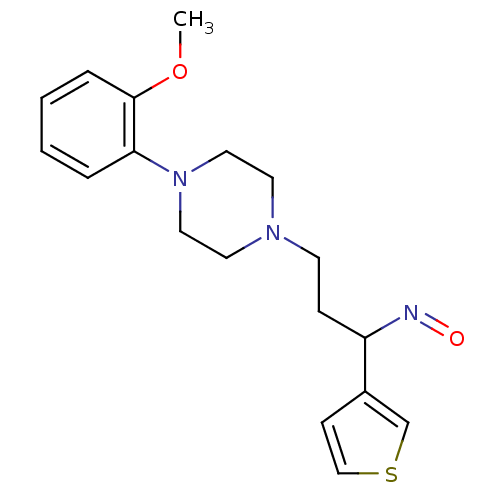 (3-(4-(2-methoxyphenyl)piperazin-1-yl)-1-(thiophen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Para£ba Curated by ChEMBL | Assay Description Antagonist activity at 5HT1A | Eur J Med Chem 45: 1508-14 (2010) Article DOI: 10.1016/j.ejmech.2009.12.059 BindingDB Entry DOI: 10.7270/Q2HX1DX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4633 total ) | Next | Last >> |