

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164242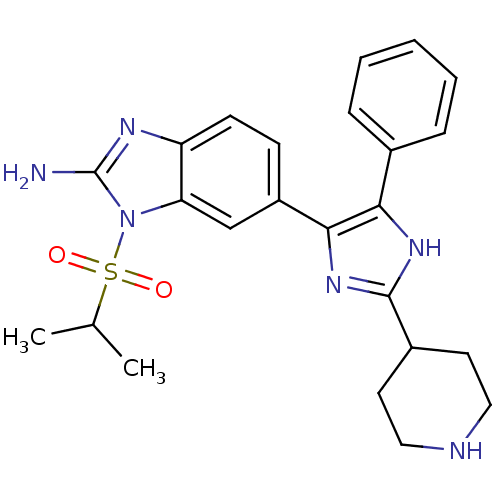 (6-(5-Phenyl-2-piperidin-4-yl-3H-imidazol-4-yl)-1-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164228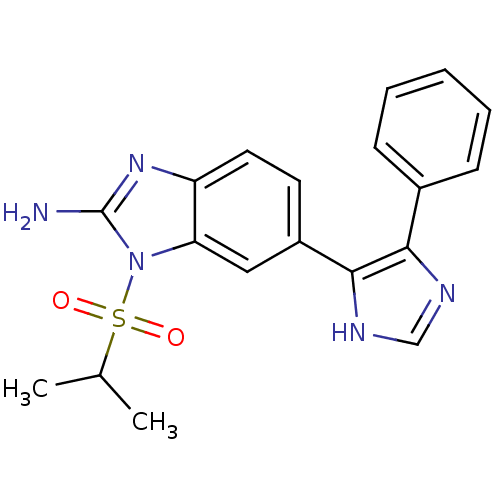 (1-(isopropylsulfonyl)-6-(4-phenyl-1H-imidazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164240 (6-[5-(4-Fluoro-phenyl)-3H-imidazol-4-yl]-1-(propan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164236 (6-[2-tert-Butyl-5-(2,4-difluoro-phenyl)-3H-imidazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164230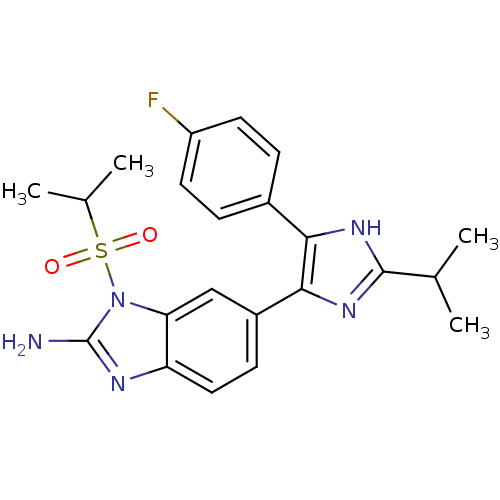 (6-[5-(4-Fluoro-phenyl)-2-isopropyl-3H-imidazol-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164239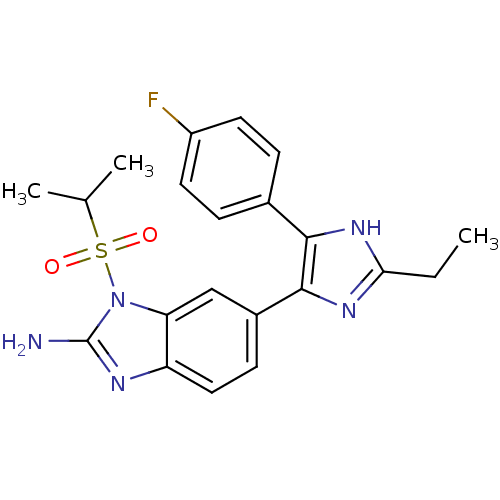 (6-[2-Ethyl-5-(4-fluoro-phenyl)-3H-imidazol-4-yl]-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164229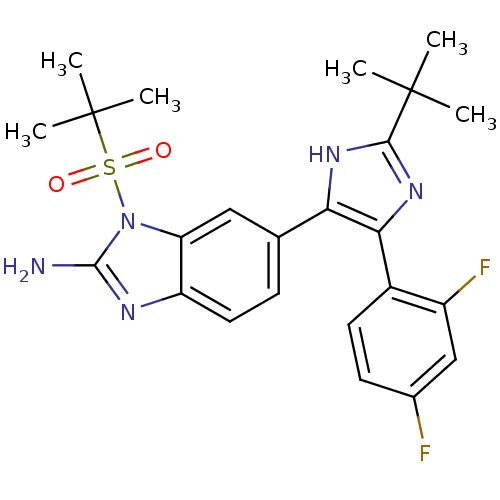 (6-[2-tert-Butyl-5-(2,4-difluoro-phenyl)-3H-imidazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164232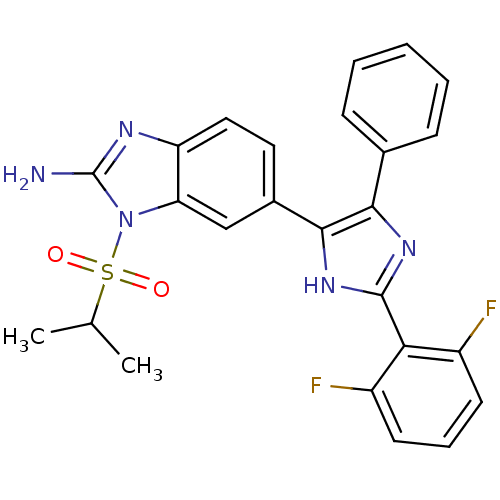 (6-(2-(2,6-difluorophenyl)-4-phenyl-1H-imidazol-5-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164231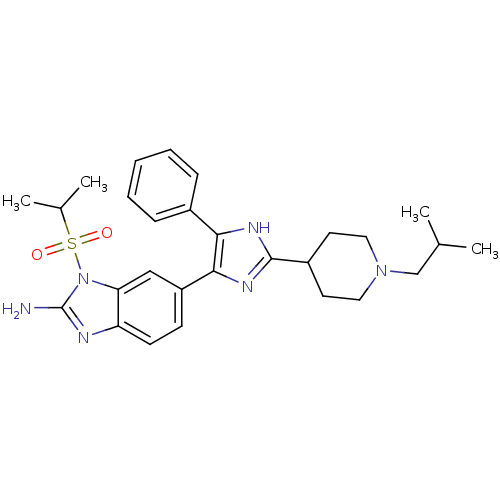 (6-[2-(1-Isobutyl-piperidin-4-yl)-5-phenyl-3H-imida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164232 (6-(2-(2,6-difluorophenyl)-4-phenyl-1H-imidazol-5-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164233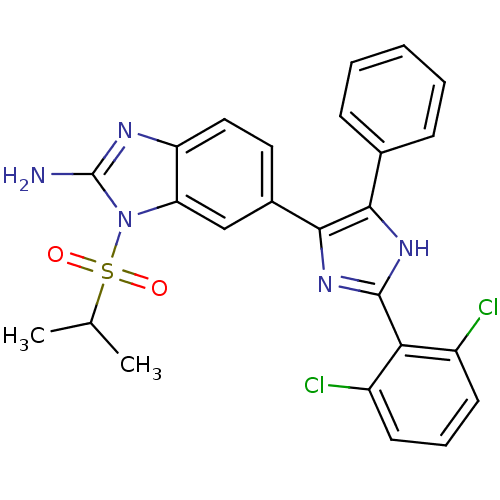 (6-(2-(2,6-dichlorophenyl)-4-phenyl-1H-imidazol-5-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164235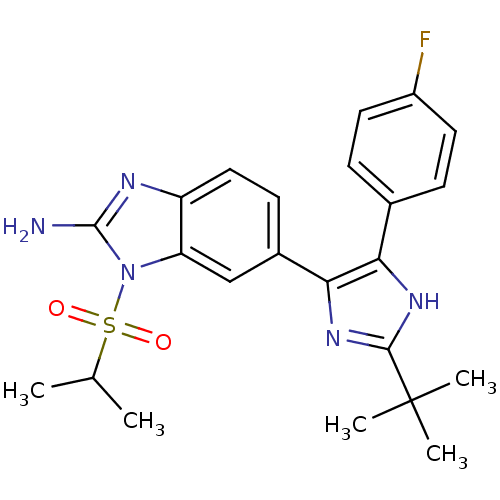 (6-[2-tert-Butyl-5-(4-fluoro-phenyl)-3H-imidazol-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164234 (1-Cyclopentanesulfonyl-6-[2-(2,6-difluoro-phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164238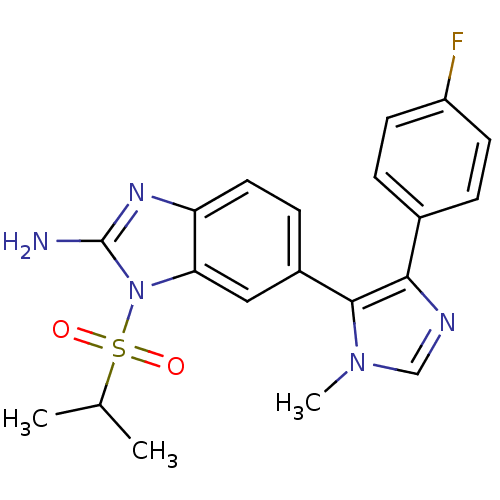 (6-[5-(4-Fluoro-phenyl)-3-methyl-3H-imidazol-4-yl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164237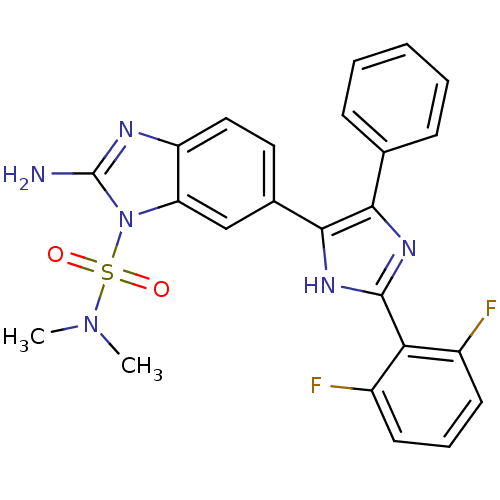 (2-Amino-6-[2-(2,6-difluoro-phenyl)-5-phenyl-3H-imi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2894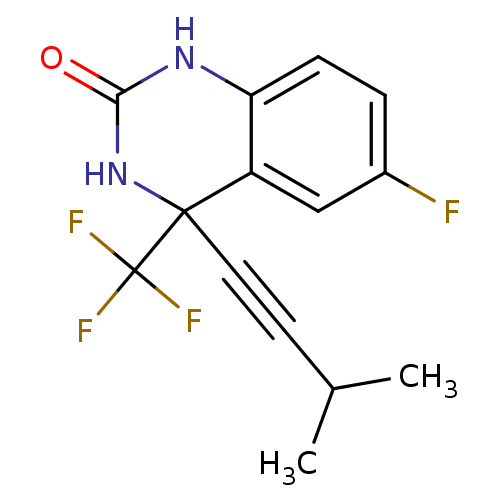 (3,4-Dihydro-6-fluoro-4-(3-methylbutyn-1-yl)-4-(tri...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2878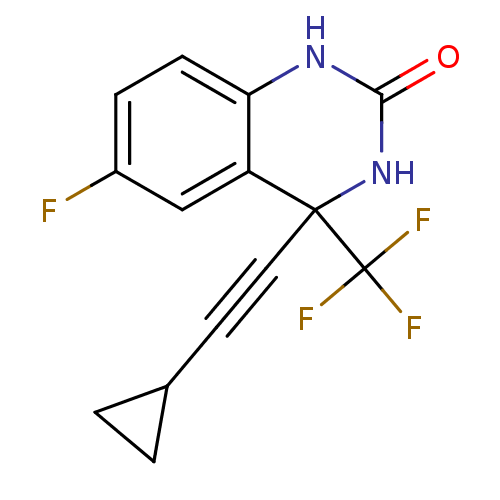 (4-(2-cyclopropylethynyl)-6-fluoro-4-(trifluorometh...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2880 (4-(1-Butyn-1-yl)-3,4-dihydro-6-fluoro-4-(trifluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2881 (5-Chloro-4-(cyclopropylethynyl)-3,4-dihydro-6-fluo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2873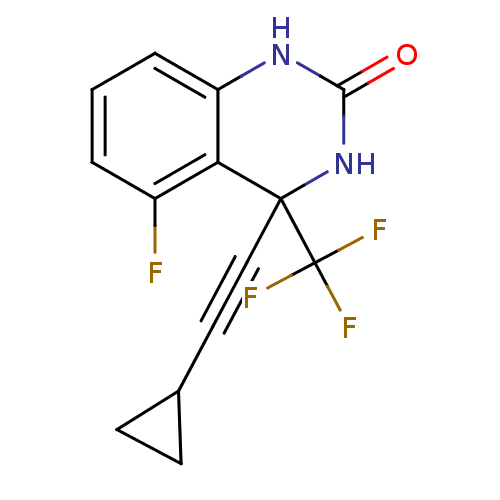 (4-(2-cyclopropylethynyl)-5-fluoro-4-(trifluorometh...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2879 (5,6-Difluoro-3.4-dihydro-4-[(2-pyridyl)ethynyl]-4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2875 (5-Chloro-4-(cyclopropylethynyl)-3,4-dihydro-4-(tri...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2872 (4-(1-Butyn-1-yl)-5,6-difuloro-3,4-dihydro-4-(trifl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2876 (4-(2-cyclopropylethynyl)-5,6-difluoro-4-(trifluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2874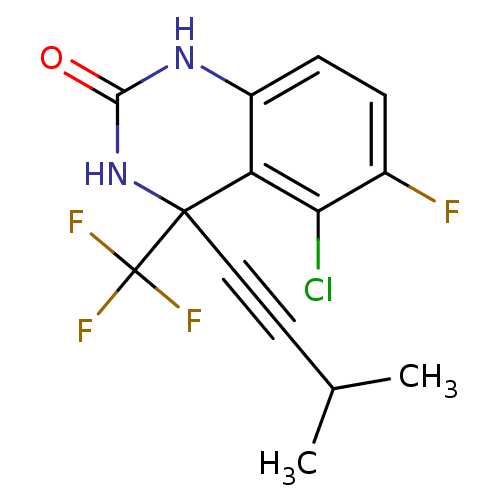 (5-Chloro-3,4-dihydro-6-fluoro-4-(3-methylbutyn-1-y...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2885 (5-Chloro-3,4-dihydro-6-fluoro-4-[(2-pyridyl)ethyny...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2877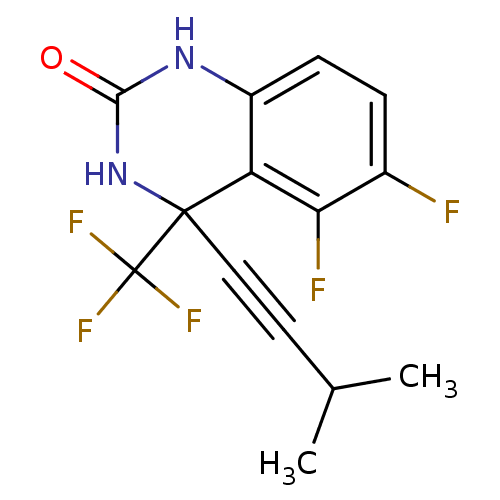 (5,6-Difluoro-3.4-dihydro-4-(3-methylbutyn-1-yl)-4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2884 (6-Chloro-4-(cyclopropylethynyl)-3,4-dihydro-5-fluo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2892 (4-(1-Butyn-1-yl)-6-chloro-3,4-dihydro-4-(trifluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2886 (6-Chloro-4-(cyclopropylethynyl)-3,4-dihydro-4-(tri...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 111 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2882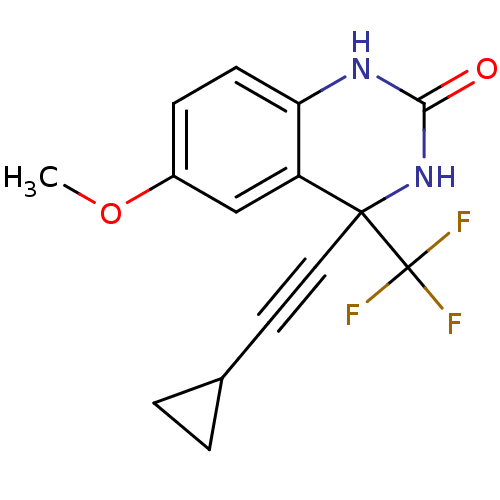 (4-(2-cyclopropylethynyl)-6-methoxy-4-(trifluoromet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164241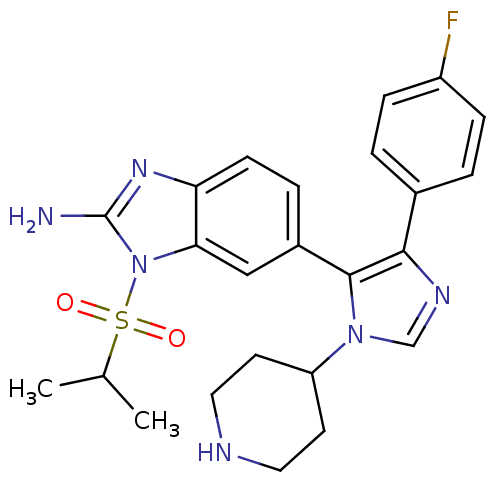 (6-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imidazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2883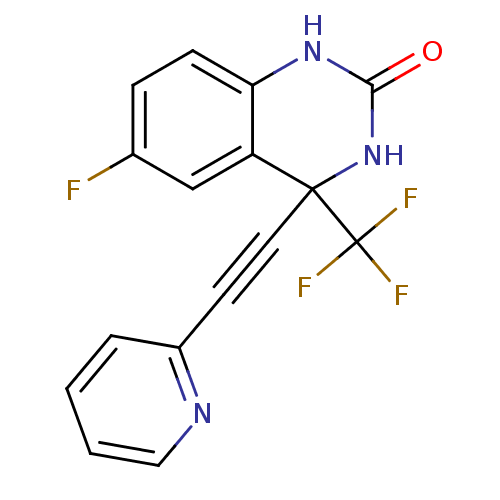 (3,4-Dihydro-6-fluoro-4-[(2-pyridyl)ethynyl]-4-(tri...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2891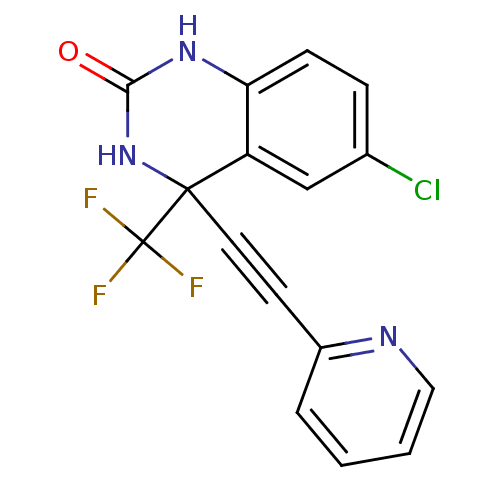 (6-Chloro-3,4-dihydro-4-[(2-pyridyl)ethynyl]-4-(tri...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2890 (3,4-Dihydro-6-fluoro-4-(phenylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2895 (4-(Cyclopropylethynyl)-5,6-dichloro-3,4-dihydro-4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 166 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2889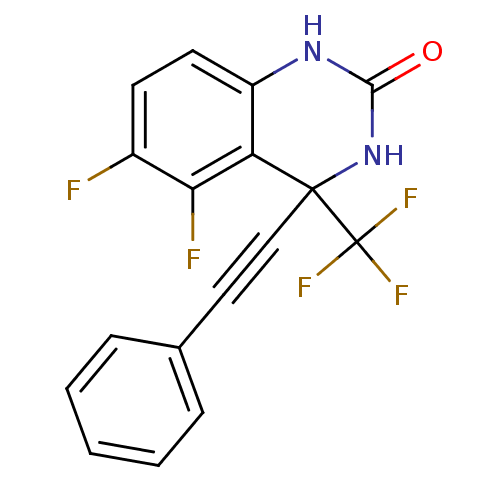 (5,6-Difluoro-3,4-dihydro-4-(phenylethynyl)-4-(trif...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2888 (3,4-Dihydro-6-methoxy-4-(phenylethynyl)-4-(trifluo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 219 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2897 (3,4-Dihydro-6-methoxy-4-[(2-pyridyl)ethynyl]-4-(tr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 237 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2893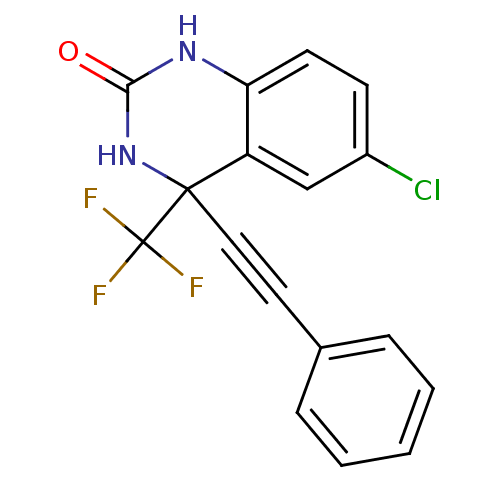 (6-Chloro-3,4-dihydro-4-(phenylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 277 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2896 (6-Chloro-3,4-dihydro-4-(3-methylbutyn-1-yl)-4-(tri...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 281 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2887 (3,4-Dihydro-6-methoxy-4-(3-methylbutyn-1-yl)-4-(tr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 401 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1944 (BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 422 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164240 (6-[5-(4-Fluoro-phenyl)-3H-imidazol-4-yl]-1-(propan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164228 (1-(isopropylsulfonyl)-6-(4-phenyl-1H-imidazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164239 (6-[2-Ethyl-5-(4-fluoro-phenyl)-3H-imidazol-4-yl]-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4.85E+3 | n/a | n/a | n/a | n/a | 8.2 | 37 |
DuPont Pharmaceuticals Company | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 2019-30 (2000) Article DOI: 10.1021/jm990580e BindingDB Entry DOI: 10.7270/Q28P5XP1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164231 (6-[2-(1-Isobutyl-piperidin-4-yl)-5-phenyl-3H-imida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164230 (6-[5-(4-Fluoro-phenyl)-2-isopropyl-3H-imidazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |