 Found 26620 hits with Last Name = 'mann' and Initial = 'g'
Found 26620 hits with Last Name = 'mann' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vesicular acetylcholine transporter
(Torpedo californica) | BDBM50039623
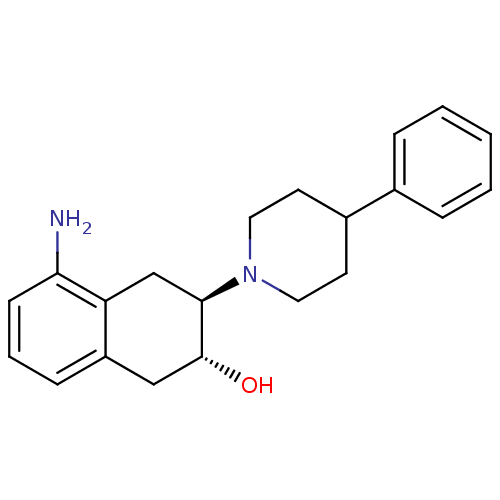
((2R,3R)-5-Amino-3-(4-phenyl-piperidin-1-yl)-1,2,3,...)Show SMILES Nc1cccc2C[C@@H](O)[C@@H](Cc12)N1CCC(CC1)c1ccccc1 Show InChI InChI=1S/C21H26N2O/c22-19-8-4-7-17-13-21(24)20(14-18(17)19)23-11-9-16(10-12-23)15-5-2-1-3-6-15/h1-8,16,20-21,24H,9-14,22H2/t20-,21-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Radiopharmaceutical Cancer Research
Curated by ChEMBL
| Assay Description
Displacement of (-)-[3H]vesamicol from VAChT in Torpedo californica electric organ synaptic vesicles |
Eur J Med Chem 100: 50-67 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.033
BindingDB Entry DOI: 10.7270/Q28C9XZ1 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17428
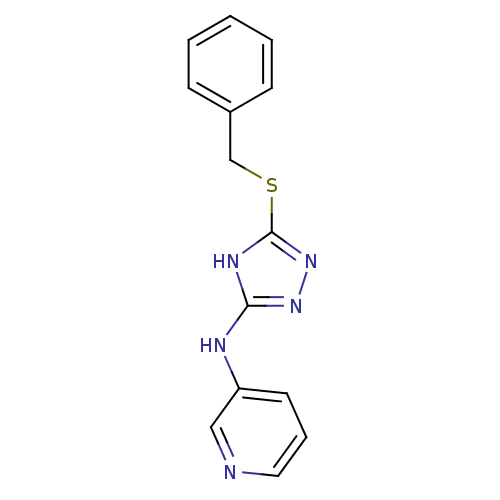
(1,2,4-Triazole Compound, 86 | N-[5-(benzylsulfanyl...)Show InChI InChI=1S/C14H13N5S/c1-2-5-11(6-3-1)10-20-14-17-13(18-19-14)16-12-7-4-8-15-9-12/h1-9H,10H2,(H2,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17355
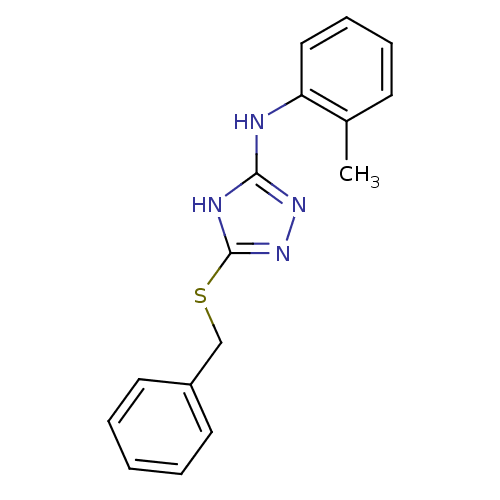
(1,2,4-Triazole Compound, 13 | 5-(benzylsulfanyl)-N...)Show InChI InChI=1S/C16H16N4S/c1-12-7-5-6-10-14(12)17-15-18-16(20-19-15)21-11-13-8-3-2-4-9-13/h2-10H,11H2,1H3,(H2,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoic acid receptor-gamma |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17388
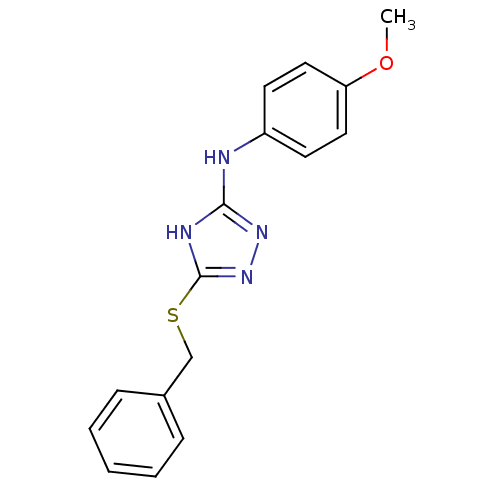
(1,2,4-Triazole Compound, 46 | 5-(benzylsulfanyl)-N...)Show InChI InChI=1S/C16H16N4OS/c1-21-14-9-7-13(8-10-14)17-15-18-16(20-19-15)22-11-12-5-3-2-4-6-12/h2-10H,11H2,1H3,(H2,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Vesicular acetylcholine transporter
(Torpedo californica) | BDBM50039613
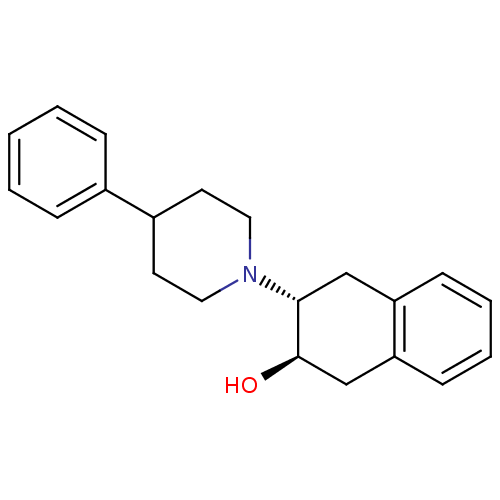
((2R,3R)-3-(4-Phenyl-piperidin-1-yl)-1,2,3,4-tetrah...)Show SMILES O[C@@H]1Cc2ccccc2C[C@H]1N1CCC(CC1)c1ccccc1 |r| Show InChI InChI=1S/C21H25NO/c23-21-15-19-9-5-4-8-18(19)14-20(21)22-12-10-17(11-13-22)16-6-2-1-3-7-16/h1-9,17,20-21,23H,10-15H2/t20-,21-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Radiopharmaceutical Cancer Research
Curated by ChEMBL
| Assay Description
Displacement of (-)-[3H]vesamicol from VAChT in Torpedo californica electric organ synaptic vesicles |
Eur J Med Chem 100: 50-67 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.033
BindingDB Entry DOI: 10.7270/Q28C9XZ1 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17365
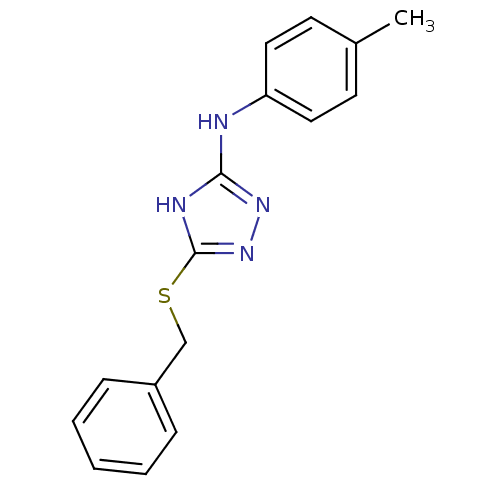
(1,2,4-Triazole Compound, 23 | 5-(benzylsulfanyl)-N...)Show InChI InChI=1S/C16H16N4S/c1-12-7-9-14(10-8-12)17-15-18-16(20-19-15)21-11-13-5-3-2-4-6-13/h2-10H,11H2,1H3,(H2,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | -57.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324510
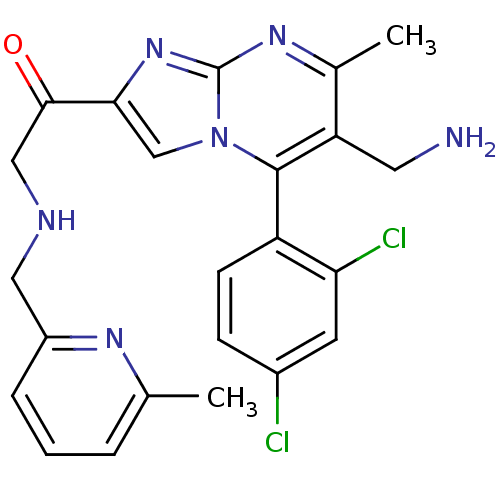
(1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...)Show SMILES Cc1cccc(CNCC(=O)c2cn3c(c(CN)c(C)nc3n2)-c2ccc(Cl)cc2Cl)n1 |(-4.59,-8.35,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,;-1.62,-5.59,;-.08,-5.54,;.65,-4.18,;2.19,-4.14,;2.92,-2.78,;2.11,-1.48,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;9.45,-1.81,;10.76,-1,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;-2.34,-6.93,)| Show InChI InChI=1S/C23H22Cl2N6O/c1-13-4-3-5-16(28-13)10-27-11-21(32)20-12-31-22(17-7-6-15(24)8-19(17)25)18(9-26)14(2)29-23(31)30-20/h3-8,12,27H,9-11,26H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212159
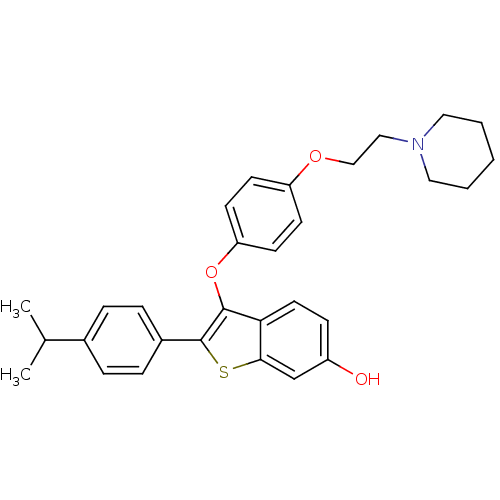
(2-(4-isopropylphenyl)-3-(4-(2-(piperidin-1-yl)etho...)Show SMILES CC(C)c1ccc(cc1)-c1sc2cc(O)ccc2c1Oc1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C30H33NO3S/c1-21(2)22-6-8-23(9-7-22)30-29(27-15-10-24(32)20-28(27)35-30)34-26-13-11-25(12-14-26)33-19-18-31-16-4-3-5-17-31/h6-15,20-21,32H,3-5,16-19H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008782
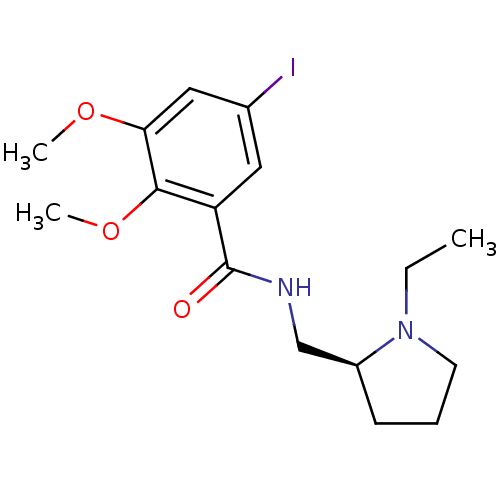
(CHEMBL44237 | EPIDEPRIDE | Epidepride;N-(1-Ethyl-p...)Show InChI InChI=1S/C16H23IN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008782

(CHEMBL44237 | EPIDEPRIDE | Epidepride;N-(1-Ethyl-p...)Show InChI InChI=1S/C16H23IN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008782

(CHEMBL44237 | EPIDEPRIDE | Epidepride;N-(1-Ethyl-p...)Show InChI InChI=1S/C16H23IN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50029978
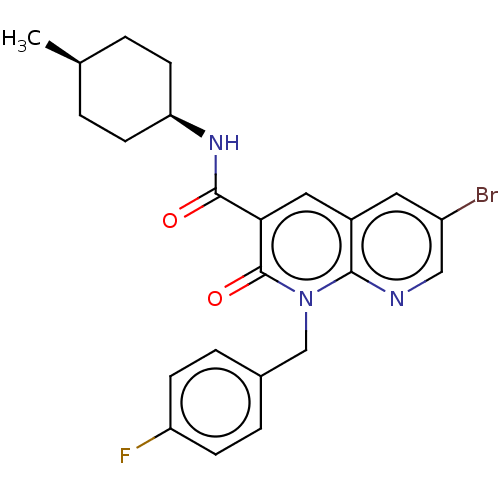
(CHEMBL3353441)Show SMILES C[C@H]1CC[C@H](CC1)NC(=O)c1cc2cc(Br)cnc2n(Cc2ccc(F)cc2)c1=O |r,wU:4.7,1.0,(16.02,-5.47,;14.69,-6.25,;14.69,-7.79,;13.37,-8.56,;12.03,-7.79,;12.02,-6.26,;13.35,-5.48,;10.7,-8.57,;9.36,-7.8,;9.35,-6.26,;8.03,-8.58,;6.69,-7.82,;5.37,-8.6,;4.03,-7.83,;2.7,-8.6,;1.37,-7.83,;2.7,-10.15,;4.04,-10.92,;5.37,-10.14,;6.7,-10.91,;6.71,-12.45,;8.04,-13.21,;8.04,-14.75,;9.37,-15.52,;10.71,-14.74,;12.04,-15.51,;10.7,-13.19,;9.36,-12.43,;8.04,-10.13,;9.38,-10.9,)| Show InChI InChI=1S/C23H23BrFN3O2/c1-14-2-8-19(9-3-14)27-22(29)20-11-16-10-17(24)12-26-21(16)28(23(20)30)13-15-4-6-18(25)7-5-15/h4-7,10-12,14,19H,2-3,8-9,13H2,1H3,(H,27,29)/t14-,19+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... |
J Med Chem 57: 8777-91 (2014)
Article DOI: 10.1021/jm500807e
BindingDB Entry DOI: 10.7270/Q2QC054S |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17390
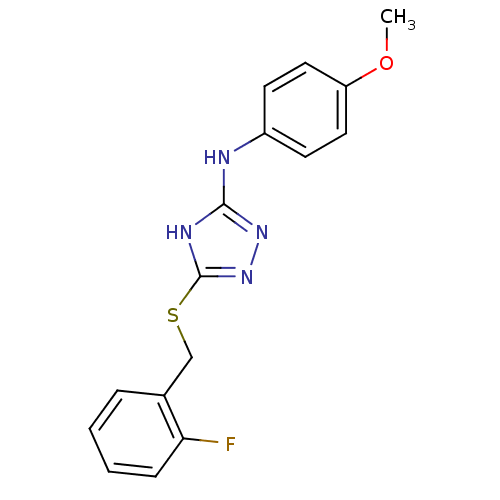
(1,2,4-Triazole Compound, 48 | 5-{[(2-fluorophenyl)...)Show InChI InChI=1S/C16H15FN4OS/c1-22-13-8-6-12(7-9-13)18-15-19-16(21-20-15)23-10-11-4-2-3-5-14(11)17/h2-9H,10H2,1H3,(H2,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50067499

((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |r,c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 6505-10 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.089
BindingDB Entry DOI: 10.7270/Q2S75H5H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM50090697
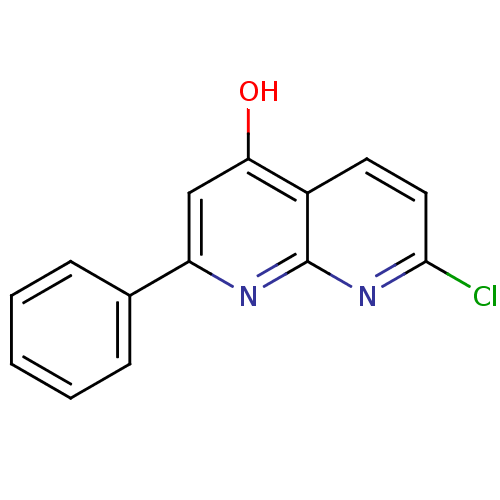
(7-Chloro-2-phenyl-[1,8]naphthyridin-4-ol | 7-chlor...)Show InChI InChI=1S/C14H9ClN2O/c15-13-7-6-10-12(18)8-11(16-14(10)17-13)9-4-2-1-3-5-9/h1-8H,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Ability to displace the specific binding of [3H]CHA to adenosine A1 receptor from bovine brain cortical membranes |
J Med Chem 43: 2814-23 (2000)
BindingDB Entry DOI: 10.7270/Q21R6R6K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM50090697

(7-Chloro-2-phenyl-[1,8]naphthyridin-4-ol | 7-chlor...)Show InChI InChI=1S/C14H9ClN2O/c15-13-7-6-10-12(18)8-11(16-14(10)17-13)9-4-2-1-3-5-9/h1-8H,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa
Curated by ChEMBL
| Assay Description
Binding affinity against adenosine A1 receptor in bovine cortical membranes using [3H]-CHA as radioligand |
J Med Chem 47: 3019-31 (2004)
Article DOI: 10.1021/jm030977p
BindingDB Entry DOI: 10.7270/Q2BC3Z07 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17362

(1,2,4-Triazole Compound, 20 | 5-[(3-methylbut-2-en...)Show SMILES CC(C)=CCSc1nnc(Nc2ccccc2C)[nH]1 |(5.94,4.3,;4.59,5.03,;4.55,6.57,;3.28,4.22,;1.81,4.7,;.67,3.67,;-.8,4.15,;-1.27,5.61,;-2.81,5.61,;-3.29,4.15,;-4.75,3.67,;-5.9,4.7,;-5.13,6.03,;-5.9,7.37,;-7.44,7.37,;-8.21,6.03,;-7.44,4.7,;-8.21,3.37,;-2.04,3.24,)| Show InChI InChI=1S/C14H18N4S/c1-10(2)8-9-19-14-16-13(17-18-14)15-12-7-5-4-6-11(12)3/h4-8H,9H2,1-3H3,(H2,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17395
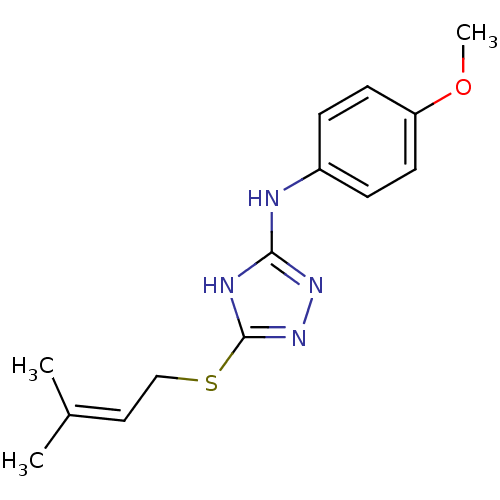
(1,2,4-Triazole Compound, 53 | N-(4-methoxyphenyl)-...)Show SMILES COc1ccc(Nc2nnc(SCC=C(C)C)[nH]2)cc1 |(-9.75,8.7,;-8.21,8.7,;-7.44,7.37,;-8.21,6.03,;-7.44,4.7,;-5.9,4.7,;-4.75,3.67,;-3.29,4.15,;-2.81,5.61,;-1.27,5.61,;-.8,4.15,;.67,3.67,;1.81,4.7,;3.28,4.22,;4.51,5.15,;5.93,4.55,;4.32,6.68,;-2.04,3.24,;-5.13,6.03,;-5.9,7.37,)| Show InChI InChI=1S/C14H18N4OS/c1-10(2)8-9-20-14-16-13(17-18-14)15-11-4-6-12(19-3)7-5-11/h4-8H,9H2,1-3H3,(H2,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50212148
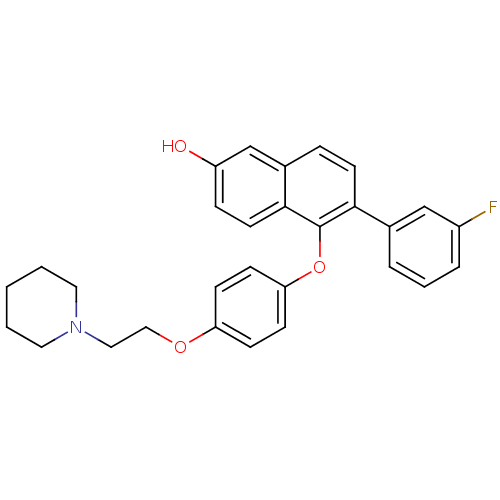
(6-(3-fluorophenyl)-5-(4-(2-(piperidin-1-yl)ethoxy)...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1cccc(F)c1 Show InChI InChI=1S/C29H28FNO3/c30-23-6-4-5-21(19-23)27-13-7-22-20-24(32)8-14-28(22)29(27)34-26-11-9-25(10-12-26)33-18-17-31-15-2-1-3-16-31/h4-14,19-20,32H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERbeta |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50029963
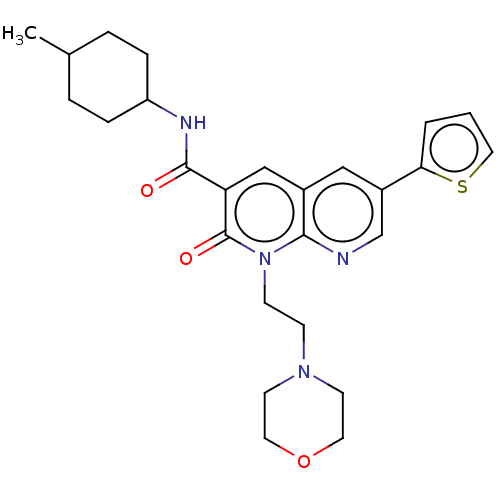
(CHEMBL3353452)Show SMILES CC1CCC(CC1)NC(=O)c1cc2cc(cnc2n(CCN2CCOCC2)c1=O)-c1cccs1 |(26.44,-2.21,;25.11,-2.98,;25.11,-4.52,;23.79,-5.29,;22.45,-4.52,;22.44,-2.99,;23.77,-2.21,;21.12,-5.3,;19.78,-4.54,;19.77,-3,;18.45,-5.31,;17.12,-4.55,;15.79,-5.33,;14.46,-4.56,;13.13,-5.33,;13.13,-6.88,;14.46,-7.65,;15.79,-6.87,;17.13,-7.64,;17.13,-9.17,;18.46,-9.94,;18.47,-11.48,;17.14,-12.25,;17.14,-13.78,;18.47,-14.56,;19.81,-13.79,;19.81,-12.24,;18.46,-6.86,;19.8,-7.63,;11.79,-4.56,;10.38,-5.19,;9.35,-4.05,;10.12,-2.71,;11.63,-3.03,)| Show InChI InChI=1S/C26H32N4O3S/c1-18-4-6-21(7-5-18)28-25(31)22-16-19-15-20(23-3-2-14-34-23)17-27-24(19)30(26(22)32)9-8-29-10-12-33-13-11-29/h2-3,14-18,21H,4-13H2,1H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... |
J Med Chem 57: 8777-91 (2014)
Article DOI: 10.1021/jm500807e
BindingDB Entry DOI: 10.7270/Q2QC054S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50067499

((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |r,c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 6505-10 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.089
BindingDB Entry DOI: 10.7270/Q2S75H5H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50029958
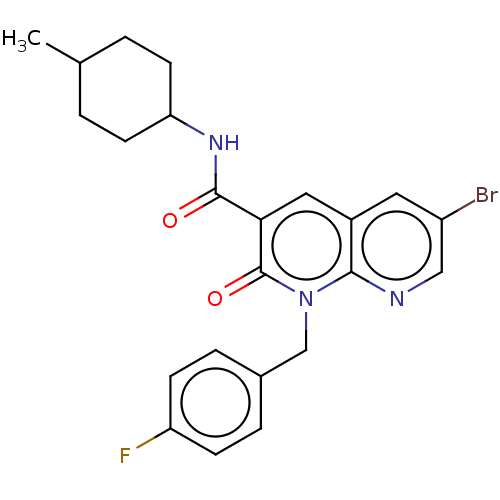
(CHEMBL3353439)Show SMILES CC1CCC(CC1)NC(=O)c1cc2cc(Br)cnc2n(Cc2ccc(F)cc2)c1=O |(16.02,-5.47,;14.69,-6.25,;14.69,-7.79,;13.37,-8.56,;12.03,-7.79,;12.02,-6.26,;13.35,-5.48,;10.7,-8.57,;9.36,-7.8,;9.35,-6.26,;8.03,-8.58,;6.69,-7.82,;5.37,-8.6,;4.03,-7.83,;2.7,-8.6,;1.37,-7.83,;2.7,-10.15,;4.04,-10.92,;5.37,-10.14,;6.7,-10.91,;6.71,-12.45,;8.04,-13.21,;8.04,-14.75,;9.37,-15.52,;10.71,-14.74,;12.04,-15.51,;10.7,-13.19,;9.36,-12.43,;8.04,-10.13,;9.38,-10.9,)| Show InChI InChI=1S/C23H23BrFN3O2/c1-14-2-8-19(9-3-14)27-22(29)20-11-16-10-17(24)12-26-21(16)28(23(20)30)13-15-4-6-18(25)7-5-15/h4-7,10-12,14,19H,2-3,8-9,13H2,1H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... |
J Med Chem 57: 8777-91 (2014)
Article DOI: 10.1021/jm500807e
BindingDB Entry DOI: 10.7270/Q2QC054S |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212148

(6-(3-fluorophenyl)-5-(4-(2-(piperidin-1-yl)ethoxy)...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1cccc(F)c1 Show InChI InChI=1S/C29H28FNO3/c30-23-6-4-5-21(19-23)27-13-7-22-20-24(32)8-14-28(22)29(27)34-26-11-9-25(10-12-26)33-18-17-31-15-2-1-3-16-31/h4-14,19-20,32H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324512
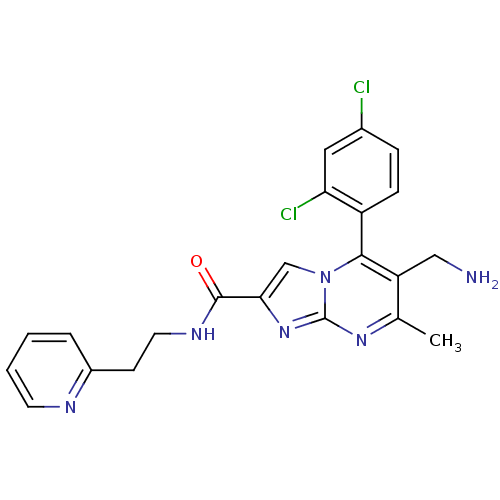
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1ccccn1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.34,-6.93,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(11-25)20(16-6-5-14(23)10-18(16)24)30-12-19(29-22(30)28-13)21(31)27-9-7-15-4-2-3-8-26-15/h2-6,8,10,12H,7,9,11,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324523
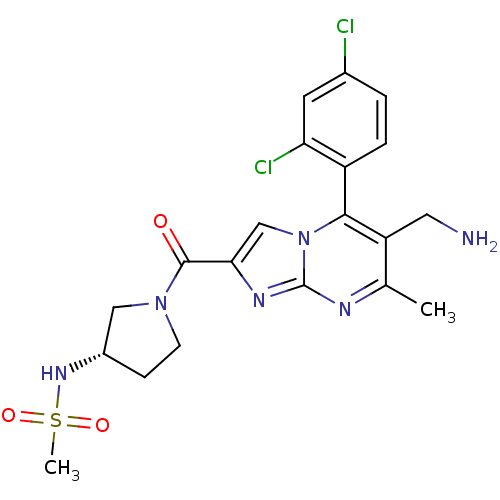
(CHEMBL1215018 | N-((3S)-1-(6-(aminomethyl)-5-(2,4-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1CC[C@@H](C1)NS(C)(=O)=O |r,wD:25.30,(7.23,-5.85,;5.92,-6.66,;4.56,-5.95,;3.26,-6.75,;1.79,-6.32,;.92,-7.59,;1.87,-8.81,;3.31,-8.29,;4.66,-9.02,;5.97,-8.21,;7.33,-8.93,;8.64,-8.12,;4.63,-10.55,;3.28,-11.29,;3.25,-12.83,;4.57,-13.63,;4.54,-15.17,;5.92,-12.87,;5.95,-11.34,;7.29,-10.6,;-.62,-7.63,;-1.43,-6.33,;-1.34,-8.99,;-.88,-10.46,;-2.12,-11.36,;-3.37,-10.46,;-2.89,-8.99,;-4.91,-10.48,;-5.99,-9.37,;-7.4,-10.02,;-6.68,-7.99,;-4.84,-8.35,)| Show InChI InChI=1S/C20H22Cl2N6O3S/c1-11-15(8-23)18(14-4-3-12(21)7-16(14)22)28-10-17(25-20(28)24-11)19(29)27-6-5-13(9-27)26-32(2,30)31/h3-4,7,10,13,26H,5-6,8-9,23H2,1-2H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50311052

(CHEMBL1079870 | ethyl 3-fluoro-9H-benzo[f]imidazo[...)Show InChI InChI=1S/C15H12FN5O2/c1-2-23-15(22)13-12-6-21-14(17-7-19-21)10-5-9(16)3-4-11(10)20(12)8-18-13/h3-5,7-8H,2,6H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]Flumazenil from human GABAalpha5beta3gamma2 receptor expressed in insect SF9 cells |
Bioorg Med Chem Lett 19: 5940-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.053
BindingDB Entry DOI: 10.7270/Q27944TT |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50311061
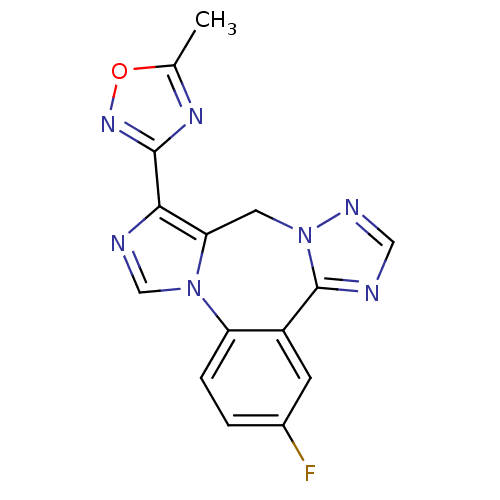
(3-(3-fluoro-9H-benzo[f]imidazo[1,5-a][1,2,4]triazo...)Show InChI InChI=1S/C15H10FN7O/c1-8-20-14(21-24-8)13-12-5-23-15(17-6-19-23)10-4-9(16)2-3-11(10)22(12)7-18-13/h2-4,6-7H,5H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]Flumazenil from cloned human GABAalpha5beta3gamma2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 19: 5940-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.053
BindingDB Entry DOI: 10.7270/Q27944TT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29321
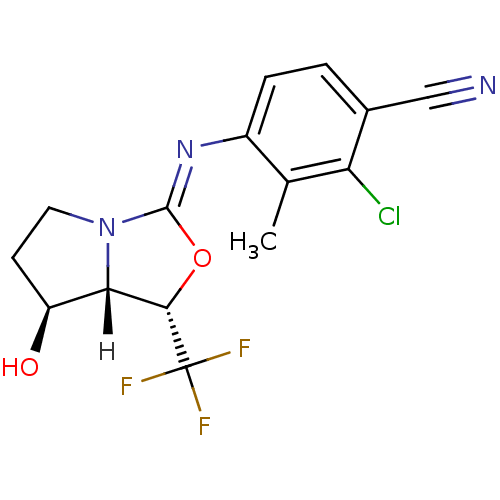
(oxazolidin-2-imine, 6d)Show SMILES [H][C@@]12[C@@H](O)CCN1\C(O[C@@H]2C(F)(F)F)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H13ClF3N3O2/c1-7-9(3-2-8(6-20)11(7)16)21-14-22-5-4-10(23)12(22)13(24-14)15(17,18)19/h2-3,10,12-13,23H,4-5H2,1H3/b21-14-/t10-,12-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | -54.8 | n/a | n/a | 19 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19964
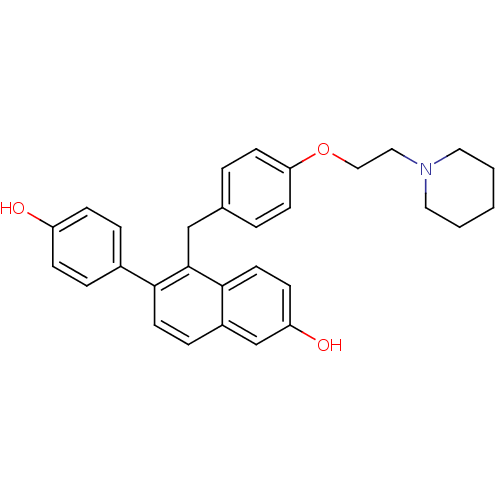
(6-(4-hydroxyphenyl)-5-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1ccc2cc(O)ccc2c1Cc1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C30H31NO3/c32-25-9-6-23(7-10-25)28-14-8-24-21-26(33)11-15-29(24)30(28)20-22-4-12-27(13-5-22)34-19-18-31-16-2-1-3-17-31/h4-15,21,32-33H,1-3,16-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.200 | -54.8 | n/a | n/a | 1.14 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories
| Assay Description
The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... |
J Med Chem 48: 6772-5 (2005)
Article DOI: 10.1021/jm050723z
BindingDB Entry DOI: 10.7270/Q2RR1WHW |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212157

(6-(3,4-difluorophenyl)-5-(4-(2-(piperidin-1-yl)eth...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1ccc(F)c(F)c1 Show InChI InChI=1S/C29H27F2NO3/c30-27-13-5-21(19-28(27)31)25-11-4-20-18-22(33)6-12-26(20)29(25)35-24-9-7-23(8-10-24)34-17-16-32-14-2-1-3-15-32/h4-13,18-19,33H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50378529

(CHEMBL566189)Show InChI InChI=1S/C15H13N5O2/c1-2-22-15(21)13-12-7-20-14(16-8-18-20)10-5-3-4-6-11(10)19(12)9-17-13/h3-6,8-9H,2,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to GABAA alpha-5-beta-3-gamma-2 receptor |
Bioorg Med Chem Lett 19: 5746-52 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.153
BindingDB Entry DOI: 10.7270/Q2PR7WZC |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50378525

(CHEMBL571466)Show InChI InChI=1S/C16H15N5O3/c1-3-24-16(22)14-13-7-21-15(17-8-19-21)11-6-10(23-2)4-5-12(11)20(13)9-18-14/h4-6,8-9H,3,7H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to GABAA alpha-5-beta-3-gamma-2 receptor |
Bioorg Med Chem Lett 19: 5746-52 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.153
BindingDB Entry DOI: 10.7270/Q2PR7WZC |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50378517
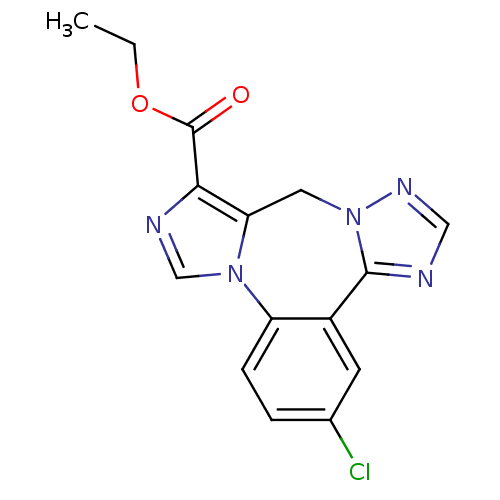
(CHEMBL567243)Show InChI InChI=1S/C15H12ClN5O2/c1-2-23-15(22)13-12-6-21-14(17-7-19-21)10-5-9(16)3-4-11(10)20(12)8-18-13/h3-5,7-8H,2,6H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to GABAA alpha-5-beta-3-gamma-2 receptor |
Bioorg Med Chem Lett 19: 5746-52 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.153
BindingDB Entry DOI: 10.7270/Q2PR7WZC |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50378486

(CHEMBL566181)Show InChI InChI=1S/C15H14N4O/c1-2-20-15-14-9-19-13(7-8-17-19)11-5-3-4-6-12(11)18(14)10-16-15/h3-8,10H,2,9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to GABAA alpha-5-beta-3-gamma-2 receptor |
Bioorg Med Chem Lett 19: 5746-52 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.153
BindingDB Entry DOI: 10.7270/Q2PR7WZC |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212149
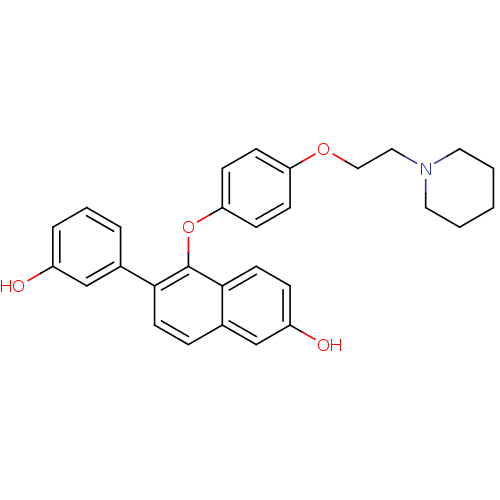
(6-(3-hydroxyphenyl)-5-(4-(2-(piperidin-1-yl)ethoxy...)Show SMILES Oc1cccc(c1)-c1ccc2cc(O)ccc2c1Oc1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C29H29NO4/c31-23-6-4-5-21(19-23)27-13-7-22-20-24(32)8-14-28(22)29(27)34-26-11-9-25(10-12-26)33-18-17-30-15-2-1-3-16-30/h4-14,19-20,31-32H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393252
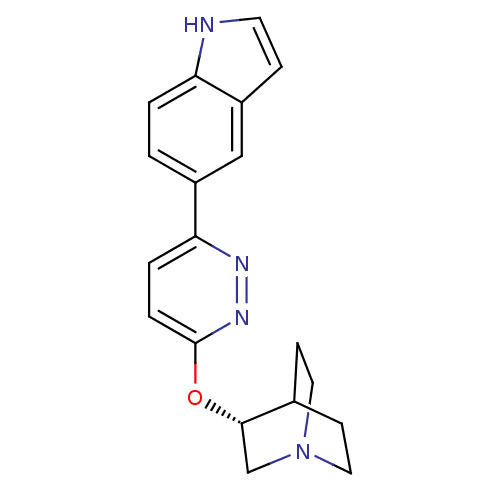
(CHEMBL2151570)Show SMILES C1CN2CCC1[C@H](C2)Oc1ccc(nn1)-c1ccc2[nH]ccc2c1 |r,wU:6.9,(10.31,-30.96,;10.31,-32.5,;11.64,-33.26,;10.9,-31.9,;12.4,-31.51,;11.64,-30.18,;12.97,-30.96,;12.97,-32.5,;14.31,-30.2,;15.64,-30.97,;16.97,-30.21,;18.3,-30.98,;18.3,-32.52,;16.96,-33.29,;15.63,-32.51,;19.62,-33.3,;19.61,-34.84,;20.94,-35.61,;22.28,-34.84,;23.75,-35.31,;24.65,-34.07,;23.75,-32.82,;22.28,-33.3,;20.95,-32.53,)| Show InChI InChI=1S/C19H20N4O/c1-2-16-15(5-8-20-16)11-14(1)17-3-4-19(22-21-17)24-18-12-23-9-6-13(18)7-10-23/h1-5,8,11,13,18,20H,6-7,9-10,12H2/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mahidol University
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-585539 from alpha7 nAChR in human cerebral cortex membranes after 75 mins by scintillation counting |
ACS Med Chem Lett 7: 890-895 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00146
BindingDB Entry DOI: 10.7270/Q2ZW1QDQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17358
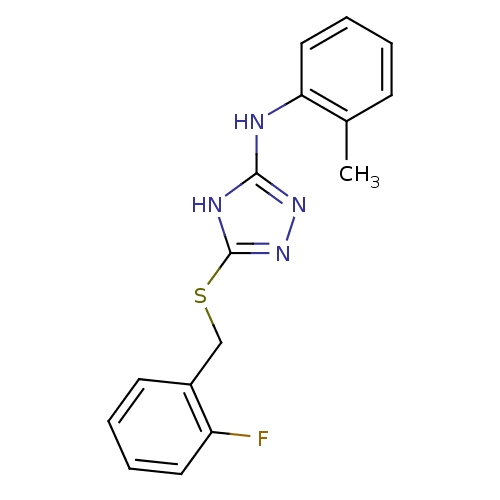
(1,2,4-Triazole Compound, 16 | 5-{[(2-fluorophenyl)...)Show InChI InChI=1S/C16H15FN4S/c1-11-6-2-5-9-14(11)18-15-19-16(21-20-15)22-10-12-7-3-4-8-13(12)17/h2-9H,10H2,1H3,(H2,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19967
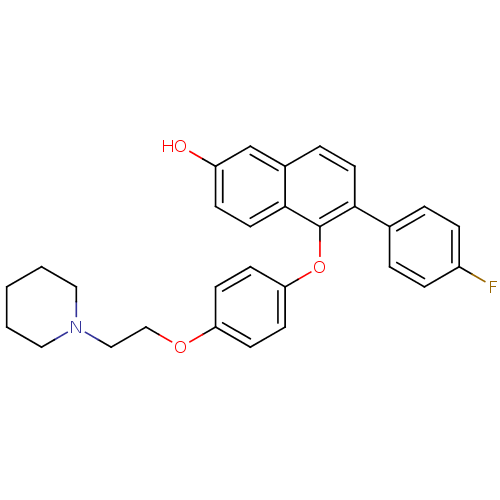
(6-(4-fluorophenyl)-5-{4-[2-(piperidin-1-yl)ethoxy]...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1ccc(F)cc1 Show InChI InChI=1S/C29H28FNO3/c30-23-7-4-21(5-8-23)27-14-6-22-20-24(32)9-15-28(22)29(27)34-26-12-10-25(11-13-26)33-19-18-31-16-2-1-3-17-31/h4-15,20,32H,1-3,16-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212160
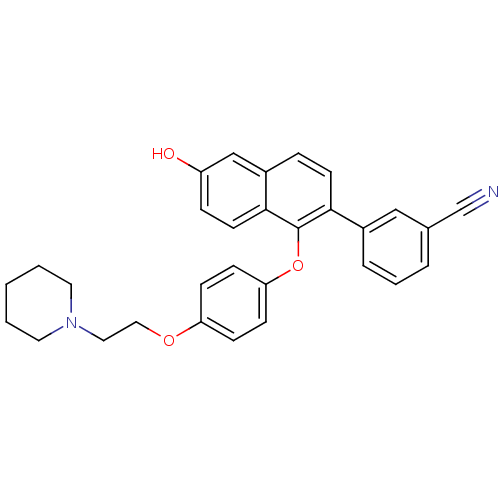
(3-(6-hydroxy-1-(4-(2-(piperidin-1-yl)ethoxy)phenox...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1cccc(c1)C#N Show InChI InChI=1S/C30H28N2O3/c31-21-22-5-4-6-23(19-22)28-13-7-24-20-25(33)8-14-29(24)30(28)35-27-11-9-26(10-12-27)34-18-17-32-15-2-1-3-16-32/h4-14,19-20,33H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50215709
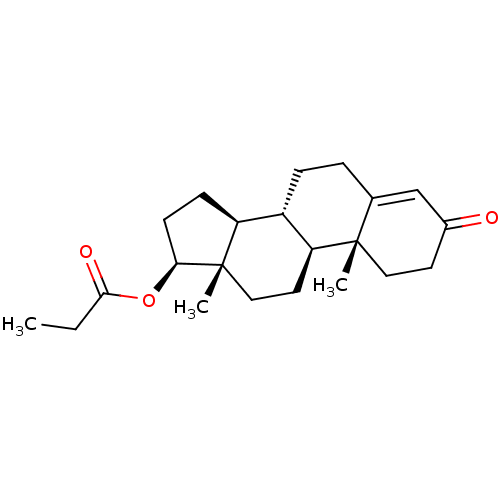
(CHEMBL1170 | Propionic acid (8R,9S,10R,13S,14S,17S...)Show SMILES CCC(=O)O[C@H]1CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@]12C |r,t:12| Show InChI InChI=1S/C22H32O3/c1-4-20(24)25-19-8-7-17-16-6-5-14-13-15(23)9-11-21(14,2)18(16)10-12-22(17,19)3/h13,16-19H,4-12H2,1-3H3/t16-,17-,18-,19-,21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 17: 4487-90 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.007
BindingDB Entry DOI: 10.7270/Q2NV9HZ9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa
Curated by ChEMBL
| Assay Description
Binding affinity against adenosine A1 receptor in bovine cortical membranes using [3H]-CHA as radioligand |
J Med Chem 47: 3019-31 (2004)
Article DOI: 10.1021/jm030977p
BindingDB Entry DOI: 10.7270/Q2BC3Z07 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50381886
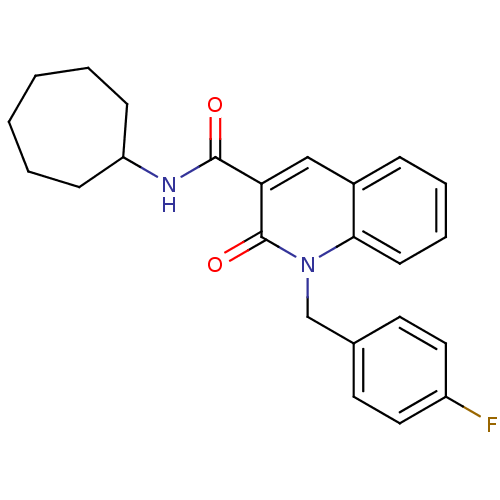
(CHEMBL2022363)Show SMILES Fc1ccc(Cn2c3ccccc3cc(C(=O)NC3CCCCCC3)c2=O)cc1 Show InChI InChI=1S/C24H25FN2O2/c25-19-13-11-17(12-14-19)16-27-22-10-6-5-7-18(22)15-21(24(27)29)23(28)26-20-8-3-1-2-4-9-20/h5-7,10-15,20H,1-4,8-9,16H2,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB2 receptor expressed in human HEK293 cell membrane after 90 mins |
Eur J Med Chem 52: 284-94 (2012)
Article DOI: 10.1016/j.ejmech.2012.03.031
BindingDB Entry DOI: 10.7270/Q2PK0H6K |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19967

(6-(4-fluorophenyl)-5-{4-[2-(piperidin-1-yl)ethoxy]...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1ccc(F)cc1 Show InChI InChI=1S/C29H28FNO3/c30-23-7-4-21(5-8-23)27-14-6-22-20-24(32)9-15-28(22)29(27)34-26-12-10-25(11-13-26)33-19-18-31-16-2-1-3-17-31/h4-15,20,32H,1-3,16-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | -54.3 | n/a | n/a | 3.18 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories
| Assay Description
The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... |
J Med Chem 48: 6772-5 (2005)
Article DOI: 10.1021/jm050723z
BindingDB Entry DOI: 10.7270/Q2RR1WHW |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356582
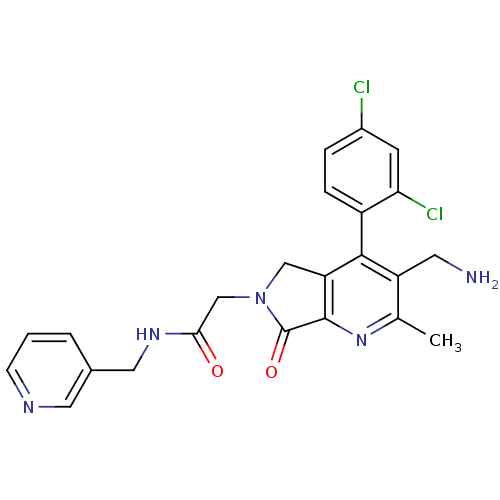
(CHEMBL1910126)Show SMILES Cc1nc2C(=O)N(CC(=O)NCc3cccnc3)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(14.01,-46.7,;12.68,-47.48,;11.34,-46.71,;10.02,-47.49,;8.55,-47.01,;8.08,-45.55,;7.65,-48.25,;6.11,-48.26,;5.34,-49.59,;6.12,-50.92,;3.8,-49.59,;3.04,-50.93,;1.5,-50.93,;.74,-52.26,;-.8,-52.27,;-1.58,-50.93,;-.81,-49.6,;.73,-49.59,;8.55,-49.5,;10.01,-49.03,;11.35,-49.8,;12.68,-49.03,;14.02,-49.79,;15.35,-49.02,;11.35,-51.33,;10.01,-52.1,;10.01,-53.64,;11.35,-54.41,;11.35,-55.95,;12.69,-53.64,;12.68,-52.1,;14.01,-51.33,)| Show InChI InChI=1S/C23H21Cl2N5O2/c1-13-17(8-26)21(16-5-4-15(24)7-19(16)25)18-11-30(23(32)22(18)29-13)12-20(31)28-10-14-3-2-6-27-9-14/h2-7,9H,8,10-12,26H2,1H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356591

(CHEMBL1910117)Show SMILES CN(C)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(23.14,-19.79,;23.91,-21.12,;23.14,-22.45,;25.45,-21.12,;26.22,-22.45,;26.22,-19.78,;27.75,-19.78,;28.65,-21.03,;30.12,-20.55,;30.12,-19.01,;31.45,-18.24,;32.78,-19,;34.12,-18.23,;32.79,-20.55,;34.13,-21.32,;35.46,-20.55,;31.45,-21.32,;31.46,-22.86,;30.12,-23.63,;30.12,-25.17,;31.45,-25.94,;31.45,-27.48,;32.79,-25.16,;32.79,-23.62,;34.12,-22.85,;28.66,-18.54,;28.18,-17.07,)| Show InChI InChI=1S/C19H20Cl2N4O2/c1-10-13(7-22)17(12-5-4-11(20)6-15(12)21)14-8-25(9-16(26)24(2)3)19(27)18(14)23-10/h4-6H,7-9,22H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50029962
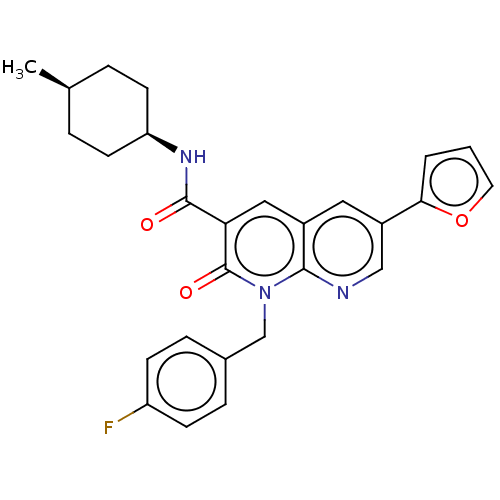
(CHEMBL3353450)Show SMILES C[C@H]1CC[C@H](CC1)NC(=O)c1cc2cc(cnc2n(Cc2ccc(F)cc2)c1=O)-c1ccco1 |r,wU:4.7,1.0,(23.36,-18.24,;22.03,-19.01,;22.03,-20.55,;20.71,-21.32,;19.37,-20.55,;19.36,-19.02,;20.69,-18.24,;18.04,-21.33,;16.7,-20.57,;16.69,-19.03,;15.37,-21.34,;14.04,-20.58,;12.71,-21.36,;11.37,-20.59,;10.05,-21.36,;10.04,-22.91,;11.38,-23.68,;12.71,-22.9,;14.05,-23.67,;14.05,-25.21,;15.38,-25.97,;15.38,-27.51,;16.71,-28.28,;18.05,-27.5,;19.38,-28.27,;18.04,-25.96,;16.71,-25.2,;15.38,-22.89,;16.72,-23.66,;8.71,-20.59,;7.3,-21.22,;6.26,-20.08,;7.03,-18.74,;8.54,-19.06,)| Show InChI InChI=1S/C27H26FN3O3/c1-17-4-10-22(11-5-17)30-26(32)23-14-19-13-20(24-3-2-12-34-24)15-29-25(19)31(27(23)33)16-18-6-8-21(28)9-7-18/h2-3,6-9,12-15,17,22H,4-5,10-11,16H2,1H3,(H,30,32)/t17-,22+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... |
J Med Chem 57: 8777-91 (2014)
Article DOI: 10.1021/jm500807e
BindingDB Entry DOI: 10.7270/Q2QC054S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data