 Found 136 hits with Last Name = 'marella' and Initial = 'ma'
Found 136 hits with Last Name = 'marella' and Initial = 'ma' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007344
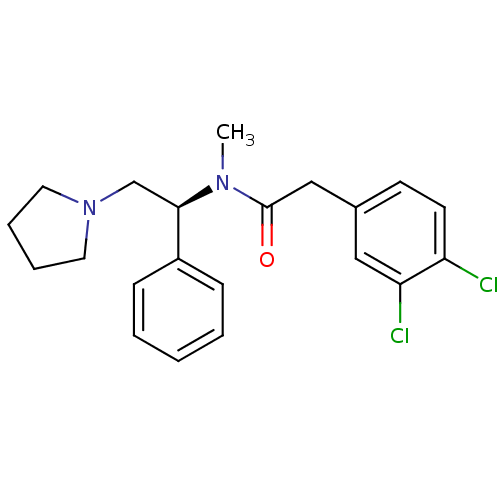
((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093965
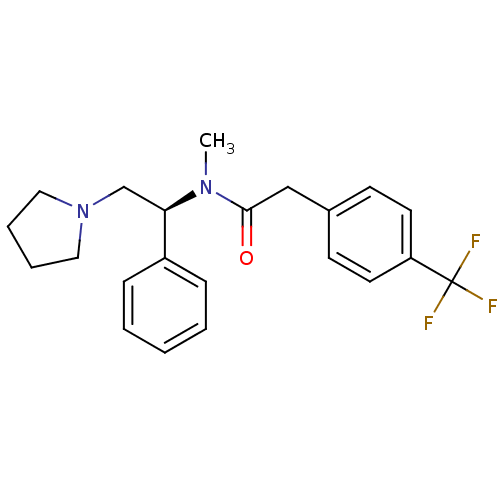
(CHEMBL86324 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-9-11-19(12-10-17)22(23,24)25)20(16-27-13-5-6-14-27)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093964
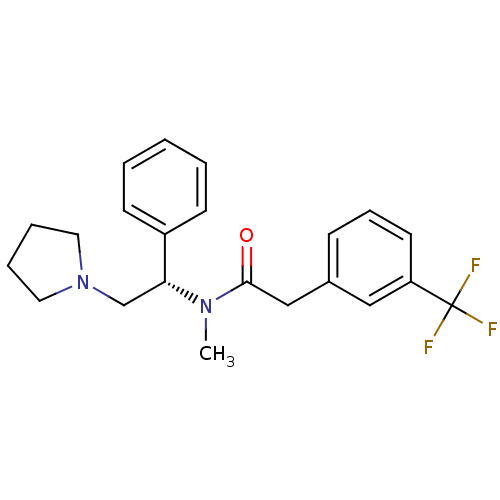
(CHEMBL313484 | N-Methyl-N-((S)-1-phenyl-2-pyrrolid...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-8-7-11-19(14-17)22(23,24)25)20(16-27-12-5-6-13-27)18-9-3-2-4-10-18/h2-4,7-11,14,20H,5-6,12-13,15-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093969
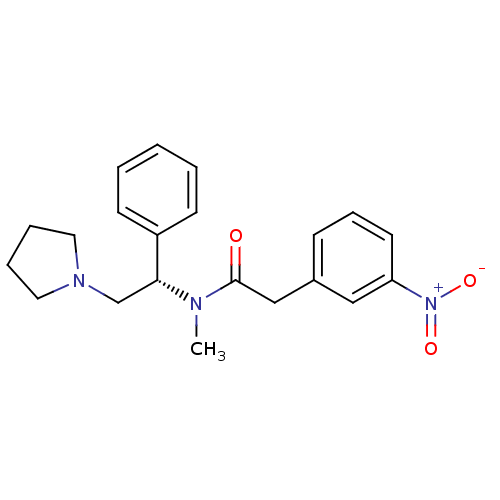
(CHEMBL82919 | N-Methyl-2-(3-nitro-phenyl)-N-((S)-1...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H25N3O3/c1-22(21(25)15-17-8-7-11-19(14-17)24(26)27)20(16-23-12-5-6-13-23)18-9-3-2-4-10-18/h2-4,7-11,14,20H,5-6,12-13,15-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093958
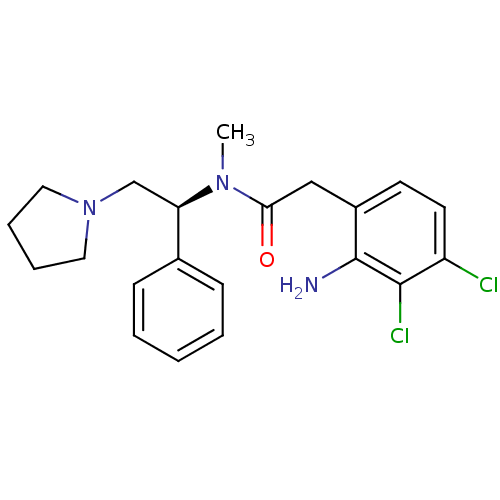
(2-(2-Amino-3,4-dichloro-phenyl)-N-methyl-N-((S)-1-...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1N Show InChI InChI=1S/C21H25Cl2N3O/c1-25(19(27)13-16-9-10-17(22)20(23)21(16)24)18(14-26-11-5-6-12-26)15-7-3-2-4-8-15/h2-4,7-10,18H,5-6,11-14,24H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093966
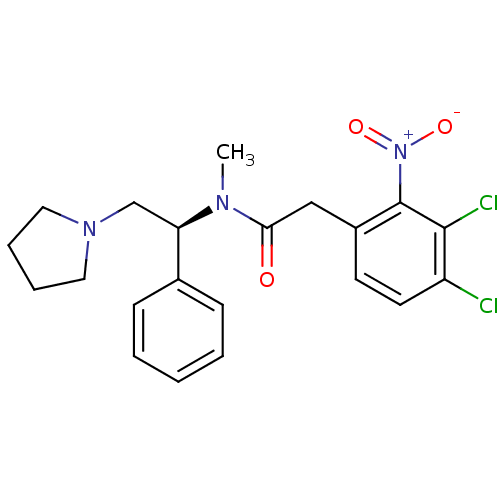
(2-(3,4-Dichloro-2-nitro-phenyl)-N-methyl-N-((S)-1-...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1[N+]([O-])=O Show InChI InChI=1S/C21H23Cl2N3O3/c1-24(18(14-25-11-5-6-12-25)15-7-3-2-4-8-15)19(27)13-16-9-10-17(22)20(23)21(16)26(28)29/h2-4,7-10,18H,5-6,11-14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093970
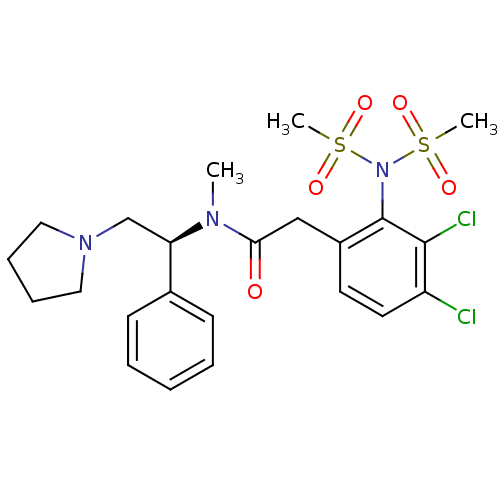
(2-(3,4-Dichloro-2-dimethanesulfonylamino-phenyl)-N...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1N(S(C)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C23H29Cl2N3O5S2/c1-26(20(16-27-13-7-8-14-27)17-9-5-4-6-10-17)21(29)15-18-11-12-19(24)22(25)23(18)28(34(2,30)31)35(3,32)33/h4-6,9-12,20H,7-8,13-16H2,1-3H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093962
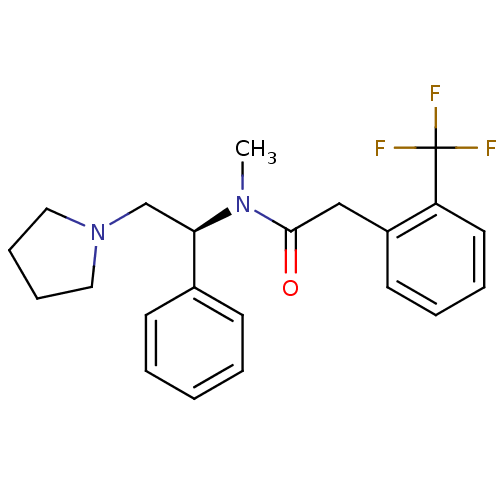
(CHEMBL87306 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccccc1C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-18-11-5-6-12-19(18)22(23,24)25)20(16-27-13-7-8-14-27)17-9-3-2-4-10-17/h2-6,9-12,20H,7-8,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093968
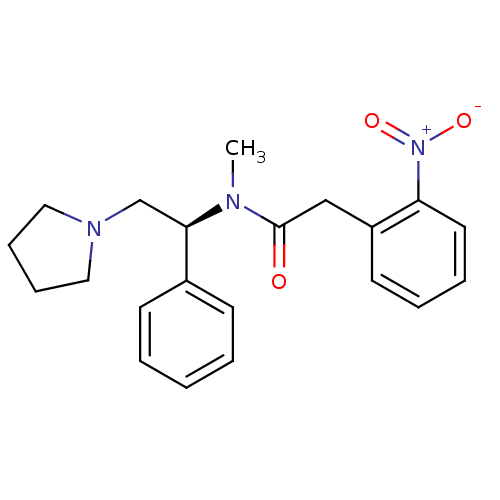
(CHEMBL87207 | N-Methyl-2-(2-nitro-phenyl)-N-((S)-1...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccccc1[N+]([O-])=O Show InChI InChI=1S/C21H25N3O3/c1-22(21(25)15-18-11-5-6-12-19(18)24(26)27)20(16-23-13-7-8-14-23)17-9-3-2-4-10-17/h2-6,9-12,20H,7-8,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093971
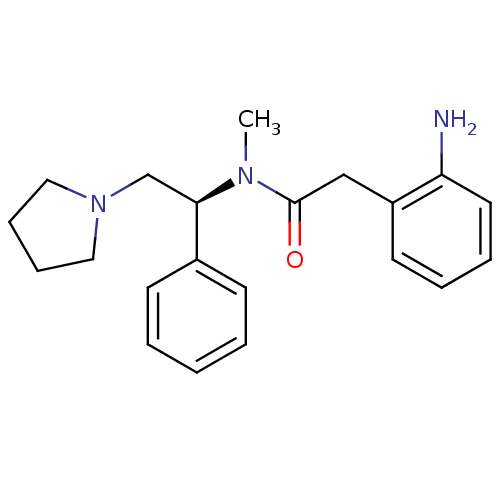
(2-(2-Amino-phenyl)-N-methyl-N-((S)-1-phenyl-2-pyrr...)Show InChI InChI=1S/C21H27N3O/c1-23(21(25)15-18-11-5-6-12-19(18)22)20(16-24-13-7-8-14-24)17-9-3-2-4-10-17/h2-6,9-12,20H,7-8,13-16,22H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093967
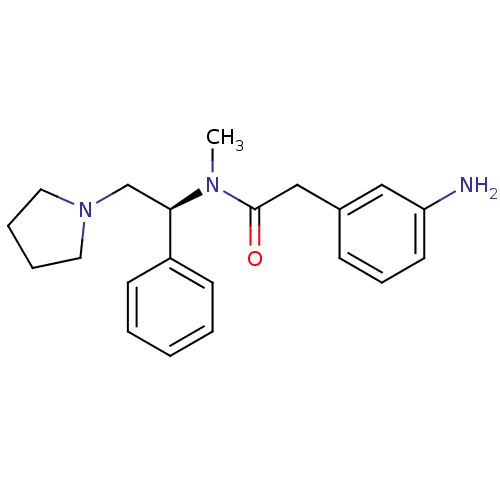
(2-(3-Amino-phenyl)-N-methyl-N-((S)-1-phenyl-2-pyrr...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(N)c1 Show InChI InChI=1S/C21H27N3O/c1-23(21(25)15-17-8-7-11-19(22)14-17)20(16-24-12-5-6-13-24)18-9-3-2-4-10-18/h2-4,7-11,14,20H,5-6,12-13,15-16,22H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093963
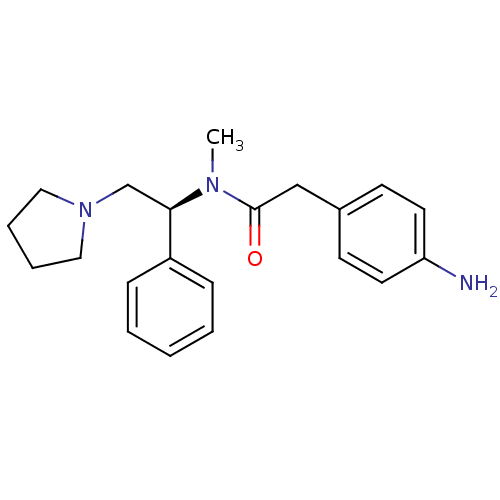
(2-(4-Amino-phenyl)-N-methyl-N-((S)-1-phenyl-2-pyrr...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(N)cc1 Show InChI InChI=1S/C21H27N3O/c1-23(21(25)15-17-9-11-19(22)12-10-17)20(16-24-13-5-6-14-24)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16,22H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093972

(CHEMBL87986 | N-Methyl-2-(4-nitro-phenyl)-N-((S)-1...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H25N3O3/c1-22(21(25)15-17-9-11-19(12-10-17)24(26)27)20(16-23-13-5-6-14-23)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093961
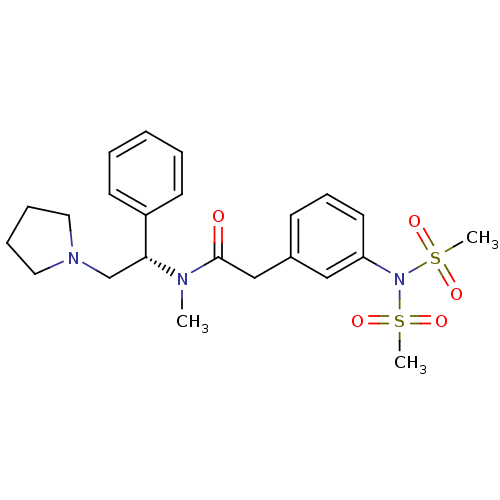
(2-(3-Dimethanesulfonylamino-phenyl)-N-methyl-N-((S...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(c1)N(S(C)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C23H31N3O5S2/c1-24(22(18-25-14-7-8-15-25)20-11-5-4-6-12-20)23(27)17-19-10-9-13-21(16-19)26(32(2,28)29)33(3,30)31/h4-6,9-13,16,22H,7-8,14-15,17-18H2,1-3H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093960
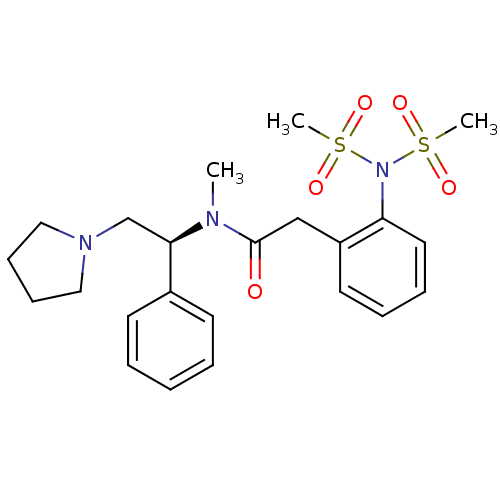
(2-(2-Dimethanesulfonylamino-phenyl)-N-methyl-N-((S...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccccc1N(S(C)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C23H31N3O5S2/c1-24(22(18-25-15-9-10-16-25)19-11-5-4-6-12-19)23(27)17-20-13-7-8-14-21(20)26(32(2,28)29)33(3,30)31/h4-8,11-14,22H,9-10,15-18H2,1-3H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093959
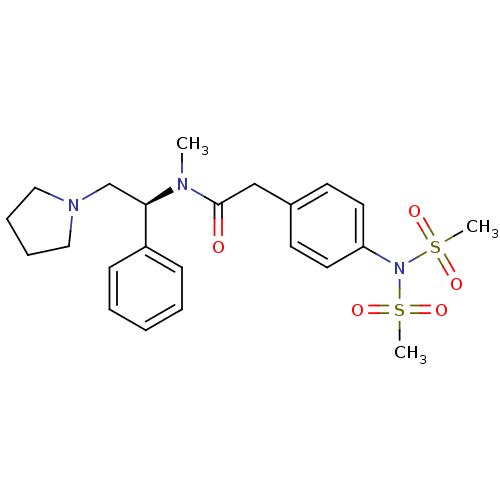
(2-(4-Dimethanesulfonylamino-phenyl)-N-methyl-N-((S...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(cc1)N(S(C)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C23H31N3O5S2/c1-24(22(18-25-15-7-8-16-25)20-9-5-4-6-10-20)23(27)17-19-11-13-21(14-12-19)26(32(2,28)29)33(3,30)31/h4-6,9-14,22H,7-8,15-18H2,1-3H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375821
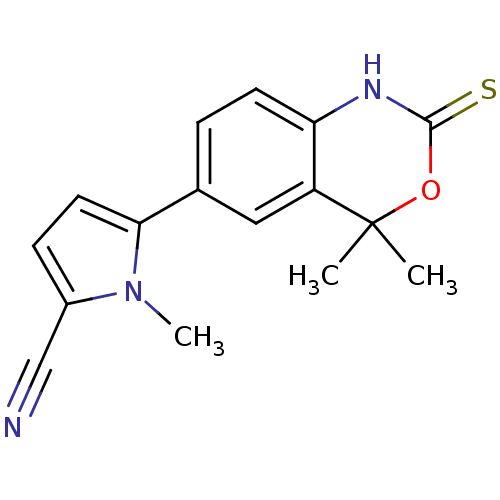
(TANAPROGET)Show InChI InChI=1S/C16H15N3OS/c1-16(2)12-8-10(4-6-13(12)18-15(21)20-16)14-7-5-11(9-17)19(14)3/h4-8H,1-3H3,(H,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human GR ligand binding domain expressed in african green monkey COS7 cells in presence of Dexamethasone by Gal4 hybrid assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM22416

((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of serotonin uptake at human SERT expressed in JAR cells |
Bioorg Med Chem Lett 18: 4929-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.060
BindingDB Entry DOI: 10.7270/Q2S183C9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of serotonin uptake at human SERT expressed in JAR cells |
Bioorg Med Chem Lett 18: 4929-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.060
BindingDB Entry DOI: 10.7270/Q2S183C9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50124566
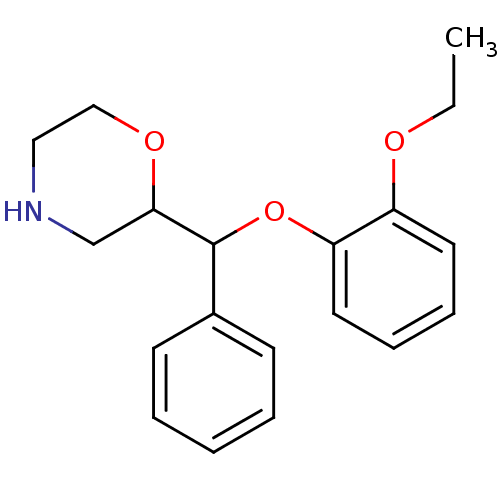
((R)-2-((R)-(2-ethoxyphenoxy)(phenyl)methyl)morphol...)Show InChI InChI=1S/C19H23NO3/c1-2-21-16-10-6-7-11-17(16)23-19(15-8-4-3-5-9-15)18-14-20-12-13-22-18/h3-11,18-20H,2,12-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK cells |
Bioorg Med Chem Lett 18: 4929-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.060
BindingDB Entry DOI: 10.7270/Q2S183C9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50366567
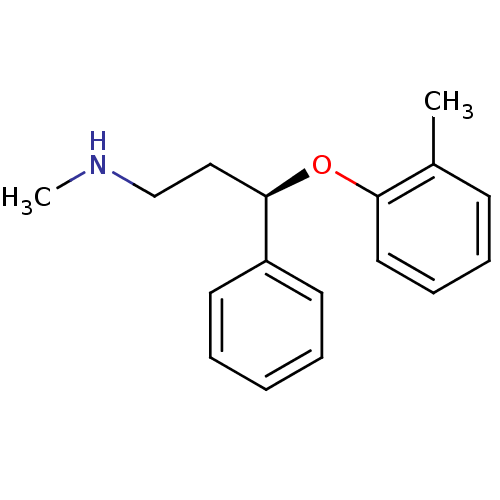
(ATOMOXETINE)Show InChI InChI=1S/C17H21NO/c1-14-8-6-7-11-16(14)19-17(12-13-18-2)15-9-4-3-5-10-15/h3-11,17-18H,12-13H2,1-2H3/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK cells |
Bioorg Med Chem Lett 18: 4929-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.060
BindingDB Entry DOI: 10.7270/Q2S183C9 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375823
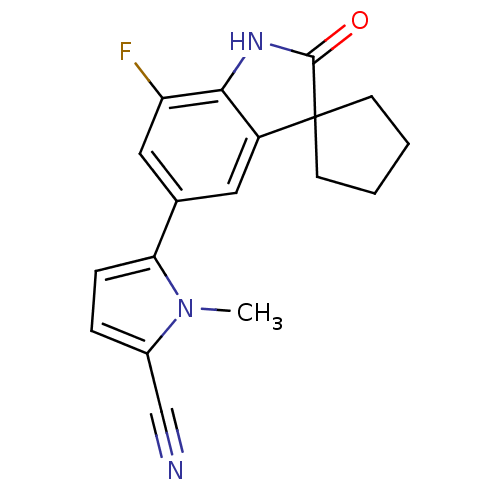
(CHEMBL407847)Show InChI InChI=1S/C18H16FN3O/c1-22-12(10-20)4-5-15(22)11-8-13-16(14(19)9-11)21-17(23)18(13)6-2-3-7-18/h4-5,8-9H,2-3,6-7H2,1H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50144435

(1-methyl-5-[2'-oxospiro[cyclohexane-1,3'-(2',3'-di...)Show InChI InChI=1S/C19H19N3O/c1-22-14(12-20)6-8-17(22)13-5-7-16-15(11-13)19(18(23)21-16)9-3-2-4-10-19/h5-8,11H,2-4,9-10H2,1H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375817

(CHEMBL272663)Show InChI InChI=1S/C18H19N3O/c1-4-18(5-2)14-10-12(6-8-15(14)20-17(18)22)16-9-7-13(11-19)21(16)3/h6-10H,4-5H2,1-3H3,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375822
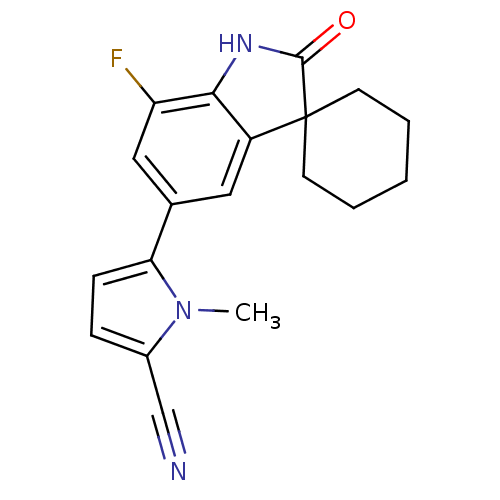
(CHEMBL408648)Show SMILES Cn1c(ccc1-c1cc2c(NC(=O)C22CCCCC2)c(F)c1)C#N Show InChI InChI=1S/C19H18FN3O/c1-23-13(11-21)5-6-16(23)12-9-14-17(15(20)10-12)22-18(24)19(14)7-3-2-4-8-19/h5-6,9-10H,2-4,7-8H2,1H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK cells |
Bioorg Med Chem Lett 18: 4929-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.060
BindingDB Entry DOI: 10.7270/Q2S183C9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK cells |
Bioorg Med Chem Lett 18: 4929-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.060
BindingDB Entry DOI: 10.7270/Q2S183C9 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375827

(CHEMBL407848 | WAY-255348)Show InChI InChI=1S/C16H14FN3O/c1-16(2)11-6-9(7-12(17)14(11)19-15(16)21)13-5-4-10(8-18)20(13)3/h4-7H,1-3H3,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375831
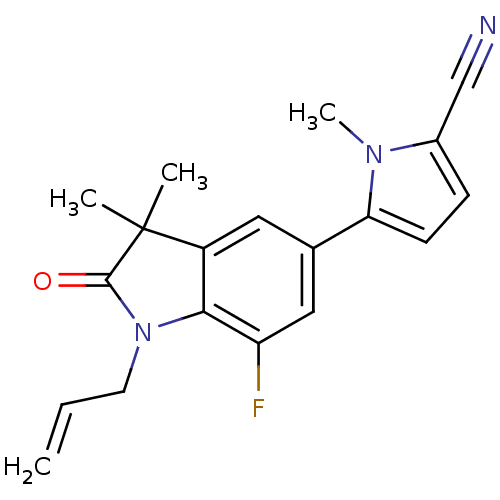
(CHEMBL429572)Show SMILES Cn1c(ccc1-c1cc2c(N(CC=C)C(=O)C2(C)C)c(F)c1)C#N Show InChI InChI=1S/C19H18FN3O/c1-5-8-23-17-14(19(2,3)18(23)24)9-12(10-15(17)20)16-7-6-13(11-21)22(16)4/h5-7,9-10H,1,8H2,2-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50144438

(5-(4,4-Dimethyl-2-oxo-1,4-dihydro-2H-benzo[d][1,3]...)Show InChI InChI=1S/C16H15N3O2/c1-16(2)12-8-10(4-6-13(12)18-15(20)21-16)14-7-5-11(9-17)19(14)3/h4-8H,1-3H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375824
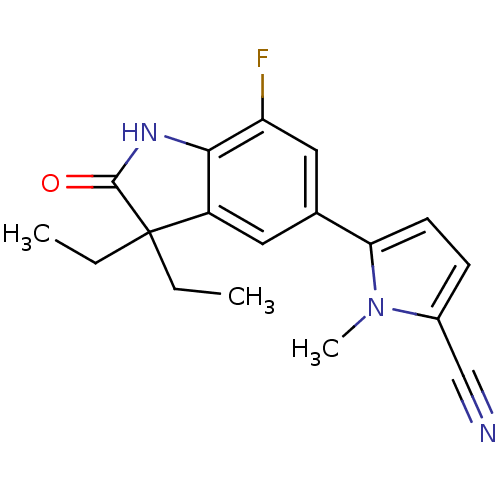
(CHEMBL429928)Show InChI InChI=1S/C18H18FN3O/c1-4-18(5-2)13-8-11(9-14(19)16(13)21-17(18)23)15-7-6-12(10-20)22(15)3/h6-9H,4-5H2,1-3H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375827

(CHEMBL407848 | WAY-255348)Show InChI InChI=1S/C16H14FN3O/c1-16(2)11-6-9(7-12(17)14(11)19-15(16)21)13-5-4-10(8-18)20(13)3/h4-7H,1-3H3,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375826
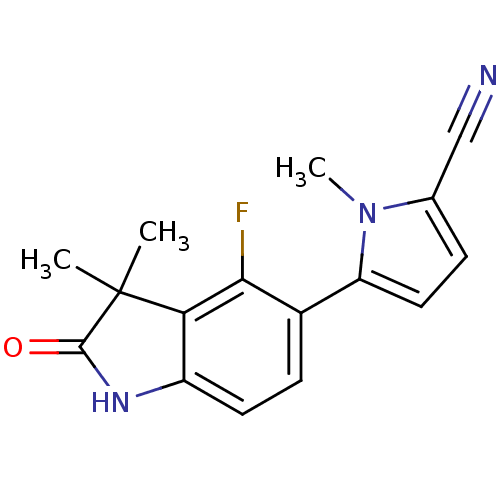
(CHEMBL270714)Show InChI InChI=1S/C16H14FN3O/c1-16(2)13-11(19-15(16)21)6-5-10(14(13)17)12-7-4-9(8-18)20(12)3/h4-7H,1-3H3,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375826

(CHEMBL270714)Show InChI InChI=1S/C16H14FN3O/c1-16(2)13-11(19-15(16)21)6-5-10(14(13)17)12-7-4-9(8-18)20(12)3/h4-7H,1-3H3,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM22417
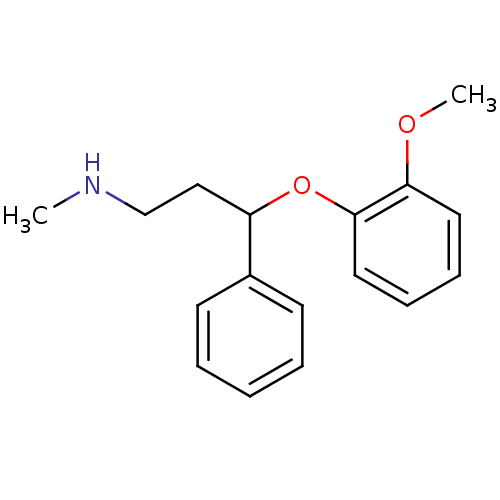
(3-(2-methoxyphenoxy)-N-methyl-3-phenylpropan-1-ami...)Show InChI InChI=1S/C17H21NO2/c1-18-13-12-15(14-8-4-3-5-9-14)20-17-11-7-6-10-16(17)19-2/h3-11,15,18H,12-13H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK cells |
Bioorg Med Chem Lett 18: 4929-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.060
BindingDB Entry DOI: 10.7270/Q2S183C9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50263540
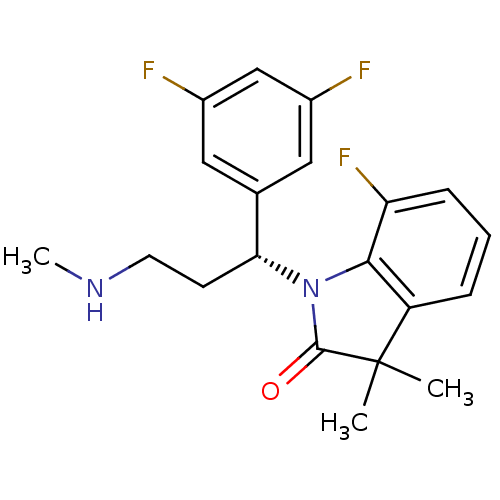
((R)-1-(1-(3,5-difluorophenyl)-3-(methylamino)propy...)Show SMILES CNCC[C@@H](N1C(=O)C(C)(C)c2cccc(F)c12)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C20H21F3N2O/c1-20(2)15-5-4-6-16(23)18(15)25(19(20)26)17(7-8-24-3)12-9-13(21)11-14(22)10-12/h4-6,9-11,17,24H,7-8H2,1-3H3/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK cells |
Bioorg Med Chem Lett 18: 4929-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.060
BindingDB Entry DOI: 10.7270/Q2S183C9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50375423
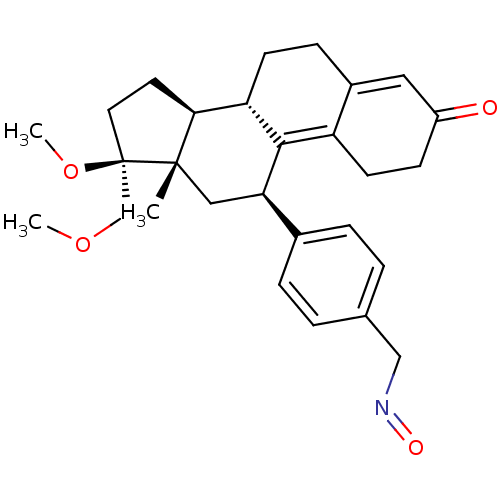
(ASOPRISNIL)Show SMILES COC[C@@]1(CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(CN=O)cc1)OC |r,c:17,t:10| Show InChI InChI=1S/C28H35NO4/c1-27-15-24(19-6-4-18(5-7-19)16-29-31)26-22-11-9-21(30)14-20(22)8-10-23(26)25(27)12-13-28(27,33-3)17-32-2/h4-7,14,23-25H,8-13,15-17H2,1-3H3/t23-,24+,25-,27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR ligand binding domain expressed in african green monkey COS7 cells in presence of 5-alpha-dihydrotestosterone by Gal4... |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR ligand binding domain expressed in african green monkey COS7 cells in presence of 5-alpha-dihydrotestosterone by Gal4... |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50263500
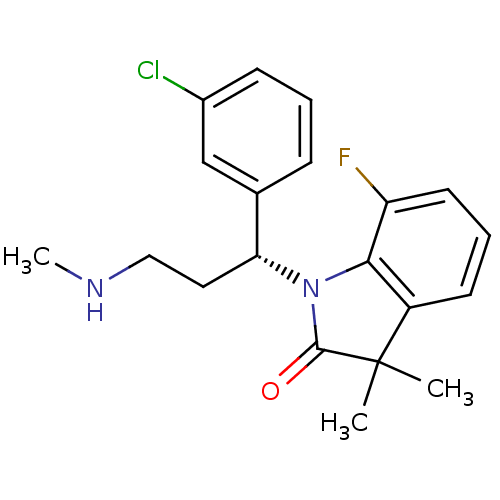
((R)-1-(1-(3-chlorophenyl)-3-(methylamino)propyl)-7...)Show SMILES CNCC[C@@H](N1C(=O)C(C)(C)c2cccc(F)c12)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C20H22ClFN2O/c1-20(2)15-8-5-9-16(22)18(15)24(19(20)25)17(10-11-23-3)13-6-4-7-14(21)12-13/h4-9,12,17,23H,10-11H2,1-3H3/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK cells |
Bioorg Med Chem Lett 18: 4929-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.060
BindingDB Entry DOI: 10.7270/Q2S183C9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50263541
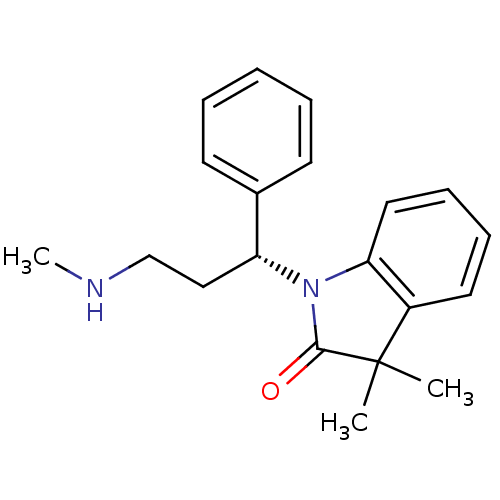
((R)-3,3-dimethyl-1-(3-(methylamino)-1-phenylpropyl...)Show SMILES CNCC[C@@H](N1C(=O)C(C)(C)c2ccccc12)c1ccccc1 |r| Show InChI InChI=1S/C20H24N2O/c1-20(2)16-11-7-8-12-18(16)22(19(20)23)17(13-14-21-3)15-9-5-4-6-10-15/h4-12,17,21H,13-14H2,1-3H3/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK cells |
Bioorg Med Chem Lett 18: 4929-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.060
BindingDB Entry DOI: 10.7270/Q2S183C9 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375816

(CHEMBL407622)Show InChI InChI=1S/C18H17N3O/c1-21-13(11-19)5-7-16(21)12-4-6-15-14(10-12)18(17(22)20-15)8-2-3-9-18/h4-7,10H,2-3,8-9H2,1H3,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375818

(CHEMBL270908)Show InChI InChI=1S/C16H15N3O/c1-16(2)12-8-10(4-6-13(12)18-15(16)20)14-7-5-11(9-17)19(14)3/h4-8H,1-3H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM30130

(CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...)Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of serotonin uptake at human SERT expressed in JAR cells |
Bioorg Med Chem Lett 18: 4929-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.060
BindingDB Entry DOI: 10.7270/Q2S183C9 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375834

(CHEMBL271804)Show SMILES CC(C)N1C(=O)C(C)(C)c2cc(cc(F)c12)-c1ccc(C#N)n1C Show InChI InChI=1S/C19H20FN3O/c1-11(2)23-17-14(19(3,4)18(23)24)8-12(9-15(17)20)16-7-6-13(10-21)22(16)5/h6-9,11H,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50263498
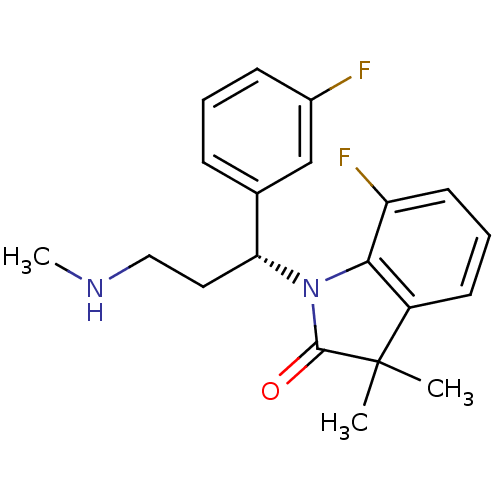
((R)-7-fluoro-1-(1-(3-fluorophenyl)-3-(methylamino)...)Show SMILES CNCC[C@@H](N1C(=O)C(C)(C)c2cccc(F)c12)c1cccc(F)c1 |r| Show InChI InChI=1S/C20H22F2N2O/c1-20(2)15-8-5-9-16(22)18(15)24(19(20)25)17(10-11-23-3)13-6-4-7-14(21)12-13/h4-9,12,17,23H,10-11H2,1-3H3/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK cells |
Bioorg Med Chem Lett 18: 4929-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.060
BindingDB Entry DOI: 10.7270/Q2S183C9 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375823

(CHEMBL407847)Show InChI InChI=1S/C18H16FN3O/c1-22-12(10-20)4-5-15(22)11-8-13-16(14(19)9-11)21-17(23)18(13)6-2-3-7-18/h4-5,8-9H,2-3,6-7H2,1H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50121163
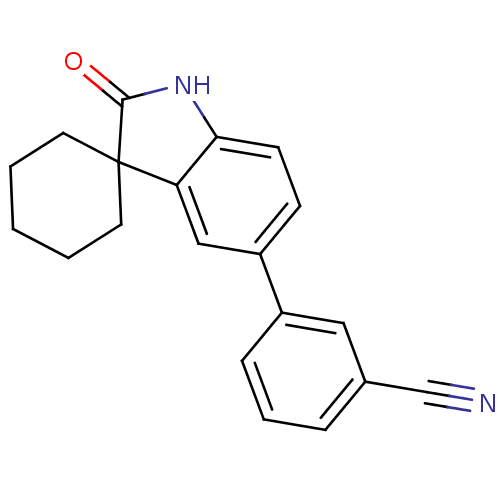
(3-(2'-oxo-1',2'-dihydrospiro[cyclohexane-1,3'-indo...)Show InChI InChI=1S/C20H18N2O/c21-13-14-5-4-6-15(11-14)16-7-8-18-17(12-16)20(19(23)22-18)9-2-1-3-10-20/h4-8,11-12H,1-3,9-10H2,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data