

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202775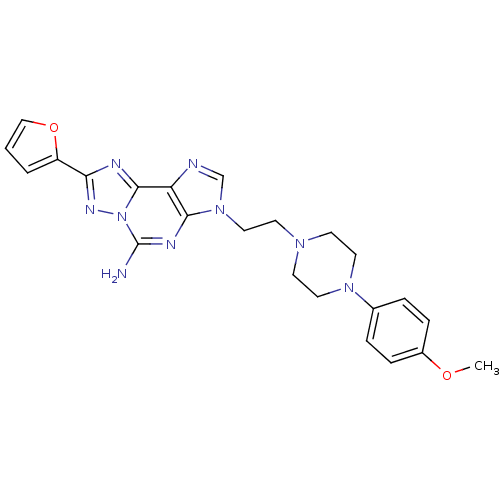 (8-(furan-2-yl)-3-(2-(4-(4-methoxyphenyl)piperazin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50051751 (1-[3-(4-Isopropenyl-phenyl)-8-aza-bicyclo[3.2.1]oc...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-paroxetine from Serotonin transporter of rat frontal cortex membrane | J Med Chem 39: 2554-8 (1996) Article DOI: 10.1021/jm9600508 BindingDB Entry DOI: 10.7270/Q2V69HP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50041293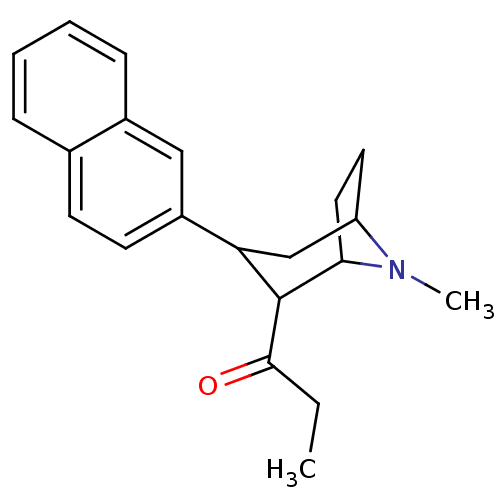 (1-(8-Methyl-3-naphthalen-2-yl-8-aza-bicyclo[3.2.1]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University Curated by ChEMBL | Assay Description Inhibition of [3H]paroxetine binding to serotonin transport sites in rat frontal cortex membranes. | J Med Chem 37: 1262-8 (1994) BindingDB Entry DOI: 10.7270/Q2TH8NBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50051753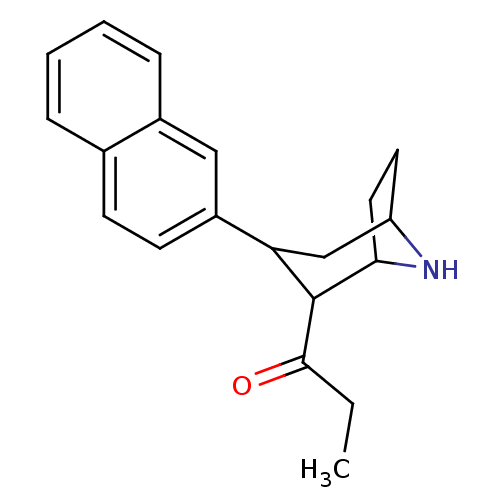 (1-(3-Naphthalen-2-yl-8-aza-bicyclo[3.2.1]oct-2-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-paroxetine from Serotonin transporter of rat frontal cortex membrane | J Med Chem 39: 2554-8 (1996) Article DOI: 10.1021/jm9600508 BindingDB Entry DOI: 10.7270/Q2V69HP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50204794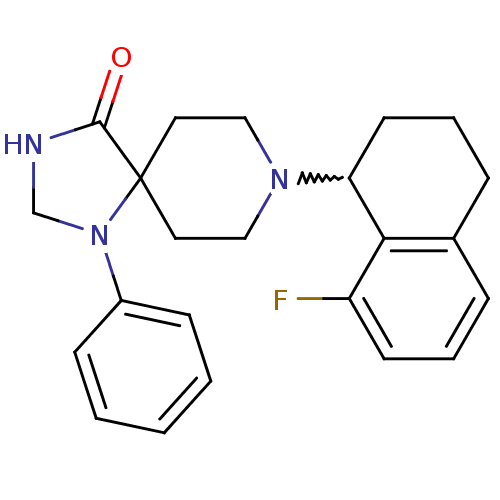 (8-(8-fluoro-1,2,3,4-tetrahydro-naphthalen-1-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 2281-4 (2007) Article DOI: 10.1016/j.bmcl.2007.01.069 BindingDB Entry DOI: 10.7270/Q2T43SRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202777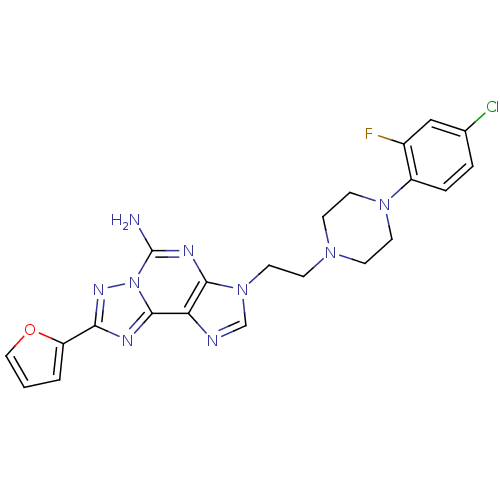 (3-(2-(4-(4-chloro-2-fluorophenyl)piperazin-1-yl)et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50051757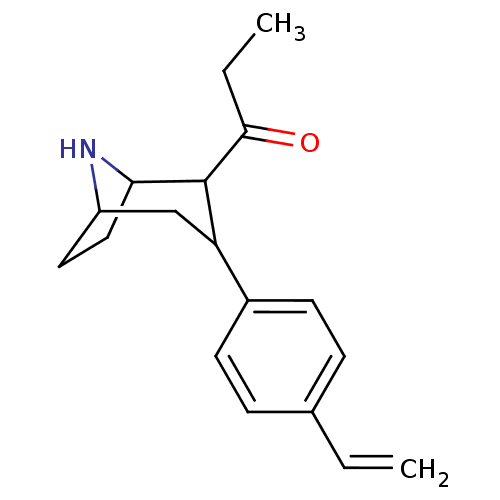 (1-[3-(4-Vinyl-phenyl)-8-aza-bicyclo[3.2.1]oct-2-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-paroxetine from Serotonin transporter of rat frontal cortex membrane | J Med Chem 39: 2554-8 (1996) Article DOI: 10.1021/jm9600508 BindingDB Entry DOI: 10.7270/Q2V69HP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50041293 (1-(8-Methyl-3-naphthalen-2-yl-8-aza-bicyclo[3.2.1]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-paroxetine from Serotonin transporter of rat frontal cortex membrane | J Med Chem 39: 2554-8 (1996) Article DOI: 10.1021/jm9600508 BindingDB Entry DOI: 10.7270/Q2V69HP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50041293 (1-(8-Methyl-3-naphthalen-2-yl-8-aza-bicyclo[3.2.1]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.394 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University Curated by ChEMBL | Assay Description Inhibition of [3H]paroxetine binding to serotonin transport sites in rat frontal cortex membranes. | J Med Chem 37: 1262-8 (1994) BindingDB Entry DOI: 10.7270/Q2TH8NBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202788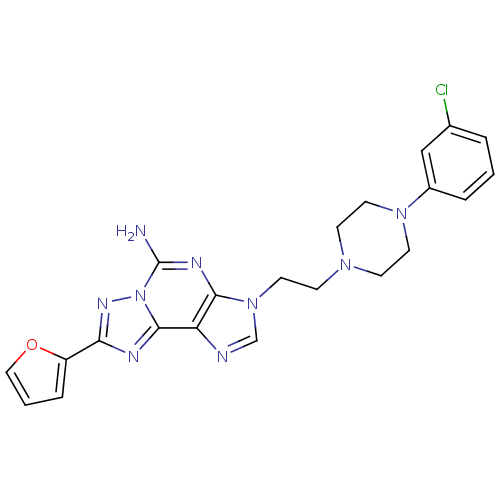 (3-(2-(4-(3-chlorophenyl)piperazin-1-yl)ethyl)-8-(f...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202771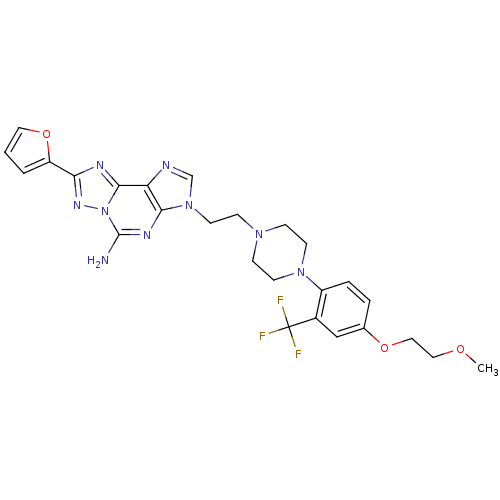 (8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)-2-(tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26946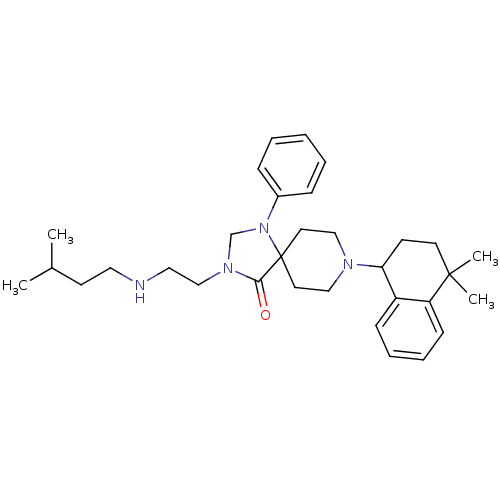 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26927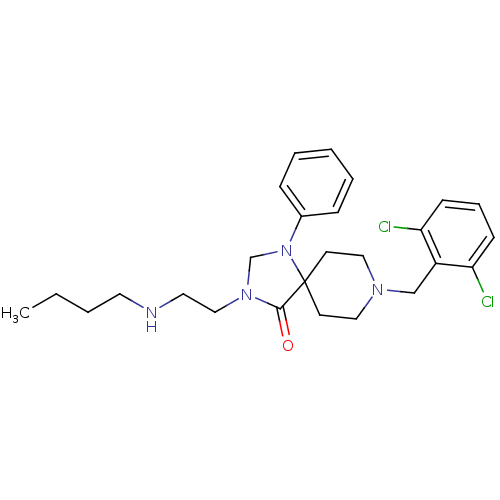 (3-[2-(butylamino)ethyl]-8-[(2,6-dichlorophenyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26926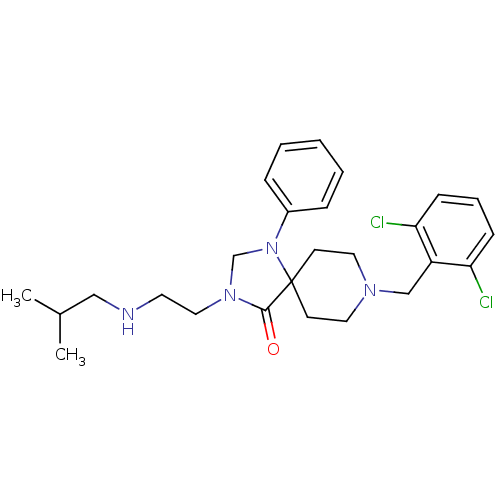 (8-[(2,6-dichlorophenyl)methyl]-3-{2-[(2-methylprop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26945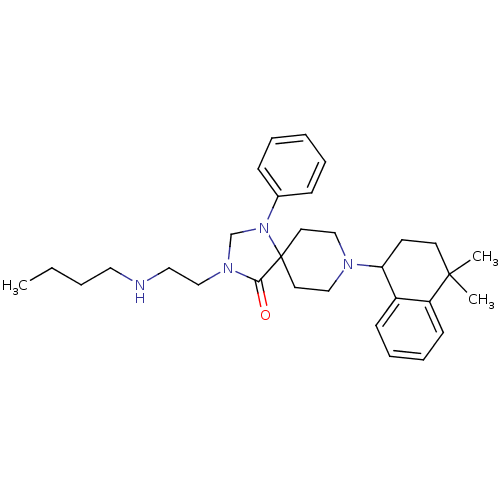 (3-[2-(butylamino)ethyl]-8-(4,4-dimethyl-1,2,3,4-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26924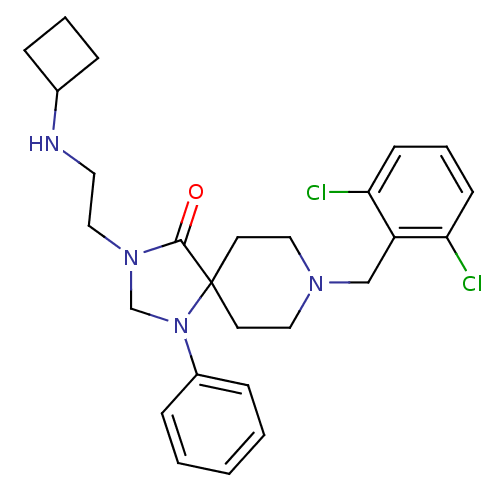 (3-[2-(cyclobutylamino)ethyl]-8-[(2,6-dichloropheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26942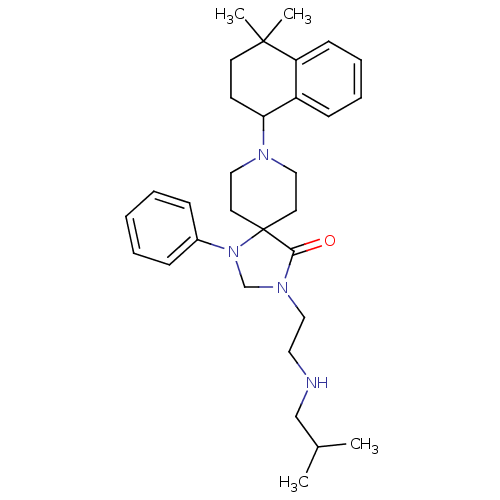 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26923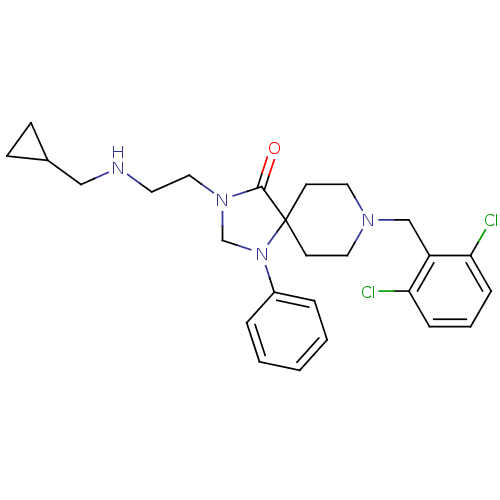 (3-{2-[(cyclopropylmethyl)amino]ethyl}-8-[(2,6-dich...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26954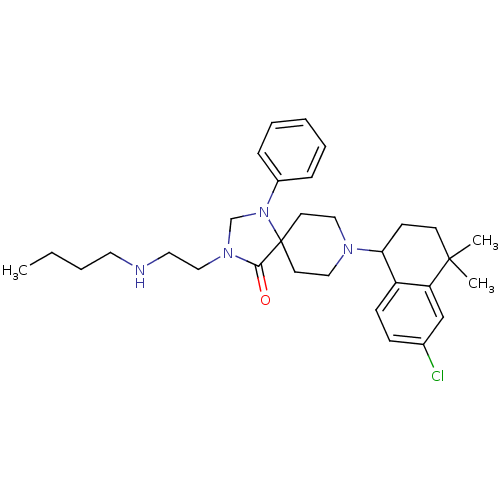 (3-[2-(butylamino)ethyl]-8-(6-chloro-4,4-dimethyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26925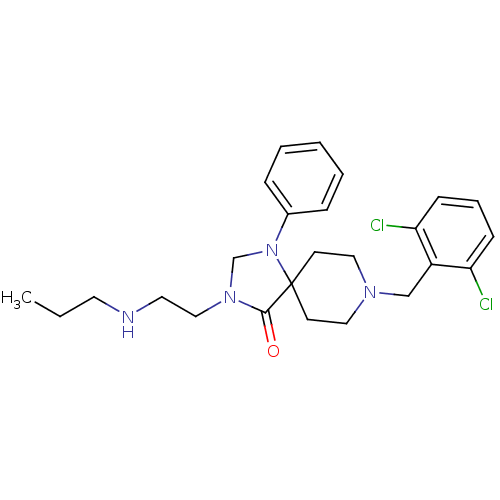 (8-[(2,6-dichlorophenyl)methyl]-1-phenyl-3-[2-(prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202779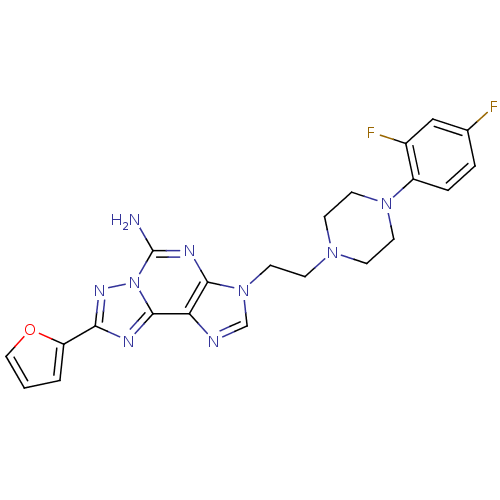 (3-(2-(4-(2,4-difluorophenyl)piperazin-1-yl)ethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202790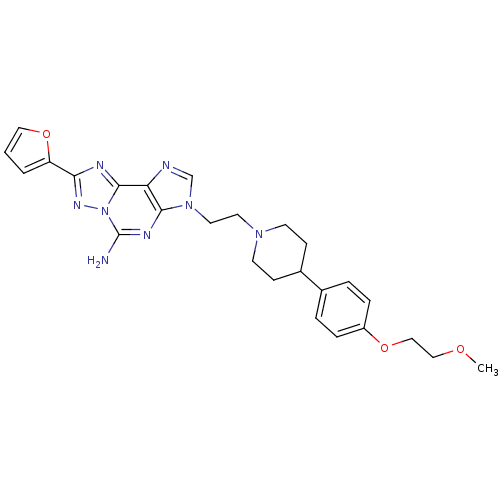 (8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26950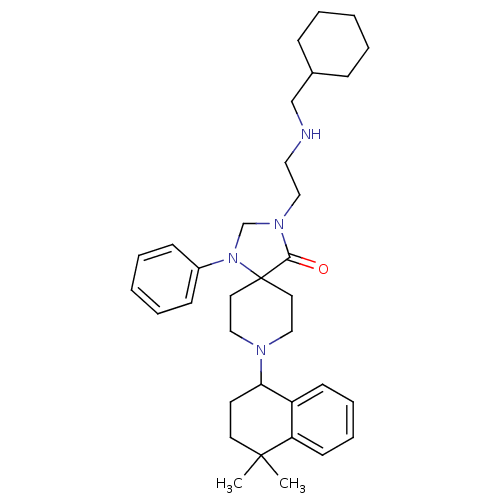 (3-{2-[(cyclohexylmethyl)amino]ethyl}-8-(4,4-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26922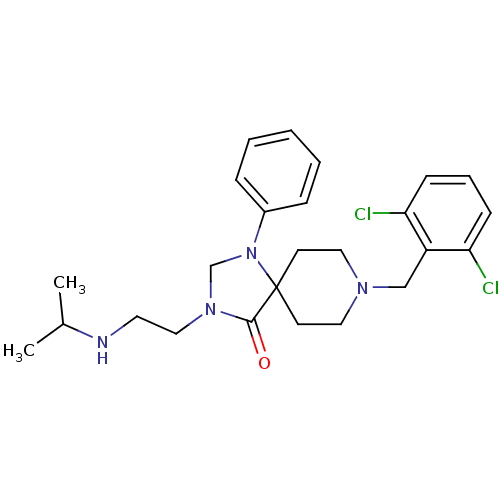 (8-[(2,6-dichlorophenyl)methyl]-1-phenyl-3-[2-(prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26921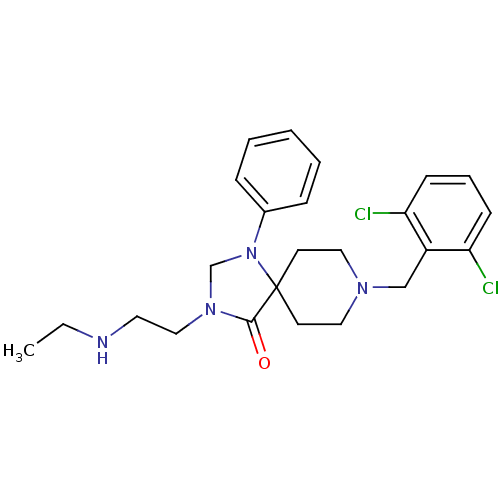 (8-[(2,6-dichlorophenyl)methyl]-3-[2-(ethylamino)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26920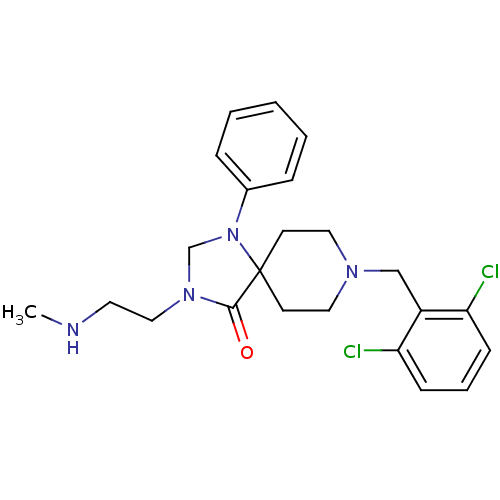 (8-[(2,6-dichlorophenyl)methyl]-3-[2-(methylamino)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26941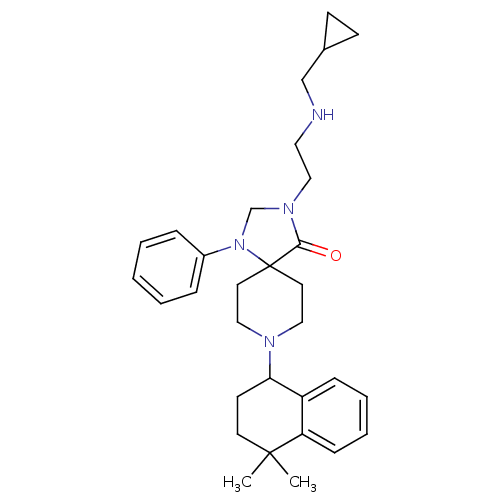 (3-{2-[(cyclopropylmethyl)amino]ethyl}-8-(4,4-dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50204769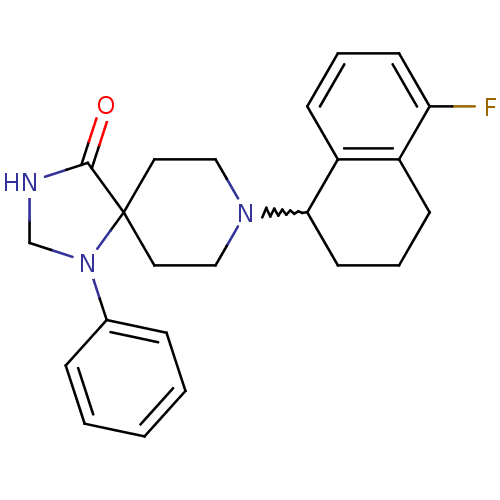 (8-(5-fluoro-1,2,3,4-tetrahydro-naphthalen-1-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 2281-4 (2007) Article DOI: 10.1016/j.bmcl.2007.01.069 BindingDB Entry DOI: 10.7270/Q2T43SRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202769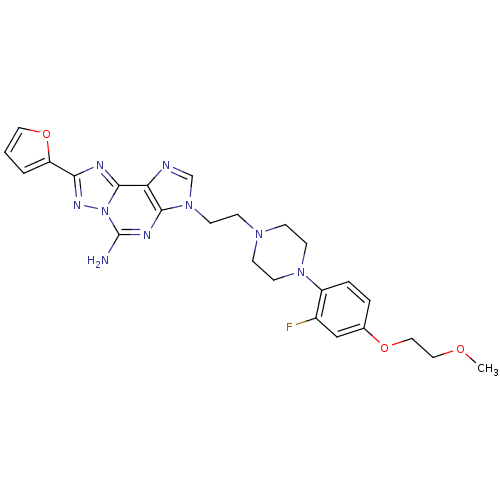 (3-(2-(4-(2-fluoro-4-(2-methoxyethoxy)phenyl)pipera...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50051750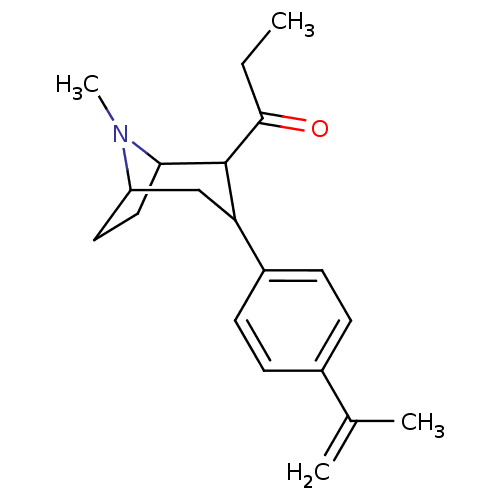 (1-[3-(4-Isopropenyl-phenyl)-8-methyl-8-aza-bicyclo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-paroxetine from Serotonin transporter of rat frontal cortex membrane | J Med Chem 39: 2554-8 (1996) Article DOI: 10.1021/jm9600508 BindingDB Entry DOI: 10.7270/Q2V69HP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202773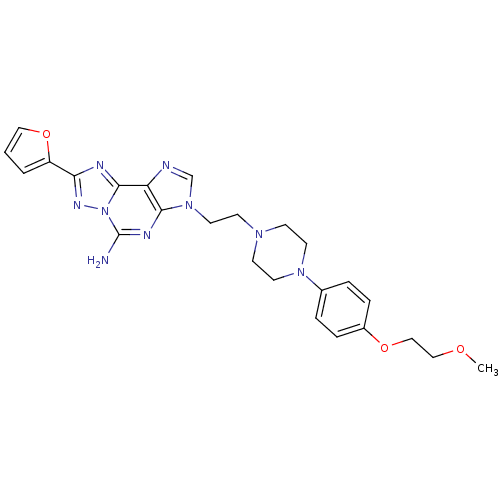 (8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50204768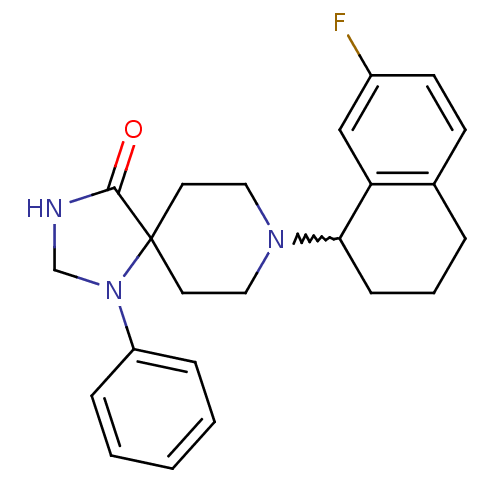 (8-(7-fluoro-1,2,3,4-tetrahydro-naphthalen-1-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 2281-4 (2007) Article DOI: 10.1016/j.bmcl.2007.01.069 BindingDB Entry DOI: 10.7270/Q2T43SRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26936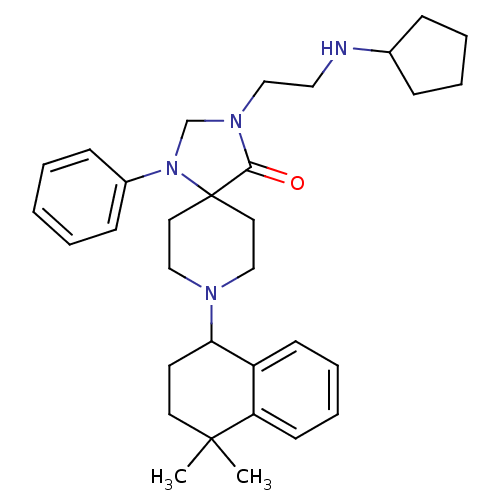 (3-[2-(cyclopentylamino)ethyl]-8-(4,4-dimethyl-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26937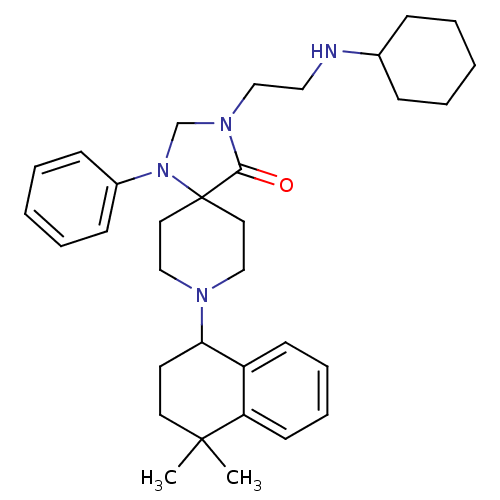 (3-[2-(cyclohexylamino)ethyl]-8-(4,4-dimethyl-1,2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26939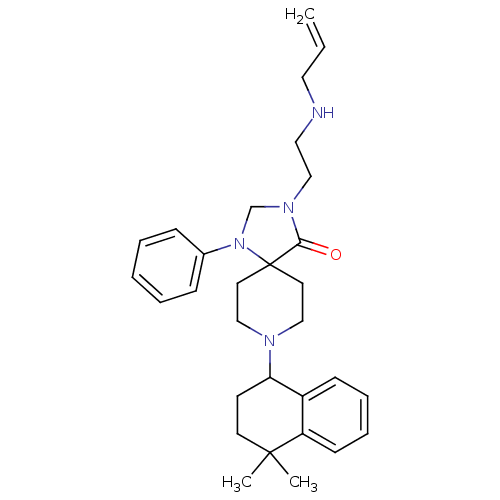 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202783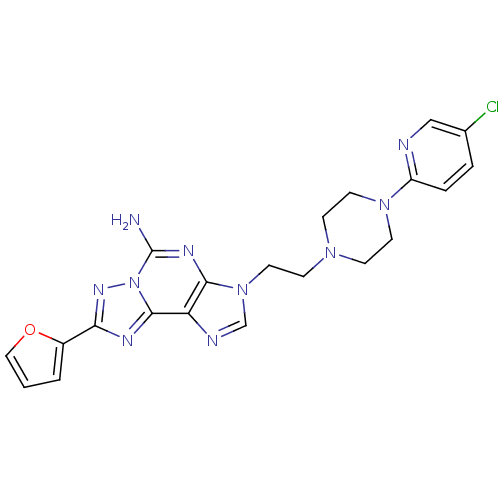 (3-(2-(4-(5-chloropyridin-2-yl)piperazin-1-yl)ethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202766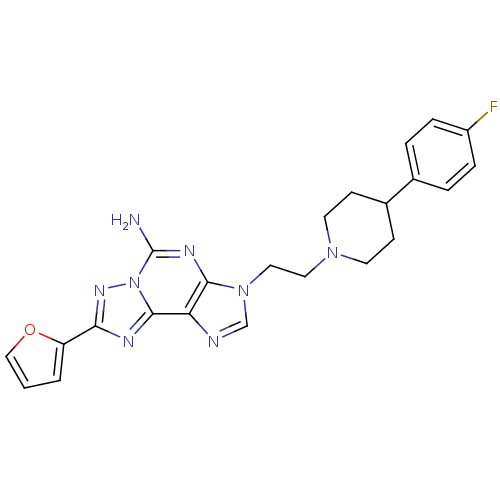 (3-(2-(4-(4-fluorophenyl)piperidin-1-yl)ethyl)-8-(f...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202767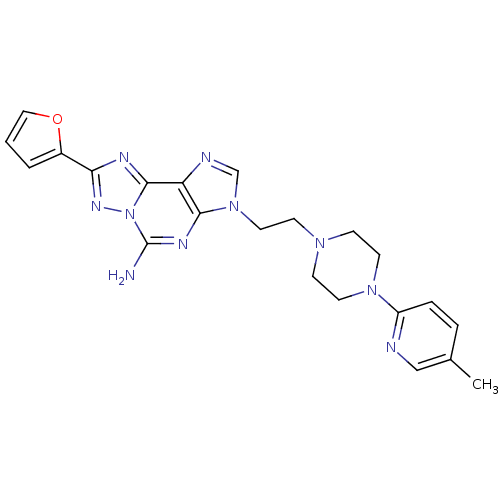 (8-(furan-2-yl)-3-(2-(4-(5-methylpyridin-2-yl)piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26938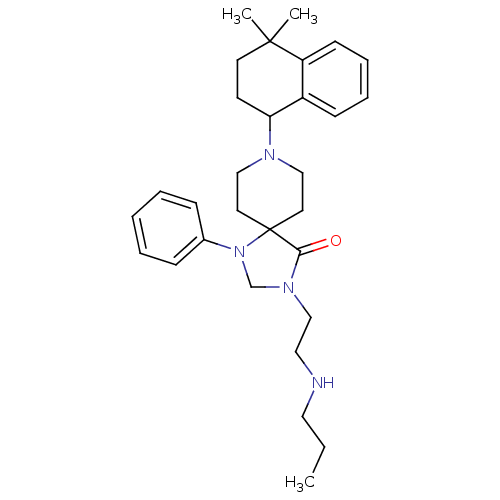 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26928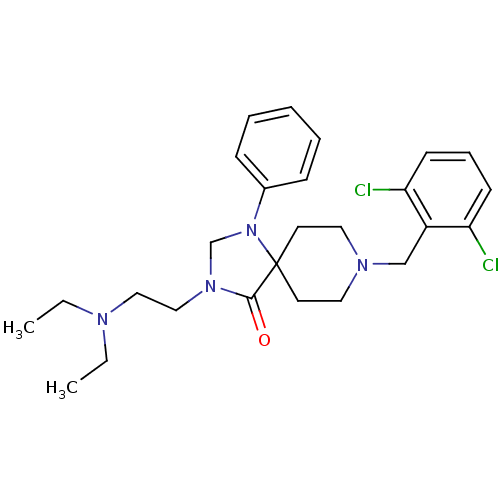 (8-[(2,6-dichlorophenyl)methyl]-3-[2-(diethylamino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50204783 (1-phenyl-8-(1-phenyl-pentyl)-1,3,8-triaza-spiro[4....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 2281-4 (2007) Article DOI: 10.1016/j.bmcl.2007.01.069 BindingDB Entry DOI: 10.7270/Q2T43SRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50041304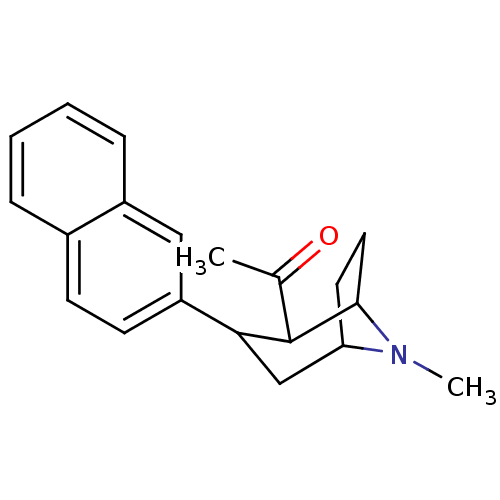 (1-(8-Methyl-3-naphthalen-2-yl-8-aza-bicyclo[3.2.1]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University Curated by ChEMBL | Assay Description Inhibition of [3H]paroxetine binding to serotonin transport sites in rat frontal cortex membranes. | J Med Chem 37: 1262-8 (1994) BindingDB Entry DOI: 10.7270/Q2TH8NBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50169328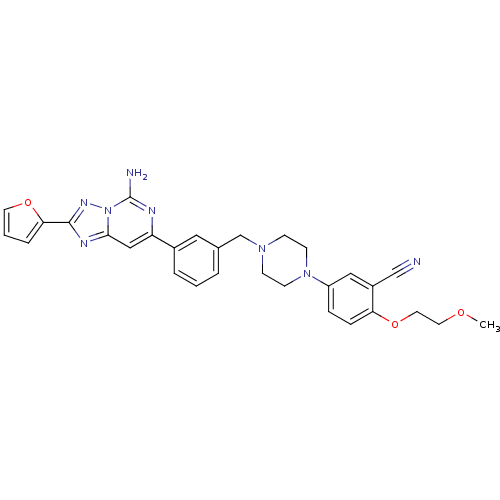 (5-{4-[3-(5-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-SCH-58,261 from A2A adenosine receptor in Rat | Bioorg Med Chem Lett 15: 3670-4 (2005) Article DOI: 10.1016/j.bmcl.2005.05.086 BindingDB Entry DOI: 10.7270/Q279446R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087008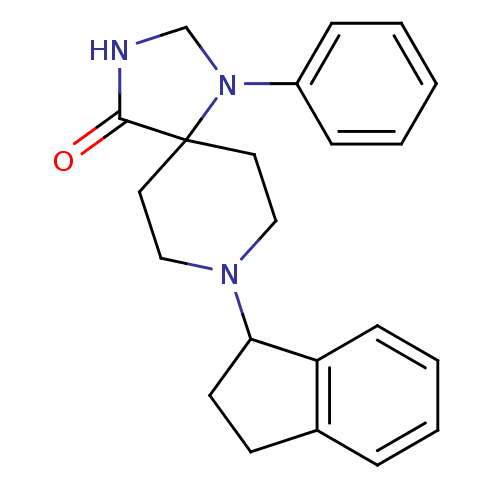 (8-indan-1-yl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 2281-4 (2007) Article DOI: 10.1016/j.bmcl.2007.01.069 BindingDB Entry DOI: 10.7270/Q2T43SRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26947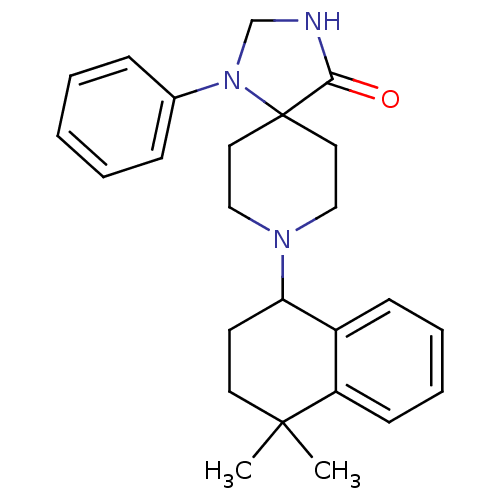 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26947 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 2281-4 (2007) Article DOI: 10.1016/j.bmcl.2007.01.069 BindingDB Entry DOI: 10.7270/Q2T43SRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202789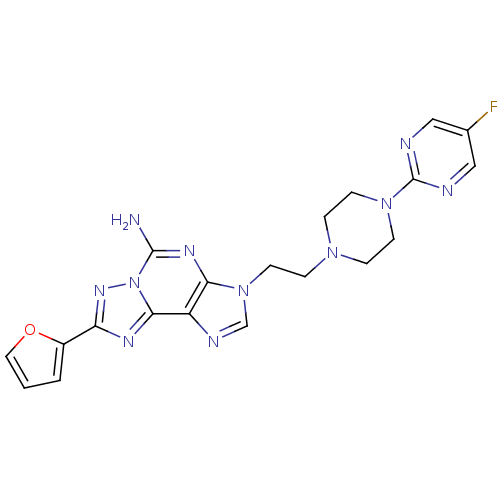 (3-(2-(4-(5-fluoropyrimidin-2-yl)piperazin-1-yl)eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26935 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087015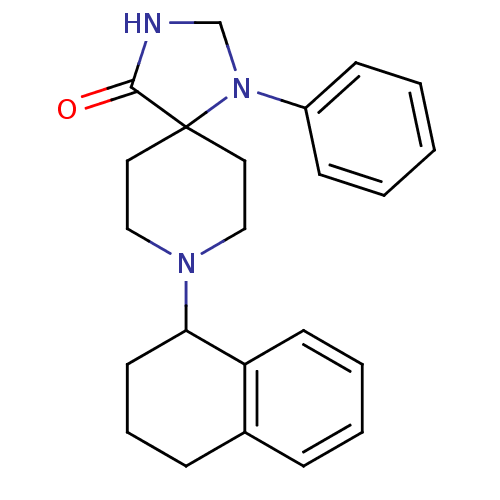 (1-Phenyl-8-(1,2,3,4-tetrahydro-naphthalen-1-yl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 2281-4 (2007) Article DOI: 10.1016/j.bmcl.2007.01.069 BindingDB Entry DOI: 10.7270/Q2T43SRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26944 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1340 total ) | Next | Last >> |