 Found 2984 hits with Last Name = 'may' and Initial = 'p'
Found 2984 hits with Last Name = 'may' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165935
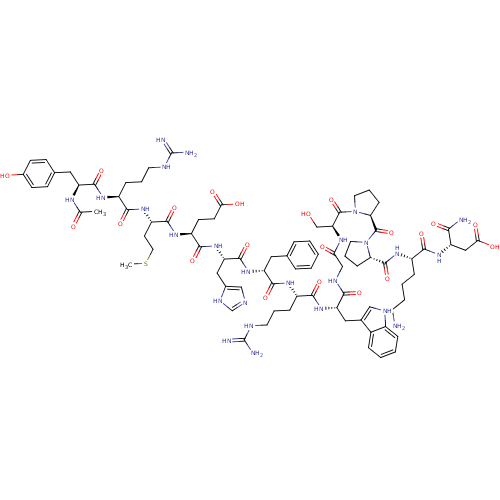
(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Rattus norvegicus) | BDBM50165931
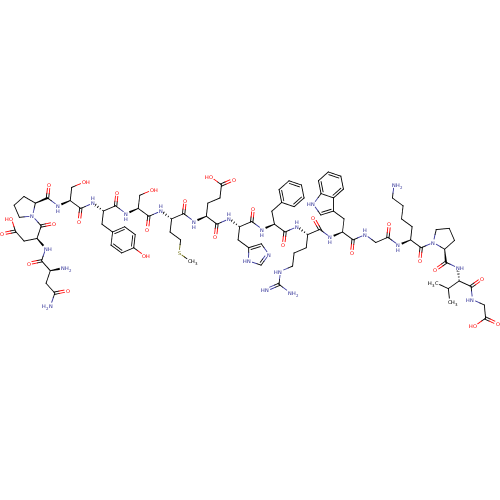
(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat melanocortin-4 receptor |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50165935

(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-3 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165929
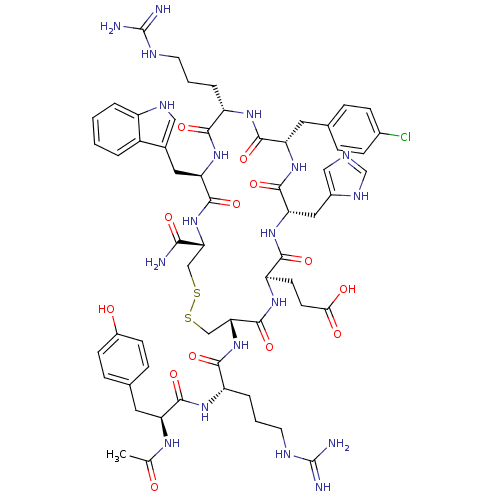
(Ac-YR[CEH(pCl-dF)RWC]-NH2 | CHEMBL415661)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C60H78ClN19O13S2/c1-31(81)72-43(22-33-12-16-37(82)17-13-33)54(89)73-41(9-5-21-69-60(65)66)52(87)80-48-29-95-94-28-47(50(62)85)79-56(91)45(24-34-26-70-39-7-3-2-6-38(34)39)77-51(86)40(8-4-20-68-59(63)64)74-55(90)44(23-32-10-14-35(61)15-11-32)76-57(92)46(25-36-27-67-30-71-36)78-53(88)42(75-58(48)93)18-19-49(83)84/h2-3,6-7,10-17,26-27,30,40-48,70,82H,4-5,8-9,18-25,28-29H2,1H3,(H2,62,85)(H,67,71)(H,72,81)(H,73,89)(H,74,90)(H,75,93)(H,76,92)(H,77,86)(H,78,88)(H,79,91)(H,80,87)(H,83,84)(H4,63,64,68)(H4,65,66,69)/t40-,41-,42+,43-,44-,45+,46-,47+,48+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-5 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165932
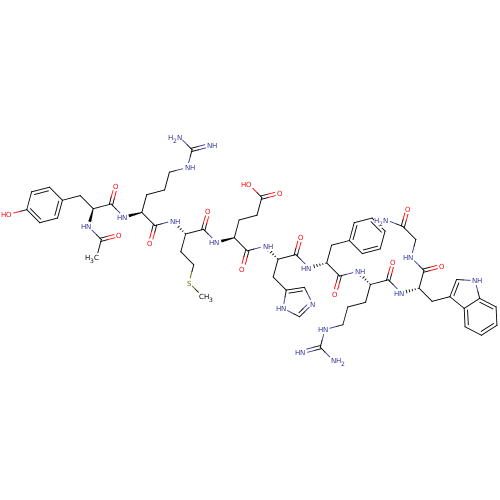
(Ac-YRMEHdFRWG-NH2 | CHEMBL266879)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(N)=O Show InChI InChI=1S/C61H83N19O13S/c1-34(81)73-46(27-36-16-18-39(82)19-17-36)57(91)74-42(14-8-23-68-60(63)64)53(87)77-45(22-25-94-2)56(90)76-44(20-21-51(84)85)55(89)80-49(29-38-31-67-33-72-38)59(93)78-47(26-35-10-4-3-5-11-35)58(92)75-43(15-9-24-69-61(65)66)54(88)79-48(52(86)71-32-50(62)83)28-37-30-70-41-13-7-6-12-40(37)41/h3-7,10-13,16-19,30-31,33,42-49,70,82H,8-9,14-15,20-29,32H2,1-2H3,(H2,62,83)(H,67,72)(H,71,86)(H,73,81)(H,74,91)(H,75,92)(H,76,90)(H,77,87)(H,78,93)(H,79,88)(H,80,89)(H,84,85)(H4,63,64,68)(H4,65,66,69)/t42-,43-,44-,45-,46-,47+,48-,49-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-3 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50165935

(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-5 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165935

(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165927
(Ac-YR[CEH(pF-dF)RWC]-NH2 | CHEMBL407809)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C60H78FN19O13S2/c1-31(81)72-43(22-33-12-16-37(82)17-13-33)54(89)73-41(9-5-21-69-60(65)66)52(87)80-48-29-95-94-28-47(50(62)85)79-56(91)45(24-34-26-70-39-7-3-2-6-38(34)39)77-51(86)40(8-4-20-68-59(63)64)74-55(90)44(23-32-10-14-35(61)15-11-32)76-57(92)46(25-36-27-67-30-71-36)78-53(88)42(75-58(48)93)18-19-49(83)84/h2-3,6-7,10-17,26-27,30,40-48,70,82H,4-5,8-9,18-25,28-29H2,1H3,(H2,62,85)(H,67,71)(H,72,81)(H,73,89)(H,74,90)(H,75,93)(H,76,92)(H,77,86)(H,78,88)(H,79,91)(H,80,87)(H,83,84)(H4,63,64,68)(H4,65,66,69)/t40-,41-,42+,43-,44-,45+,46-,47+,48+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165924

(Ac-YR[CEH(d-2alpha-Nal)RWC]-NH2 | CHEMBL412523)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C64H81N19O13S2/c1-34(84)75-47(25-35-15-18-41(85)19-16-35)58(92)76-45(13-7-23-72-64(68)69)56(90)83-52-32-98-97-31-51(54(65)88)82-60(94)49(27-39-29-73-43-11-5-4-10-42(39)43)80-55(89)44(12-6-22-71-63(66)67)77-59(93)48(26-36-14-17-37-8-2-3-9-38(37)24-36)79-61(95)50(28-40-30-70-33-74-40)81-57(91)46(78-62(52)96)20-21-53(86)87/h2-5,8-11,14-19,24,29-30,33,44-52,73,85H,6-7,12-13,20-23,25-28,31-32H2,1H3,(H2,65,88)(H,70,74)(H,75,84)(H,76,92)(H,77,93)(H,78,96)(H,79,95)(H,80,89)(H,81,91)(H,82,94)(H,83,90)(H,86,87)(H4,66,67,71)(H4,68,69,72)/t44-,45-,46+,47-,48-,49+,50-,51+,52+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Rattus norvegicus) | BDBM50165936
(Ac-dR[CEHdFRWC]-NH2 | CHEMBL267900)Show SMILES CC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C51H70N18O11S2/c1-27(70)62-33(13-7-17-58-50(53)54)43(74)69-40-25-82-81-24-39(42(52)73)68-47(78)37(20-29-22-60-32-12-6-5-11-31(29)32)66-44(75)34(14-8-18-59-51(55)56)63-46(77)36(19-28-9-3-2-4-10-28)65-48(79)38(21-30-23-57-26-61-30)67-45(76)35(64-49(40)80)15-16-41(71)72/h2-6,9-12,22-23,26,33-40,60H,7-8,13-21,24-25H2,1H3,(H2,52,73)(H,57,61)(H,62,70)(H,63,77)(H,64,80)(H,65,79)(H,66,75)(H,67,76)(H,68,78)(H,69,74)(H,71,72)(H4,53,54,58)(H4,55,56,59)/t33-,34+,35-,36+,37-,38+,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat melanocortin-4 receptor |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165941

(Ac-R[CEHdFRWC]-NH2 | CHEMBL408257)Show SMILES CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C51H70N18O11S2/c1-27(70)62-33(13-7-17-58-50(53)54)43(74)69-40-25-82-81-24-39(42(52)73)68-47(78)37(20-29-22-60-32-12-6-5-11-31(29)32)66-44(75)34(14-8-18-59-51(55)56)63-46(77)36(19-28-9-3-2-4-10-28)65-48(79)38(21-30-23-57-26-61-30)67-45(76)35(64-49(40)80)15-16-41(71)72/h2-6,9-12,22-23,26,33-40,60H,7-8,13-21,24-25H2,1H3,(H2,52,73)(H,57,61)(H,62,70)(H,63,77)(H,64,80)(H,65,79)(H,66,75)(H,67,76)(H,68,78)(H,69,74)(H,71,72)(H4,53,54,58)(H4,55,56,59)/t33-,34-,35+,36-,37+,38-,39+,40+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165933
(CHEMBL428326 | GPYRMEHFRWGSPPKD-NH2)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)CN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C89H127N27O22S/c1-139-37-30-60(107-76(127)57(19-9-32-98-88(93)94)104-82(133)63(39-50-24-26-53(118)27-25-50)113-84(135)67-21-11-34-114(67)71(120)43-91)80(131)106-59(28-29-72(121)122)79(130)112-65(41-52-45-97-48-102-52)83(134)110-62(38-49-14-3-2-4-15-49)81(132)105-58(20-10-33-99-89(95)96)78(129)111-64(40-51-44-100-55-17-6-5-16-54(51)55)75(126)101-46-70(119)103-66(47-117)86(137)116-36-13-23-69(116)87(138)115-35-12-22-68(115)85(136)108-56(18-7-8-31-90)77(128)109-61(74(92)125)42-73(123)124/h2-6,14-17,24-27,44-45,48,56-69,100,117-118H,7-13,18-23,28-43,46-47,90-91H2,1H3,(H2,92,125)(H,97,102)(H,101,126)(H,103,119)(H,104,133)(H,105,132)(H,106,131)(H,107,127)(H,108,136)(H,109,128)(H,110,134)(H,111,129)(H,112,130)(H,113,135)(H,121,122)(H,123,124)(H4,93,94,98)(H4,95,96,99)/t56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165926

(Ac-YR[CEHdFRWC]SPPKD-NH2 | CHEMBL2373515)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O |wU:103.110,32.96,27.27,4.3,72.76,114.121,61.64,50.53,wD:110.118,94.99,16.16,123.130,36.37,82.87,(-1.2,-14.32,;-.65,-12.88,;.87,-12.64,;-1.63,-11.7,;-1.07,-10.25,;-2.62,-10.24,;-3.96,-9.47,;-5.2,-10.39,;-6.61,-9.78,;-6.79,-8.24,;-8.2,-7.63,;-5.55,-7.32,;-4.14,-7.93,;.45,-10.01,;1.41,-11.19,;.99,-8.56,;.02,-7.37,;1.51,-6.97,;3.03,-6.87,;3.9,-8.14,;5.44,-8.04,;6.29,-9.31,;7.83,-9.22,;5.61,-10.7,;-1.49,-7.62,;-2.04,-9.06,;-2.48,-6.43,;-1.91,-4.99,;-.42,-5.33,;1.11,-5.27,;2.58,-4.78,;3.85,-3.92,;4.85,-2.75,;5.49,-1.36,;5.74,.16,;7.28,.13,;5.56,1.69,;7.06,2.08,;8.14,3.18,;9.06,4.42,;10.51,3.93,;10.5,2.38,;11.63,1.35,;11.28,-.15,;9.82,-.62,;8.7,.44,;9.03,1.94,;4.98,3.11,;4.04,4.33,;5.12,5.43,;2.81,5.24,;3.54,6.6,;5.09,6.63,;5.82,7.98,;7.36,8,;8.1,9.36,;9.64,9.4,;7.3,10.67,;1.37,5.79,;-.16,5.95,;-.23,7.47,;-1.68,5.67,;-2.16,7.12,;-1.14,8.26,;.38,7.93,;1.39,9.08,;.91,10.56,;-.6,10.86,;-1.62,9.71,;-3.06,4.97,;-4.21,3.95,;-5.38,4.96,;-5.04,2.66,;-6.44,3.31,;-6.58,4.84,;-5.42,5.85,;-6.02,7.26,;-7.56,7.12,;-7.89,5.62,;-5.48,1.19,;-5.52,-.35,;-7.05,-.52,;-5.13,-1.84,;-6.56,-2.42,;-7.8,-1.48,;-9.21,-2.06,;-10.42,-1.11,;-9.42,-3.59,;-4.38,-3.17,;-3.27,-4.25,;-4.19,-5.49,;6.15,-3.59,;6.08,-5.12,;7.51,-2.88,;8.81,-3.7,;8.73,-5.24,;10.04,-6.07,;10.17,-3,;10.25,-1.46,;11.47,-3.71,;10.78,-2.02,;11.57,-.8,;12.9,-1.58,;12.83,-3.12,;14.14,-3.95,;14.07,-5.49,;15.34,-3.36,;14.94,-4.86,;15.22,-6.01,;16.72,-5.61,;16.79,-4.06,;18.17,-3.36,;18.22,-1.83,;19.46,-4.2,;20.82,-3.48,;20.9,-1.95,;22.25,-1.24,;22.33,.3,;23.7,1,;23.77,2.55,;22.11,-4.31,;22.04,-5.85,;23.49,-3.61,;24.79,-4.43,;24.71,-5.98,;26.01,-6.8,;27.51,-7.2,;25.94,-8.33,;26.15,-3.73,;27.45,-4.56,;26.22,-2.19,)| Show InChI InChI=1S/C83H115N25O21S2/c1-44(110)95-57(34-46-22-24-49(111)25-23-46)73(121)96-54(19-10-30-92-83(88)89)71(119)105-62-41-130-131-42-63(78(126)104-61(40-109)80(128)108-32-12-21-65(108)81(129)107-31-11-20-64(107)79(127)99-52(17-7-8-28-84)69(117)100-56(68(85)116)37-67(114)115)106-75(123)59(35-47-38-93-51-16-6-5-15-50(47)51)102-70(118)53(18-9-29-91-82(86)87)97-74(122)58(33-45-13-3-2-4-14-45)101-76(124)60(36-48-39-90-43-94-48)103-72(120)55(98-77(62)125)26-27-66(112)113/h2-6,13-16,22-25,38-39,43,52-65,93,109,111H,7-12,17-21,26-37,40-42,84H2,1H3,(H2,85,116)(H,90,94)(H,95,110)(H,96,121)(H,97,122)(H,98,125)(H,99,127)(H,100,117)(H,101,124)(H,102,118)(H,103,120)(H,104,126)(H,105,119)(H,106,123)(H,112,113)(H,114,115)(H4,86,87,91)(H4,88,89,92)/t52-,53-,54?,55+,56-,57-,58-,59+,60-,61-,62+,63+,64-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Rattus norvegicus) | BDBM50165930
(Ac-YR[CEHdFRWC]-NH2 | CHEMBL264352)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C60H79N19O13S2/c1-32(80)71-43(24-34-15-17-37(81)18-16-34)54(88)72-41(14-8-22-68-60(64)65)52(86)79-48-30-94-93-29-47(50(61)84)78-56(90)45(25-35-27-69-39-12-6-5-11-38(35)39)76-51(85)40(13-7-21-67-59(62)63)73-55(89)44(23-33-9-3-2-4-10-33)75-57(91)46(26-36-28-66-31-70-36)77-53(87)42(74-58(48)92)19-20-49(82)83/h2-6,9-12,15-18,27-28,31,40-48,69,81H,7-8,13-14,19-26,29-30H2,1H3,(H2,61,84)(H,66,70)(H,71,80)(H,72,88)(H,73,89)(H,74,92)(H,75,91)(H,76,85)(H,77,87)(H,78,90)(H,79,86)(H,82,83)(H4,62,63,67)(H4,64,65,68)/t40-,41-,42+,43-,44-,45+,46-,47+,48+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat melanocortin-4 receptor |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165932

(Ac-YRMEHdFRWG-NH2 | CHEMBL266879)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(N)=O Show InChI InChI=1S/C61H83N19O13S/c1-34(81)73-46(27-36-16-18-39(82)19-17-36)57(91)74-42(14-8-23-68-60(63)64)53(87)77-45(22-25-94-2)56(90)76-44(20-21-51(84)85)55(89)80-49(29-38-31-67-33-72-38)59(93)78-47(26-35-10-4-3-5-11-35)58(92)75-43(15-9-24-69-61(65)66)54(88)79-48(52(86)71-32-50(62)83)28-37-30-70-41-13-7-6-12-40(37)41/h3-7,10-13,16-19,30-31,33,42-49,70,82H,8-9,14-15,20-29,32H2,1-2H3,(H2,62,83)(H,67,72)(H,71,86)(H,73,81)(H,74,91)(H,75,92)(H,76,90)(H,77,87)(H,78,93)(H,79,88)(H,80,89)(H,84,85)(H4,63,64,68)(H4,65,66,69)/t42-,43-,44-,45-,46-,47+,48-,49-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165936

(Ac-dR[CEHdFRWC]-NH2 | CHEMBL267900)Show SMILES CC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C51H70N18O11S2/c1-27(70)62-33(13-7-17-58-50(53)54)43(74)69-40-25-82-81-24-39(42(52)73)68-47(78)37(20-29-22-60-32-12-6-5-11-31(29)32)66-44(75)34(14-8-18-59-51(55)56)63-46(77)36(19-28-9-3-2-4-10-28)65-48(79)38(21-30-23-57-26-61-30)67-45(76)35(64-49(40)80)15-16-41(71)72/h2-6,9-12,22-23,26,33-40,60H,7-8,13-21,24-25H2,1H3,(H2,52,73)(H,57,61)(H,62,70)(H,63,77)(H,64,80)(H,65,79)(H,66,75)(H,67,76)(H,68,78)(H,69,74)(H,71,72)(H4,53,54,58)(H4,55,56,59)/t33-,34+,35-,36+,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165926

(Ac-YR[CEHdFRWC]SPPKD-NH2 | CHEMBL2373515)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O |wU:103.110,32.96,27.27,4.3,72.76,114.121,61.64,50.53,wD:110.118,94.99,16.16,123.130,36.37,82.87,(-1.2,-14.32,;-.65,-12.88,;.87,-12.64,;-1.63,-11.7,;-1.07,-10.25,;-2.62,-10.24,;-3.96,-9.47,;-5.2,-10.39,;-6.61,-9.78,;-6.79,-8.24,;-8.2,-7.63,;-5.55,-7.32,;-4.14,-7.93,;.45,-10.01,;1.41,-11.19,;.99,-8.56,;.02,-7.37,;1.51,-6.97,;3.03,-6.87,;3.9,-8.14,;5.44,-8.04,;6.29,-9.31,;7.83,-9.22,;5.61,-10.7,;-1.49,-7.62,;-2.04,-9.06,;-2.48,-6.43,;-1.91,-4.99,;-.42,-5.33,;1.11,-5.27,;2.58,-4.78,;3.85,-3.92,;4.85,-2.75,;5.49,-1.36,;5.74,.16,;7.28,.13,;5.56,1.69,;7.06,2.08,;8.14,3.18,;9.06,4.42,;10.51,3.93,;10.5,2.38,;11.63,1.35,;11.28,-.15,;9.82,-.62,;8.7,.44,;9.03,1.94,;4.98,3.11,;4.04,4.33,;5.12,5.43,;2.81,5.24,;3.54,6.6,;5.09,6.63,;5.82,7.98,;7.36,8,;8.1,9.36,;9.64,9.4,;7.3,10.67,;1.37,5.79,;-.16,5.95,;-.23,7.47,;-1.68,5.67,;-2.16,7.12,;-1.14,8.26,;.38,7.93,;1.39,9.08,;.91,10.56,;-.6,10.86,;-1.62,9.71,;-3.06,4.97,;-4.21,3.95,;-5.38,4.96,;-5.04,2.66,;-6.44,3.31,;-6.58,4.84,;-5.42,5.85,;-6.02,7.26,;-7.56,7.12,;-7.89,5.62,;-5.48,1.19,;-5.52,-.35,;-7.05,-.52,;-5.13,-1.84,;-6.56,-2.42,;-7.8,-1.48,;-9.21,-2.06,;-10.42,-1.11,;-9.42,-3.59,;-4.38,-3.17,;-3.27,-4.25,;-4.19,-5.49,;6.15,-3.59,;6.08,-5.12,;7.51,-2.88,;8.81,-3.7,;8.73,-5.24,;10.04,-6.07,;10.17,-3,;10.25,-1.46,;11.47,-3.71,;10.78,-2.02,;11.57,-.8,;12.9,-1.58,;12.83,-3.12,;14.14,-3.95,;14.07,-5.49,;15.34,-3.36,;14.94,-4.86,;15.22,-6.01,;16.72,-5.61,;16.79,-4.06,;18.17,-3.36,;18.22,-1.83,;19.46,-4.2,;20.82,-3.48,;20.9,-1.95,;22.25,-1.24,;22.33,.3,;23.7,1,;23.77,2.55,;22.11,-4.31,;22.04,-5.85,;23.49,-3.61,;24.79,-4.43,;24.71,-5.98,;26.01,-6.8,;27.51,-7.2,;25.94,-8.33,;26.15,-3.73,;27.45,-4.56,;26.22,-2.19,)| Show InChI InChI=1S/C83H115N25O21S2/c1-44(110)95-57(34-46-22-24-49(111)25-23-46)73(121)96-54(19-10-30-92-83(88)89)71(119)105-62-41-130-131-42-63(78(126)104-61(40-109)80(128)108-32-12-21-65(108)81(129)107-31-11-20-64(107)79(127)99-52(17-7-8-28-84)69(117)100-56(68(85)116)37-67(114)115)106-75(123)59(35-47-38-93-51-16-6-5-15-50(47)51)102-70(118)53(18-9-29-91-82(86)87)97-74(122)58(33-45-13-3-2-4-14-45)101-76(124)60(36-48-39-90-43-94-48)103-72(120)55(98-77(62)125)26-27-66(112)113/h2-6,13-16,22-25,38-39,43,52-65,93,109,111H,7-12,17-21,26-37,40-42,84H2,1H3,(H2,85,116)(H,90,94)(H,95,110)(H,96,121)(H,97,122)(H,98,125)(H,99,127)(H,100,117)(H,101,124)(H,102,118)(H,103,120)(H,104,126)(H,105,119)(H,106,123)(H,112,113)(H,114,115)(H4,86,87,91)(H4,88,89,92)/t52-,53-,54?,55+,56-,57-,58-,59+,60-,61-,62+,63+,64-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038757
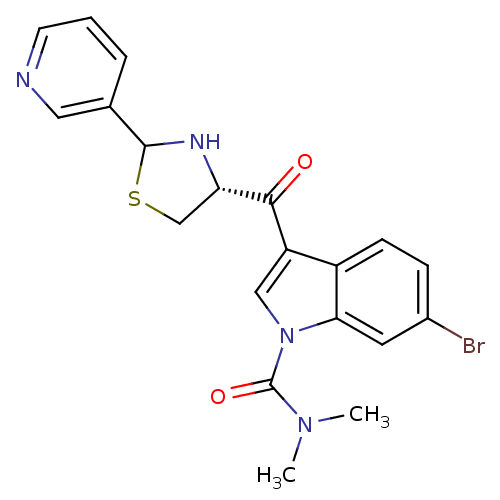
(6-Bromo-3-((R)-2-pyridin-3-yl-thiazolidine-4-carbo...)Show SMILES CN(C)C(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccc(Br)cc12 Show InChI InChI=1S/C20H19BrN4O2S/c1-24(2)20(27)25-10-15(14-6-5-13(21)8-17(14)25)18(26)16-11-28-19(23-16)12-4-3-7-22-9-12/h3-10,16,19,23H,11H2,1-2H3/t16-,19?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165930

(Ac-YR[CEHdFRWC]-NH2 | CHEMBL264352)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C60H79N19O13S2/c1-32(80)71-43(24-34-15-17-37(81)18-16-34)54(88)72-41(14-8-22-68-60(64)65)52(86)79-48-30-94-93-29-47(50(61)84)78-56(90)45(25-35-27-69-39-12-6-5-11-38(35)39)76-51(85)40(13-7-21-67-59(62)63)73-55(89)44(23-33-9-3-2-4-10-33)75-57(91)46(26-36-28-66-31-70-36)77-53(87)42(74-58(48)92)19-20-49(82)83/h2-6,9-12,15-18,27-28,31,40-48,69,81H,7-8,13-14,19-26,29-30H2,1H3,(H2,61,84)(H,66,70)(H,71,80)(H,72,88)(H,73,89)(H,74,92)(H,75,91)(H,76,85)(H,77,87)(H,78,90)(H,79,86)(H,82,83)(H4,62,63,67)(H4,64,65,68)/t40-,41-,42+,43-,44-,45+,46-,47+,48+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038809
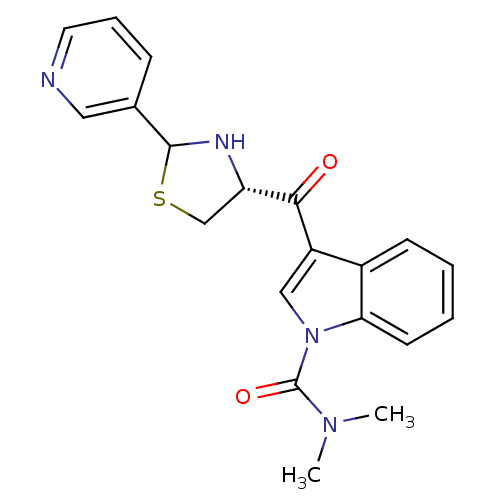
(3-((R)-2-Pyridin-3-yl-thiazolidine-4-carbonyl)-ind...)Show SMILES CN(C)C(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccccc12 Show InChI InChI=1S/C20H20N4O2S/c1-23(2)20(26)24-11-15(14-7-3-4-8-17(14)24)18(25)16-12-27-19(22-16)13-6-5-9-21-10-13/h3-11,16,19,22H,12H2,1-2H3/t16-,19?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50165932

(Ac-YRMEHdFRWG-NH2 | CHEMBL266879)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(N)=O Show InChI InChI=1S/C61H83N19O13S/c1-34(81)73-46(27-36-16-18-39(82)19-17-36)57(91)74-42(14-8-23-68-60(63)64)53(87)77-45(22-25-94-2)56(90)76-44(20-21-51(84)85)55(89)80-49(29-38-31-67-33-72-38)59(93)78-47(26-35-10-4-3-5-11-35)58(92)75-43(15-9-24-69-61(65)66)54(88)79-48(52(86)71-32-50(62)83)28-37-30-70-41-13-7-6-12-40(37)41/h3-7,10-13,16-19,30-31,33,42-49,70,82H,8-9,14-15,20-29,32H2,1-2H3,(H2,62,83)(H,67,72)(H,71,86)(H,73,81)(H,74,91)(H,75,92)(H,76,90)(H,77,87)(H,78,93)(H,79,88)(H,80,89)(H,84,85)(H4,63,64,68)(H4,65,66,69)/t42-,43-,44-,45-,46-,47+,48-,49-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-5 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165939

(AEKKDEGPYRMEHFRWGSPPKD | CHEMBL412536)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O Show InChI InChI=1S/C118H174N34O35S/c1-63(122)97(167)135-77(36-39-93(159)160)105(175)137-71(23-8-11-42-119)100(170)136-72(24-9-12-43-120)102(172)147-84(55-95(163)164)111(181)140-76(35-38-92(157)158)98(168)132-60-91(156)150-47-16-28-87(150)112(182)148-81(52-65-31-33-68(154)34-32-65)109(179)138-74(26-14-45-128-117(123)124)101(171)142-79(41-50-188-2)107(177)141-78(37-40-94(161)162)106(176)146-83(54-67-58-127-62-133-67)110(180)144-80(51-64-19-4-3-5-20-64)108(178)139-75(27-15-46-129-118(125)126)103(173)145-82(53-66-57-130-70-22-7-6-21-69(66)70)99(169)131-59-90(155)134-86(61-153)114(184)152-49-18-30-89(152)115(185)151-48-17-29-88(151)113(183)143-73(25-10-13-44-121)104(174)149-85(116(186)187)56-96(165)166/h3-7,19-22,31-34,57-58,62-63,71-89,130,153-154H,8-18,23-30,35-56,59-61,119-122H2,1-2H3,(H,127,133)(H,131,169)(H,132,168)(H,134,155)(H,135,167)(H,136,170)(H,137,175)(H,138,179)(H,139,178)(H,140,181)(H,141,177)(H,142,171)(H,143,183)(H,144,180)(H,145,173)(H,146,176)(H,147,172)(H,148,182)(H,149,174)(H,157,158)(H,159,160)(H,161,162)(H,163,164)(H,165,166)(H,186,187)(H4,123,124,128)(H4,125,126,129)/t63-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038792

(6-Benzyloxy-3-((R)-2-pyridin-3-yl-thiazolidine-4-c...)Show SMILES CC(C)(C)OC(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccc(OCc3ccccc3)cc12 Show InChI InChI=1S/C29H29N3O4S/c1-29(2,3)36-28(34)32-16-23(26(33)24-18-37-27(31-24)20-10-7-13-30-15-20)22-12-11-21(14-25(22)32)35-17-19-8-5-4-6-9-19/h4-16,24,27,31H,17-18H2,1-3H3/t24-,27?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038834
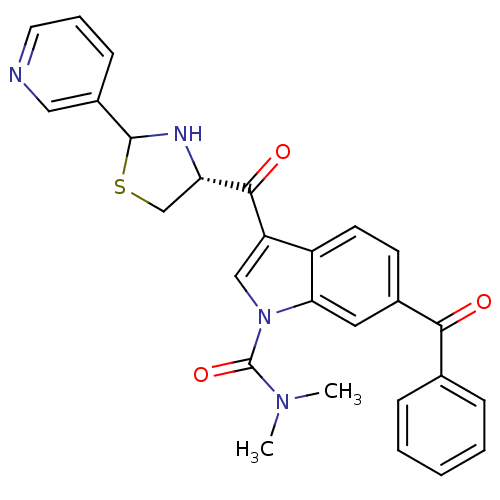
(6-Benzoyl-3-((R)-2-pyridin-3-yl-thiazolidine-4-car...)Show SMILES CN(C)C(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccc(cc12)C(=O)c1ccccc1 Show InChI InChI=1S/C27H24N4O3S/c1-30(2)27(34)31-15-21(25(33)22-16-35-26(29-22)19-9-6-12-28-14-19)20-11-10-18(13-23(20)31)24(32)17-7-4-3-5-8-17/h3-15,22,26,29H,16H2,1-2H3/t22-,26?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038750
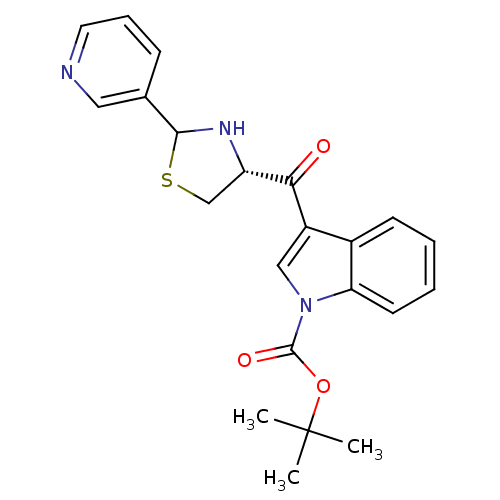
(3-((R)-2-Pyridin-3-yl-thiazolidine-4-carbonyl)-ind...)Show SMILES CC(C)(C)OC(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccccc12 Show InChI InChI=1S/C22H23N3O3S/c1-22(2,3)28-21(27)25-12-16(15-8-4-5-9-18(15)25)19(26)17-13-29-20(24-17)14-7-6-10-23-11-14/h4-12,17,20,24H,13H2,1-3H3/t17-,20?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038806
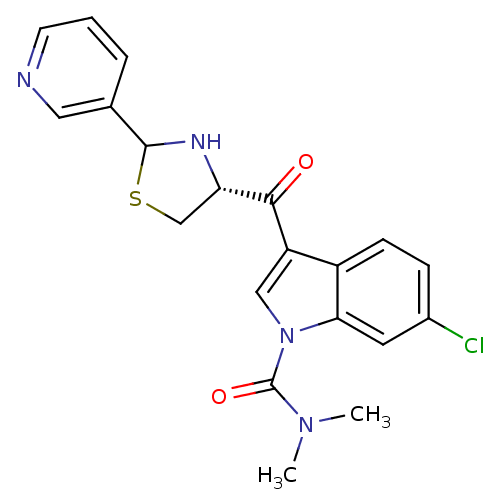
(6-Chloro-3-((R)-2-pyridin-3-yl-thiazolidine-4-carb...)Show SMILES CN(C)C(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccc(Cl)cc12 Show InChI InChI=1S/C20H19ClN4O2S/c1-24(2)20(27)25-10-15(14-6-5-13(21)8-17(14)25)18(26)16-11-28-19(23-16)12-4-3-7-22-9-12/h3-10,16,19,23H,11H2,1-2H3/t16-,19?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038777
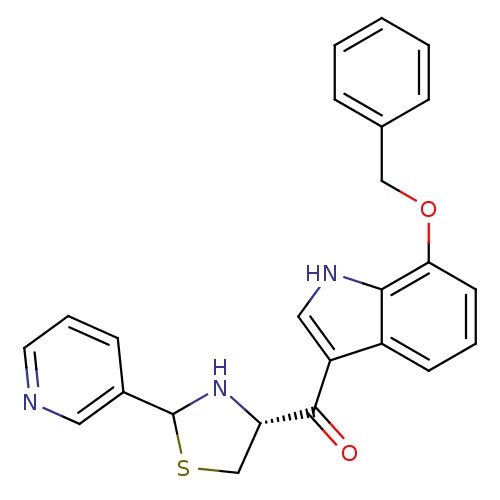
((7-Benzyloxy-1H-indol-3-yl)-((R)-2-pyridin-3-yl-th...)Show SMILES O=C([C@@H]1CSC(N1)c1cccnc1)c1c[nH]c2c(OCc3ccccc3)cccc12 Show InChI InChI=1S/C24H21N3O2S/c28-23(20-15-30-24(27-20)17-8-5-11-25-12-17)19-13-26-22-18(19)9-4-10-21(22)29-14-16-6-2-1-3-7-16/h1-13,20,24,26-27H,14-15H2/t20-,24?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038780
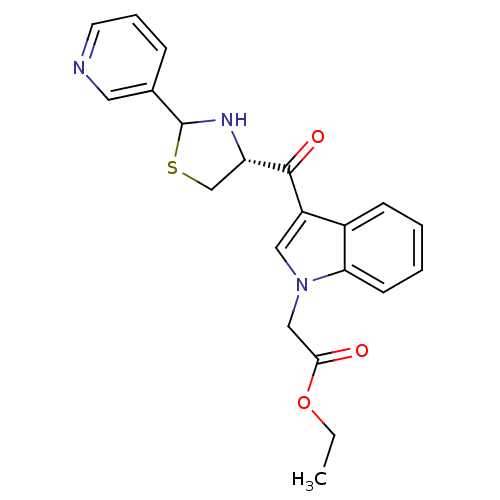
(CHEMBL64428 | [3-((R)-2-Pyridin-3-yl-thiazolidine-...)Show SMILES CCOC(=O)Cn1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccccc12 Show InChI InChI=1S/C21H21N3O3S/c1-2-27-19(25)12-24-11-16(15-7-3-4-8-18(15)24)20(26)17-13-28-21(23-17)14-6-5-9-22-10-14/h3-11,17,21,23H,2,12-13H2,1H3/t17-,21?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165937
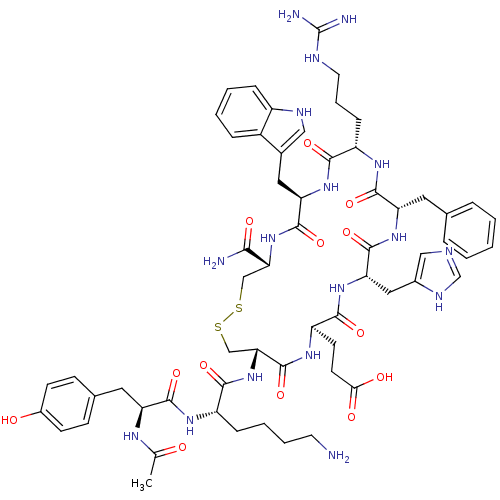
(Ac-YK[CEHdFRWC]-NH2 | CHEMBL412495)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C60H79N17O13S2/c1-33(78)69-44(25-35-16-18-38(79)19-17-35)55(86)70-41(14-7-8-22-61)53(84)77-49-31-92-91-30-48(51(62)82)76-57(88)46(26-36-28-67-40-13-6-5-12-39(36)40)74-52(83)42(15-9-23-66-60(63)64)71-56(87)45(24-34-10-3-2-4-11-34)73-58(89)47(27-37-29-65-32-68-37)75-54(85)43(72-59(49)90)20-21-50(80)81/h2-6,10-13,16-19,28-29,32,41-49,67,79H,7-9,14-15,20-27,30-31,61H2,1H3,(H2,62,82)(H,65,68)(H,69,78)(H,70,86)(H,71,87)(H,72,90)(H,73,89)(H,74,83)(H,75,85)(H,76,88)(H,77,84)(H,80,81)(H4,63,64,66)/t41-,42-,43+,44-,45-,46+,47-,48+,49+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50407675
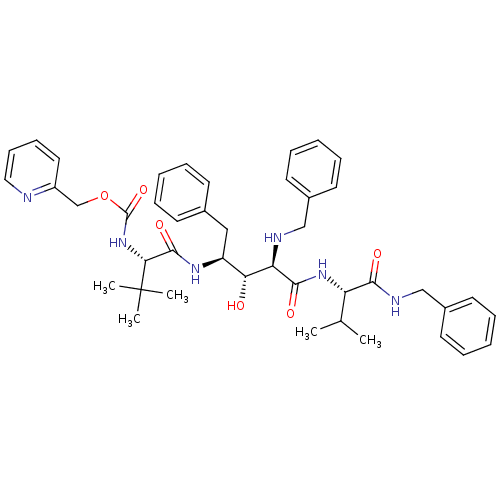
(CHEMBL342293)Show SMILES CC(C)[C@H](NC(=O)[C@H](NCc1ccccc1)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccn1)C(C)(C)C)C(=O)NCc1ccccc1 Show InChI InChI=1S/C43H54N6O6/c1-29(2)35(39(51)46-27-32-21-13-8-14-22-32)48-40(52)36(45-26-31-19-11-7-12-20-31)37(50)34(25-30-17-9-6-10-18-30)47-41(53)38(43(3,4)5)49-42(54)55-28-33-23-15-16-24-44-33/h6-24,29,34-38,45,50H,25-28H2,1-5H3,(H,46,51)(H,47,53)(H,48,52)(H,49,54)/t34-,35-,36+,37+,38+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV type 1 protease |
J Med Chem 38: 4917-28 (1996)
BindingDB Entry DOI: 10.7270/Q2FX7BN8 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038786
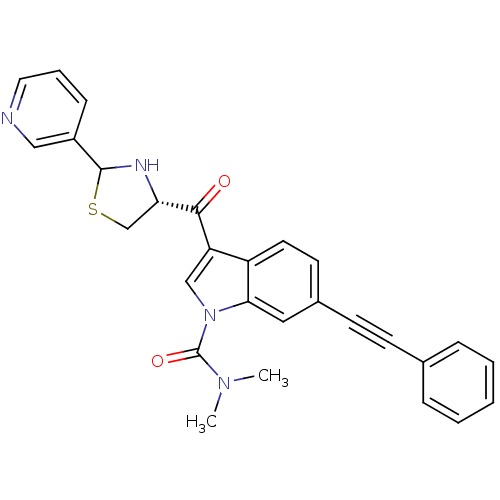
(6-Phenylethynyl-3-((R)-2-pyridin-3-yl-thiazolidine...)Show SMILES CN(C)C(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccc(cc12)C#Cc1ccccc1 Show InChI InChI=1S/C28H24N4O2S/c1-31(2)28(34)32-17-23(26(33)24-18-35-27(30-24)21-9-6-14-29-16-21)22-13-12-20(15-25(22)32)11-10-19-7-4-3-5-8-19/h3-9,12-17,24,27,30H,18H2,1-2H3/t24-,27?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038810
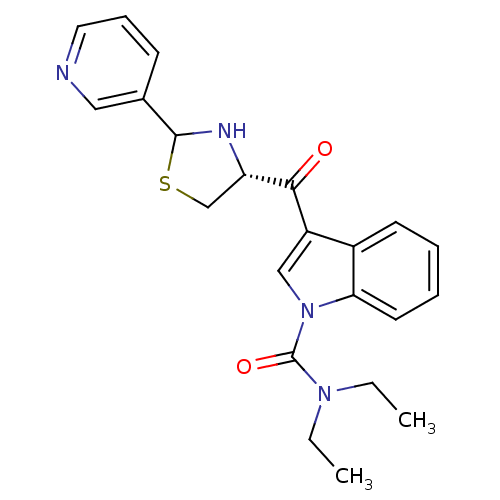
(3-((R)-2-Pyridin-3-yl-thiazolidine-4-carbonyl)-ind...)Show SMILES CCN(CC)C(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccccc12 Show InChI InChI=1S/C22H24N4O2S/c1-3-25(4-2)22(28)26-13-17(16-9-5-6-10-19(16)26)20(27)18-14-29-21(24-18)15-8-7-11-23-12-15/h5-13,18,21,24H,3-4,14H2,1-2H3/t18-,21?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038825
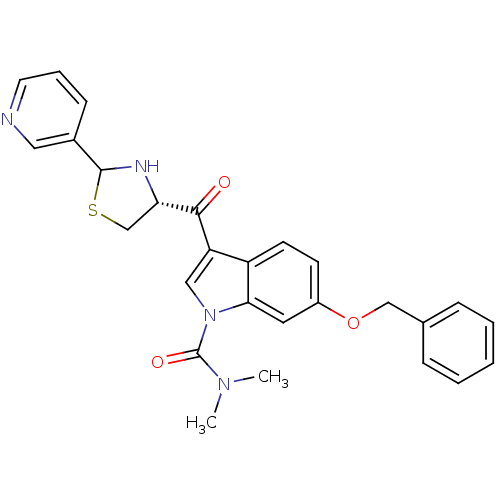
(6-Benzyloxy-3-((R)-2-pyridin-3-yl-thiazolidine-4-c...)Show SMILES CN(C)C(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccc(OCc3ccccc3)cc12 Show InChI InChI=1S/C27H26N4O3S/c1-30(2)27(33)31-15-22(25(32)23-17-35-26(29-23)19-9-6-12-28-14-19)21-11-10-20(13-24(21)31)34-16-18-7-4-3-5-8-18/h3-15,23,26,29H,16-17H2,1-2H3/t23-,26?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50165932

(Ac-YRMEHdFRWG-NH2 | CHEMBL266879)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(N)=O Show InChI InChI=1S/C61H83N19O13S/c1-34(81)73-46(27-36-16-18-39(82)19-17-36)57(91)74-42(14-8-23-68-60(63)64)53(87)77-45(22-25-94-2)56(90)76-44(20-21-51(84)85)55(89)80-49(29-38-31-67-33-72-38)59(93)78-47(26-35-10-4-3-5-11-35)58(92)75-43(15-9-24-69-61(65)66)54(88)79-48(52(86)71-32-50(62)83)28-37-30-70-41-13-7-6-12-40(37)41/h3-7,10-13,16-19,30-31,33,42-49,70,82H,8-9,14-15,20-29,32H2,1-2H3,(H2,62,83)(H,67,72)(H,71,86)(H,73,81)(H,74,91)(H,75,92)(H,76,90)(H,77,87)(H,78,93)(H,79,88)(H,80,89)(H,84,85)(H4,63,64,68)(H4,65,66,69)/t42-,43-,44-,45-,46-,47+,48-,49-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-3 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038819
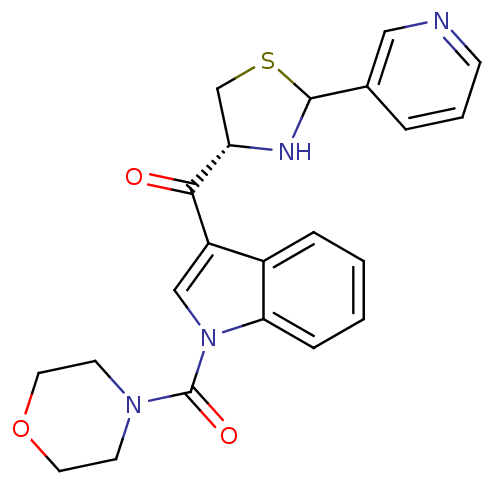
(CHEMBL64464 | Morpholin-4-yl-[3-((R)-2-pyridin-3-y...)Show SMILES O=C([C@@H]1CSC(N1)c1cccnc1)c1cn(C(=O)N2CCOCC2)c2ccccc12 Show InChI InChI=1S/C22H22N4O3S/c27-20(18-14-30-21(24-18)15-4-3-7-23-12-15)17-13-26(19-6-2-1-5-16(17)19)22(28)25-8-10-29-11-9-25/h1-7,12-13,18,21,24H,8-11,14H2/t18-,21?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038749
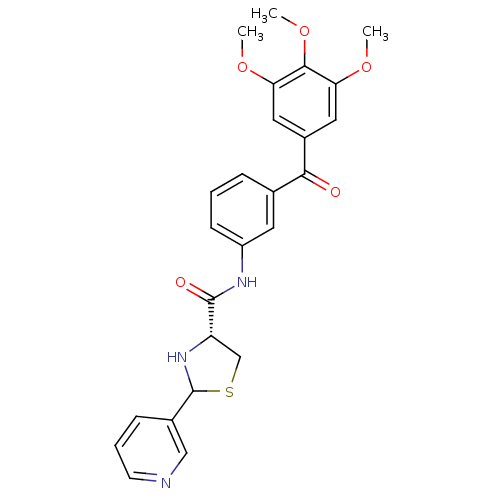
((R)-2-Pyridin-3-yl-thiazolidine-4-carboxylic acid ...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)c1cccc(NC(=O)[C@@H]2CSC(N2)c2cccnc2)c1 Show InChI InChI=1S/C25H25N3O5S/c1-31-20-11-17(12-21(32-2)23(20)33-3)22(29)15-6-4-8-18(10-15)27-24(30)19-14-34-25(28-19)16-7-5-9-26-13-16/h4-13,19,25,28H,14H2,1-3H3,(H,27,30)/t19-,25?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038801
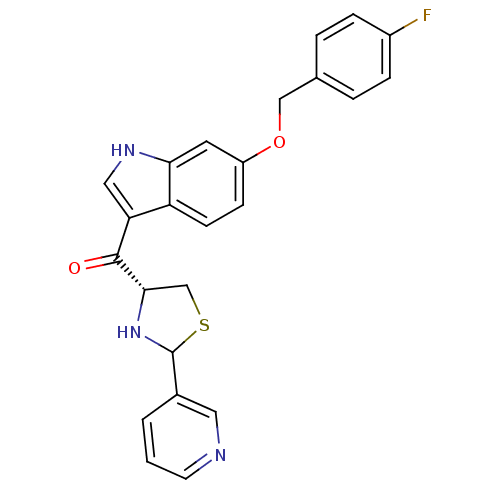
(CHEMBL62830 | [6-(4-Fluoro-benzyloxy)-1H-indol-3-y...)Show SMILES Fc1ccc(COc2ccc3c(c[nH]c3c2)C(=O)[C@@H]2CSC(N2)c2cccnc2)cc1 Show InChI InChI=1S/C24H20FN3O2S/c25-17-5-3-15(4-6-17)13-30-18-7-8-19-20(12-27-21(19)10-18)23(29)22-14-31-24(28-22)16-2-1-9-26-11-16/h1-12,22,24,27-28H,13-14H2/t22-,24?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16047
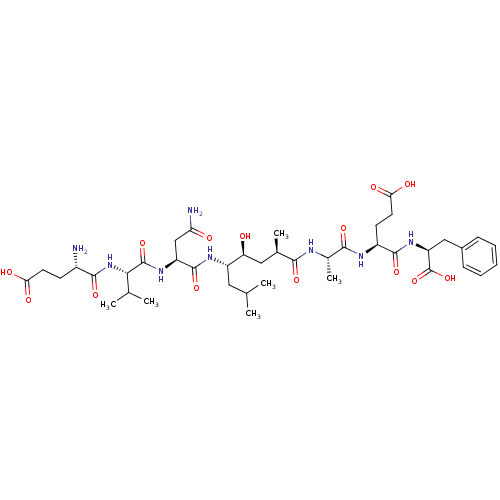
((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H64N8O14/c1-20(2)16-27(46-39(60)28(19-31(43)51)47-40(61)34(21(3)4)49-37(58)25(42)12-14-32(52)53)30(50)17-22(5)35(56)44-23(6)36(57)45-26(13-15-33(54)55)38(59)48-29(41(62)63)18-24-10-8-7-9-11-24/h7-11,20-23,25-30,34,50H,12-19,42H2,1-6H3,(H2,43,51)(H,44,56)(H,45,57)(H,46,60)(H,47,61)(H,48,59)(H,49,58)(H,52,53)(H,54,55)(H,62,63)/t22-,23+,25+,26+,27+,28+,29+,30+,34+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human beta-secretase |
Bioorg Med Chem Lett 13: 4335-9 (2003)
BindingDB Entry DOI: 10.7270/Q2513ZR3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038759
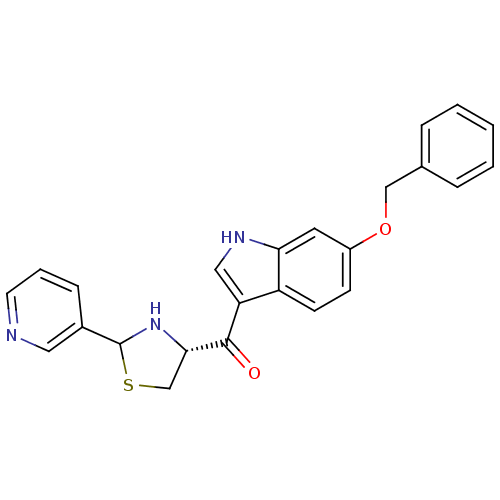
((6-Benzyloxy-1H-indol-3-yl)-((R)-2-pyridin-3-yl-th...)Show SMILES O=C([C@@H]1CSC(N1)c1cccnc1)c1c[nH]c2cc(OCc3ccccc3)ccc12 Show InChI InChI=1S/C24H21N3O2S/c28-23(22-15-30-24(27-22)17-7-4-10-25-12-17)20-13-26-21-11-18(8-9-19(20)21)29-14-16-5-2-1-3-6-16/h1-13,22,24,26-27H,14-15H2/t22-,24?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038835
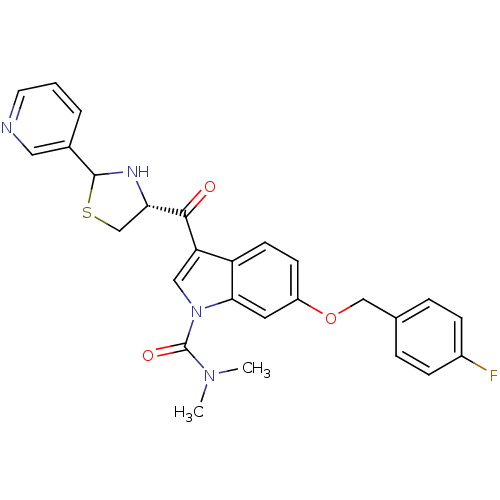
(6-(4-Fluoro-benzyloxy)-3-((R)-2-pyridin-3-yl-thiaz...)Show SMILES CN(C)C(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccc(OCc3ccc(F)cc3)cc12 Show InChI InChI=1S/C27H25FN4O3S/c1-31(2)27(34)32-14-22(25(33)23-16-36-26(30-23)18-4-3-11-29-13-18)21-10-9-20(12-24(21)32)35-15-17-5-7-19(28)8-6-17/h3-14,23,26,30H,15-16H2,1-2H3/t23-,26?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038760
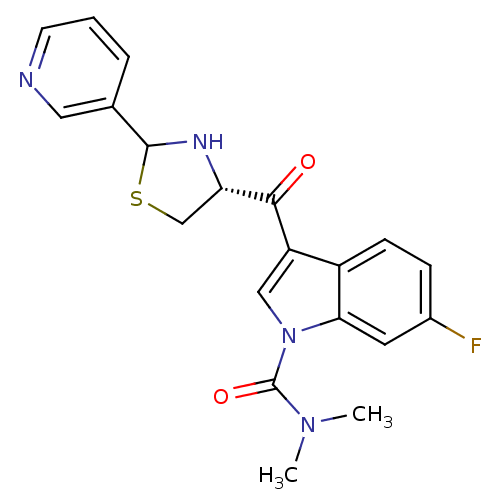
(6-Fluoro-3-((R)-2-pyridin-3-yl-thiazolidine-4-carb...)Show SMILES CN(C)C(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccc(F)cc12 Show InChI InChI=1S/C20H19FN4O2S/c1-24(2)20(27)25-10-15(14-6-5-13(21)8-17(14)25)18(26)16-11-28-19(23-16)12-4-3-7-22-9-12/h3-10,16,19,23H,11H2,1-2H3/t16-,19?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038752
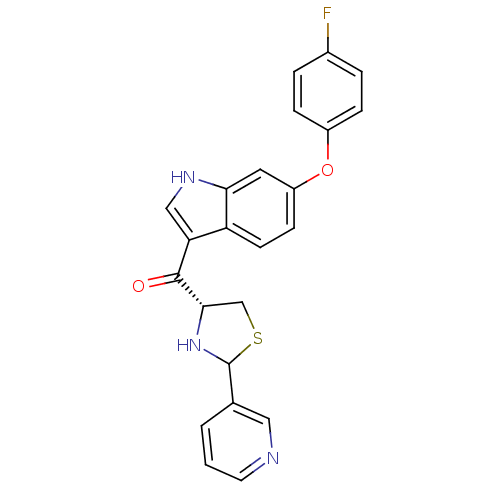
(CHEMBL64636 | [6-(4-Fluoro-phenoxy)-1H-indol-3-yl]...)Show SMILES Fc1ccc(Oc2ccc3c(c[nH]c3c2)C(=O)[C@@H]2CSC(N2)c2cccnc2)cc1 Show InChI InChI=1S/C23H18FN3O2S/c24-15-3-5-16(6-4-15)29-17-7-8-18-19(12-26-20(18)10-17)22(28)21-13-30-23(27-21)14-2-1-9-25-11-14/h1-12,21,23,26-27H,13H2/t21-,23?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038788
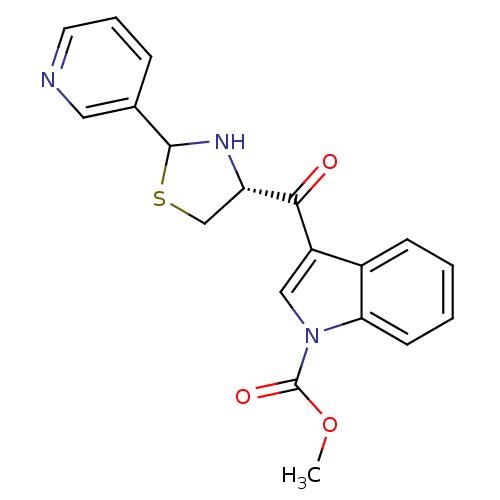
(3-((R)-2-Pyridin-3-yl-thiazolidine-4-carbonyl)-ind...)Show SMILES COC(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccccc12 Show InChI InChI=1S/C19H17N3O3S/c1-25-19(24)22-10-14(13-6-2-3-7-16(13)22)17(23)15-11-26-18(21-15)12-5-4-8-20-9-12/h2-10,15,18,21H,11H2,1H3/t15-,18?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
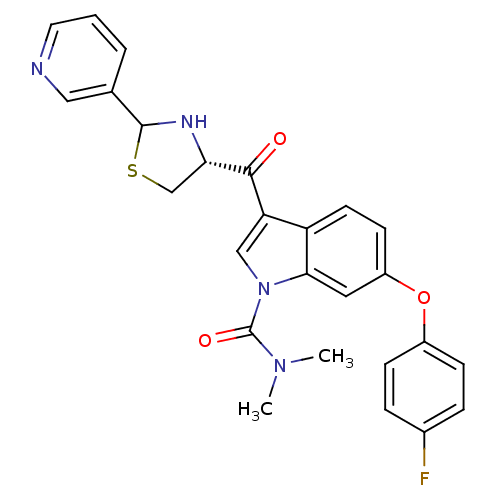
(Cavia porcellus) | BDBM50038754
(6-(4-Fluoro-phenoxy)-3-((R)-2-pyridin-3-yl-thiazol...)Show SMILES CN(C)C(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccc(Oc3ccc(F)cc3)cc12 Show InChI InChI=1S/C26H23FN4O3S/c1-30(2)26(33)31-14-21(24(32)22-15-35-25(29-22)16-4-3-11-28-13-16)20-10-9-19(12-23(20)31)34-18-7-5-17(27)6-8-18/h3-14,22,25,29H,15H2,1-2H3/t22-,25?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165928
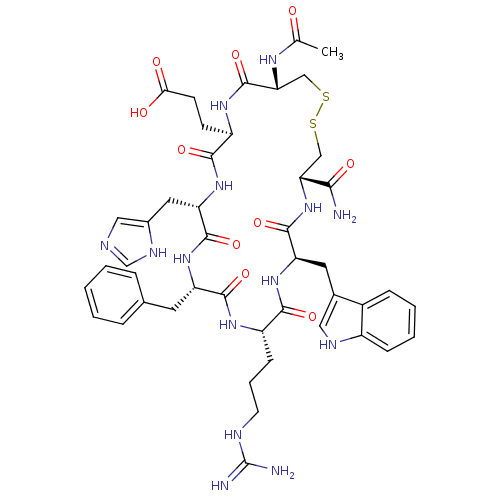
(Ac-[CEHdFRWC]-NH2 | CHEMBL385556)Show SMILES CC(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C45H58N14O10S2/c1-24(60)53-36-22-71-70-21-35(38(46)63)59-42(67)33(17-26-19-51-29-11-6-5-10-28(26)29)57-39(64)30(12-7-15-50-45(47)48)54-41(66)32(16-25-8-3-2-4-9-25)56-43(68)34(18-27-20-49-23-52-27)58-40(65)31(55-44(36)69)13-14-37(61)62/h2-6,8-11,19-20,23,30-36,51H,7,12-18,21-22H2,1H3,(H2,46,63)(H,49,52)(H,53,60)(H,54,66)(H,55,69)(H,56,68)(H,57,64)(H,58,65)(H,59,67)(H,61,62)(H4,47,48,50)/t30-,31+,32-,33+,34-,35+,36+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
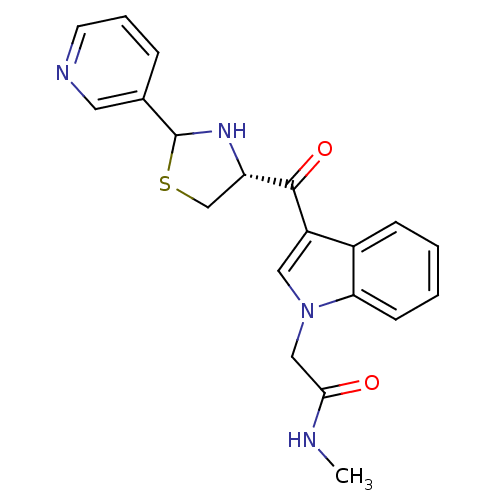
(Cavia porcellus) | BDBM50038763
(CHEMBL293259 | N-Methyl-2-[3-((R)-2-pyridin-3-yl-t...)Show SMILES CNC(=O)Cn1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccccc12 Show InChI InChI=1S/C20H20N4O2S/c1-21-18(25)11-24-10-15(14-6-2-3-7-17(14)24)19(26)16-12-27-20(23-16)13-5-4-8-22-9-13/h2-10,16,20,23H,11-12H2,1H3,(H,21,25)/t16-,20?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


















































