 Found 370 hits with Last Name = 'mcallister' and Initial = 'l'
Found 370 hits with Last Name = 'mcallister' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50432208
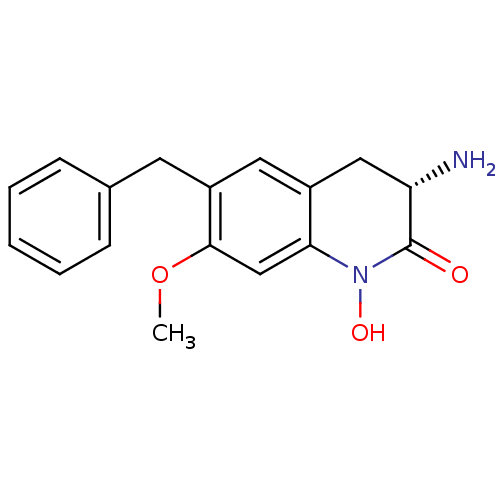
(CHEMBL2347110)Show SMILES COc1cc2N(O)C(=O)[C@@H](N)Cc2cc1Cc1ccccc1 |r| Show InChI InChI=1S/C17H18N2O3/c1-22-16-10-15-12(9-14(18)17(20)19(15)21)8-13(16)7-11-5-3-2-4-6-11/h2-6,8,10,14,21H,7,9,18H2,1H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426340
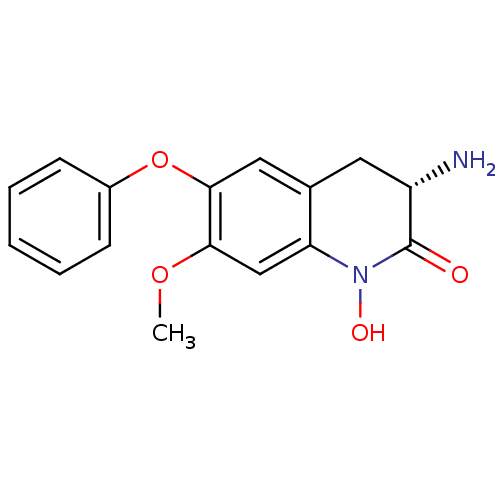
(CHEMBL2321943)Show SMILES COc1cc2N(O)C(=O)[C@@H](N)Cc2cc1Oc1ccccc1 |r| Show InChI InChI=1S/C16H16N2O4/c1-21-14-9-13-10(7-12(17)16(19)18(13)20)8-15(14)22-11-5-3-2-4-6-11/h2-6,8-9,12,20H,7,17H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107730
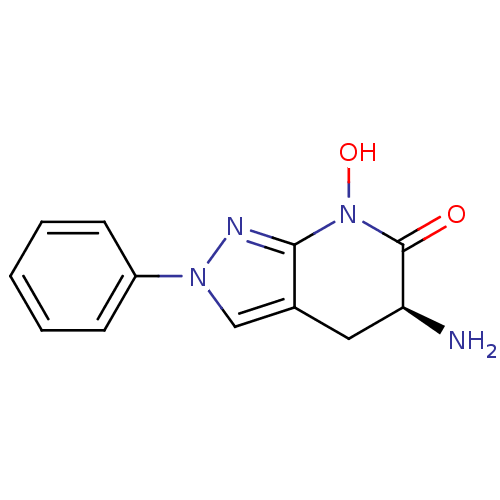
(CHEMBL2347108 | US8933095, 14)Show InChI InChI=1S/C12H12N4O2/c13-10-6-8-7-15(9-4-2-1-3-5-9)14-11(8)16(18)12(10)17/h1-5,7,10,18H,6,13H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426341
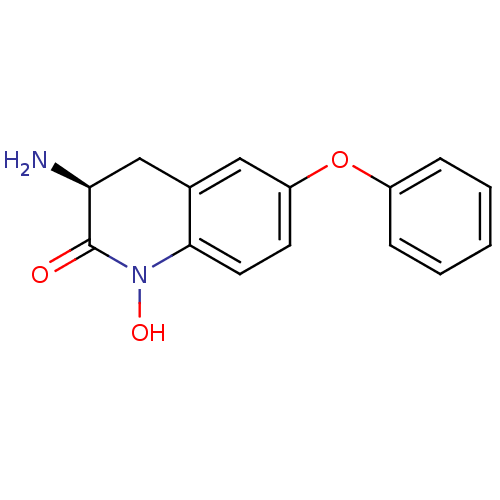
(CHEMBL2321944)Show InChI InChI=1S/C15H14N2O3/c16-13-9-10-8-12(20-11-4-2-1-3-5-11)6-7-14(10)17(19)15(13)18/h1-8,13,19H,9,16H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386310
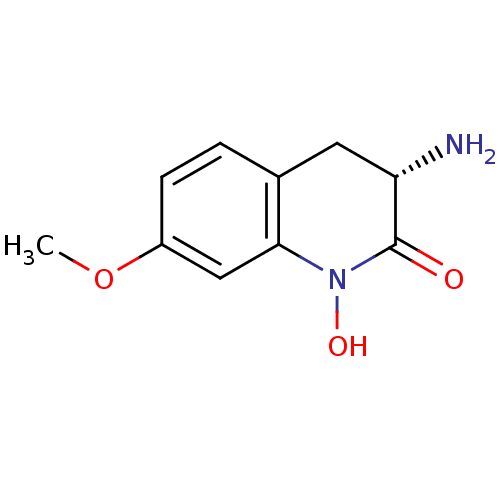
(CHEMBL2049092)Show InChI InChI=1S/C10H12N2O3/c1-15-7-3-2-6-4-8(11)10(13)12(14)9(6)5-7/h2-3,5,8,14H,4,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107747
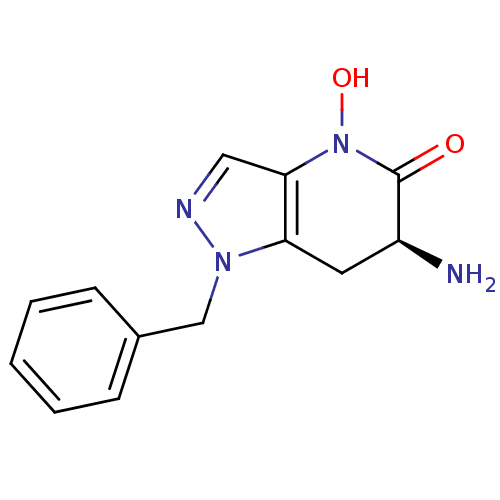
(CHEMBL2347115 | US8933095, 4)Show InChI InChI=1S/C13H14N4O2/c14-10-6-11-12(17(19)13(10)18)7-15-16(11)8-9-4-2-1-3-5-9/h1-5,7,10,19H,6,8,14H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292
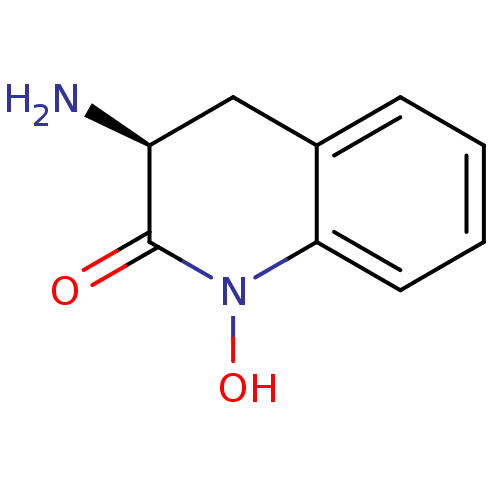
(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292

(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107738
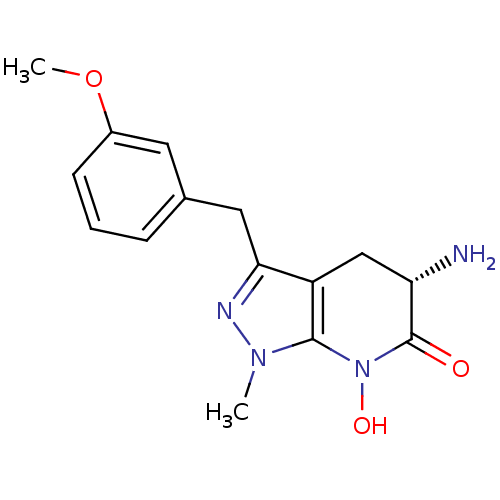
(CHEMBL2347113 | US8933095, 22)Show SMILES COc1cccc(Cc2nn(C)c3N(O)C(=O)[C@@H](N)Cc23)c1 |r| Show InChI InChI=1S/C15H18N4O3/c1-18-14-11(8-12(16)15(20)19(14)21)13(17-18)7-9-4-3-5-10(6-9)22-2/h3-6,12,21H,7-8,16H2,1-2H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107746
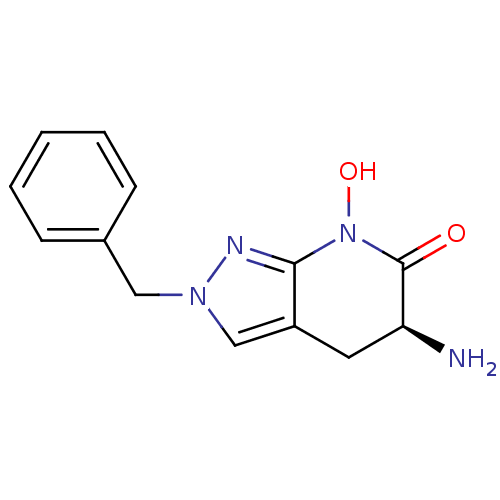
(CHEMBL2347107 | US8933095, 1)Show InChI InChI=1S/C13H14N4O2/c14-11-6-10-8-16(7-9-4-2-1-3-5-9)15-12(10)17(19)13(11)18/h1-5,8,11,19H,6-7,14H2/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107720
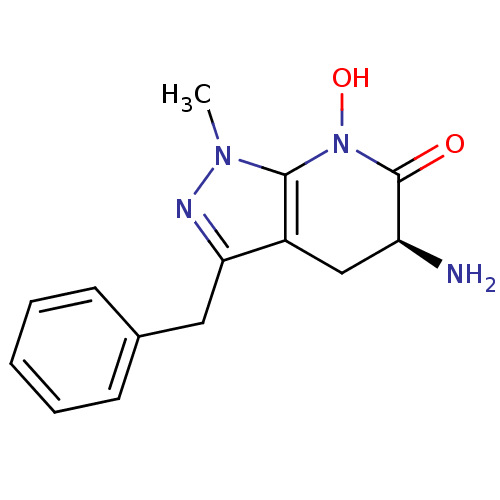
(CHEMBL2347112 | US8598200, 2)Show SMILES Cn1nc(Cc2ccccc2)c2C[C@H](N)C(=O)N(O)c12 |r| Show InChI InChI=1S/C14H16N4O2/c1-17-13-10(8-11(15)14(19)18(13)20)12(16-17)7-9-5-3-2-4-6-9/h2-6,11,20H,7-8,15H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50432200
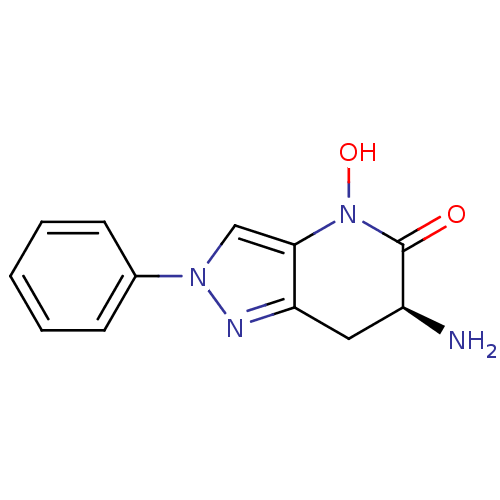
(CHEMBL2347109 | US8933095, 16)Show InChI InChI=1S/C12H12N4O2/c13-9-6-10-11(16(18)12(9)17)7-15(14-10)8-4-2-1-3-5-8/h1-5,7,9,18H,6,13H2/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107722
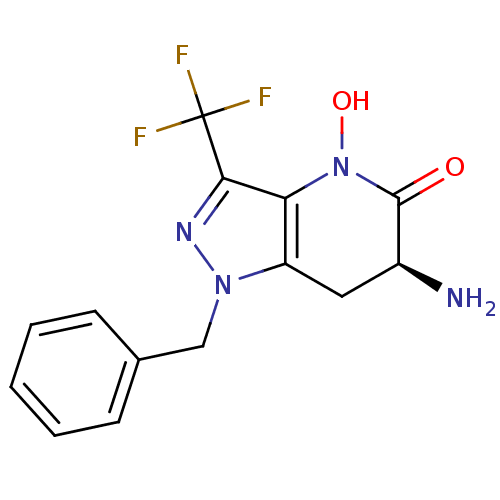
(CHEMBL2347114 | US8933095, 5)Show SMILES N[C@H]1Cc2c(N(O)C1=O)c(nn2Cc1ccccc1)C(F)(F)F |r| Show InChI InChI=1S/C14H13F3N4O2/c15-14(16,17)12-11-10(6-9(18)13(22)21(11)23)20(19-12)7-8-4-2-1-3-5-8/h1-5,9,23H,6-7,18H2/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis |
Bioorg Med Chem Lett 23: 1961-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.039
BindingDB Entry DOI: 10.7270/Q2N87C48 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50250852
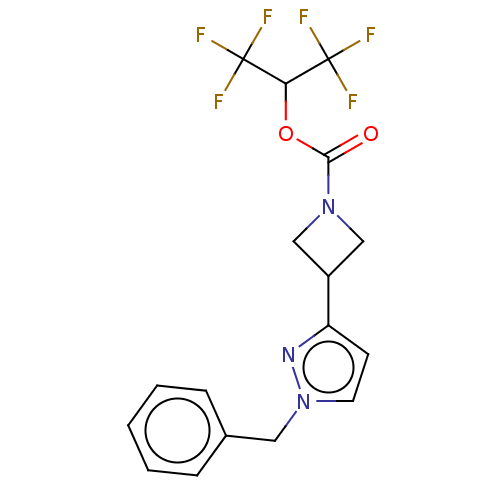
(CHEMBL4078217)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1ccn(Cc2ccccc2)n1)C(F)(F)F Show InChI InChI=1S/C17H15F6N3O2/c18-16(19,20)14(17(21,22)23)28-15(27)25-9-12(10-25)13-6-7-26(24-13)8-11-4-2-1-3-5-11/h1-7,12,14H,8-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250848
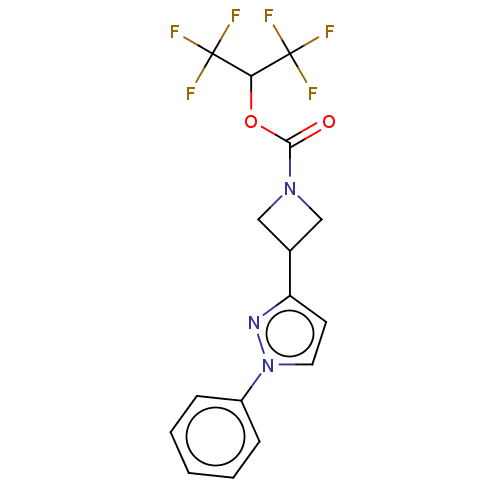
(CHEMBL4059676)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1ccn(n1)-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C16H13F6N3O2/c17-15(18,19)13(16(20,21)22)27-14(26)24-8-10(9-24)12-6-7-25(23-12)11-4-2-1-3-5-11/h1-7,10,13H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250859
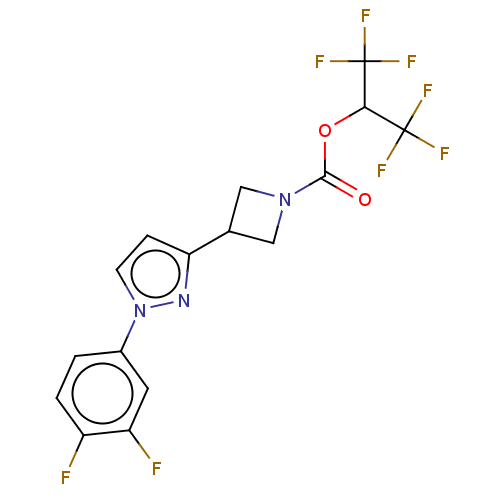
(CHEMBL4097203)Show SMILES Fc1ccc(cc1F)-n1ccc(n1)C1CN(C1)C(=O)OC(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C16H11F8N3O2/c17-10-2-1-9(5-11(10)18)27-4-3-12(25-27)8-6-26(7-8)14(28)29-13(15(19,20)21)16(22,23)24/h1-5,8,13H,6-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM474403
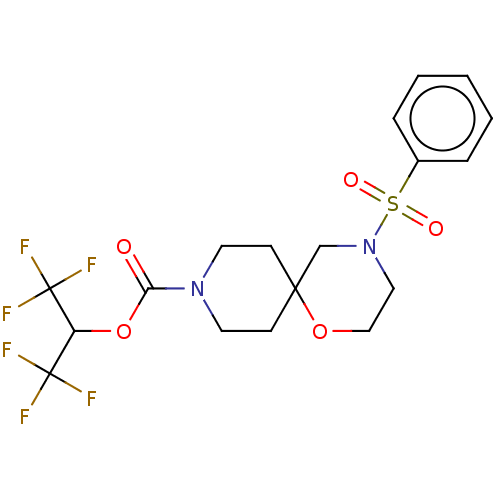
(1,1,1,3,3,3-Hexafluoropropan-2-yl 4-(phenylsulfony...)Show SMILES FC(F)(F)C(OC(=O)N1CCC2(CC1)CN(CCO2)S(=O)(=O)c1ccccc1)C(F)(F)F Show InChI InChI=1S/C18H20F6N2O5S/c19-17(20,21)14(18(22,23)24)31-15(27)25-8-6-16(7-9-25)12-26(10-11-30-16)32(28,29)13-4-2-1-3-5-13/h1-5,14H,6-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.259 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
T = 30 minutes. |
US Patent US10858373 (2020)
BindingDB Entry DOI: 10.7270/Q2B27ZCK |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250855
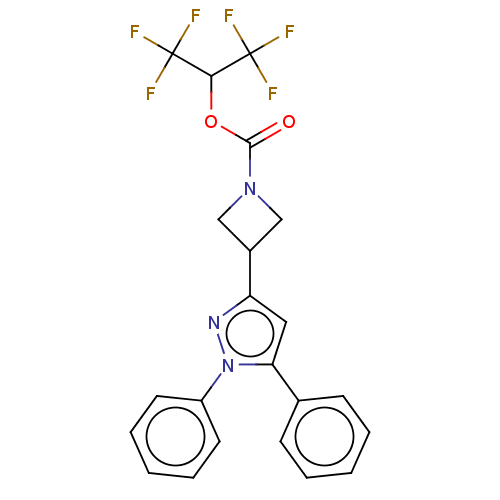
(CHEMBL4077745)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1cc(-c2ccccc2)n(n1)-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C22H17F6N3O2/c23-21(24,25)19(22(26,27)28)33-20(32)30-12-15(13-30)17-11-18(14-7-3-1-4-8-14)31(29-17)16-9-5-2-6-10-16/h1-11,15,19H,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250850
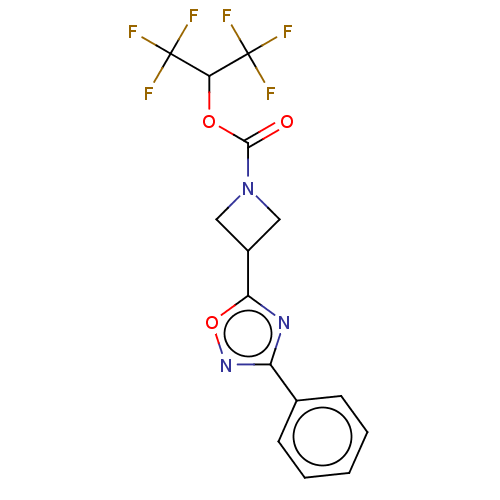
(CHEMBL4089505)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1nc(no1)-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C15H11F6N3O3/c16-14(17,18)12(15(19,20)21)26-13(25)24-6-9(7-24)11-22-10(23-27-11)8-4-2-1-3-5-8/h1-5,9,12H,6-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250852

(CHEMBL4078217)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1ccn(Cc2ccccc2)n1)C(F)(F)F Show InChI InChI=1S/C17H15F6N3O2/c18-16(19,20)14(17(21,22)23)28-15(27)25-9-12(10-25)13-6-7-26(24-13)8-11-4-2-1-3-5-11/h1-7,12,14H,8-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM474408
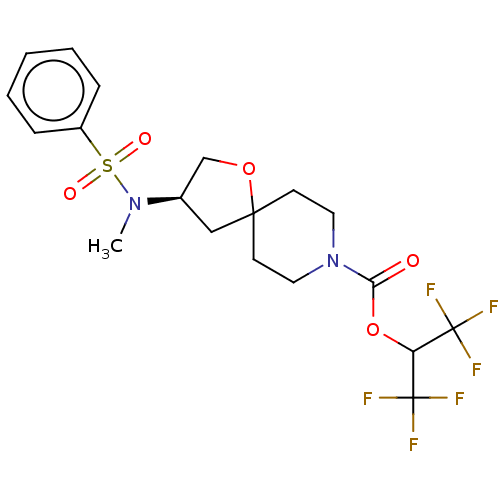
(1,1,1,3,3,3-Hexafluoropropan-2-yl (3R)-3-[methyl(p...)Show SMILES CN([C@H]1COC2(C1)CCN(CC2)C(=O)OC(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C19H22F6N2O5S/c1-26(33(29,30)14-5-3-2-4-6-14)13-11-17(31-12-13)7-9-27(10-8-17)16(28)32-15(18(20,21)22)19(23,24)25/h2-6,13,15H,7-12H2,1H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
T = 30 minutes. |
US Patent US10858373 (2020)
BindingDB Entry DOI: 10.7270/Q2B27ZCK |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50250855

(CHEMBL4077745)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1cc(-c2ccccc2)n(n1)-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C22H17F6N3O2/c23-21(24,25)19(22(26,27)28)33-20(32)30-12-15(13-30)17-11-18(14-7-3-1-4-8-14)31(29-17)16-9-5-2-6-10-16/h1-11,15,19H,12-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395921
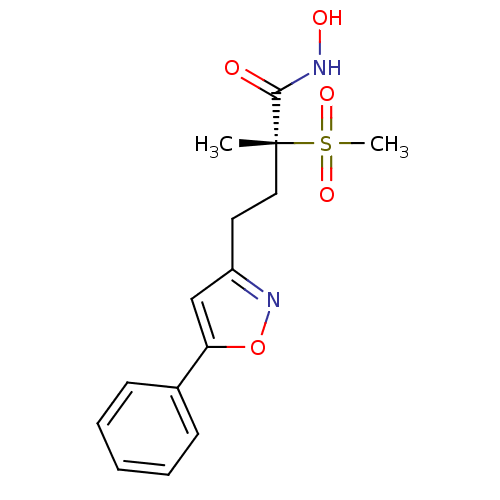
(CHEMBL2164511)Show SMILES C[C@@](CCc1cc(on1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C15H18N2O5S/c1-15(14(18)16-19,23(2,20)21)9-8-12-10-13(22-17-12)11-6-4-3-5-7-11/h3-7,10,19H,8-9H2,1-2H3,(H,16,18)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.511 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485077
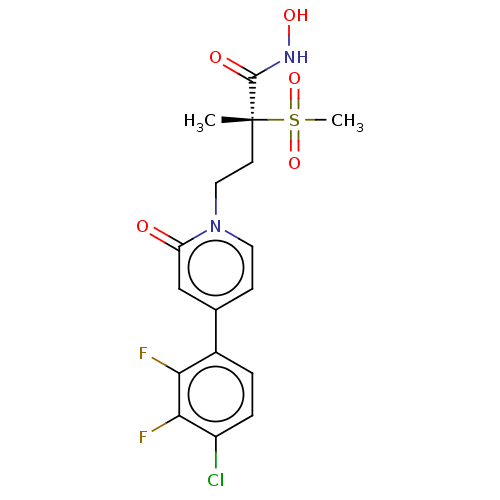
(CHEMBL2023517)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(Cl)c(F)c1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C17H17ClF2N2O5S/c1-17(16(24)21-25,28(2,26)27)6-8-22-7-5-10(9-13(22)23)11-3-4-12(18)15(20)14(11)19/h3-5,7,9,25H,6,8H2,1-2H3,(H,21,24)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485061

(CHEMBL2023524)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-n1nccn1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C19H21N5O5S/c1-19(18(26)22-27,30(2,28)29)8-12-23-11-7-15(13-17(23)25)14-3-5-16(6-4-14)24-20-9-10-21-24/h3-7,9-11,13,27H,8,12H2,1-2H3,(H,22,26)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM474444
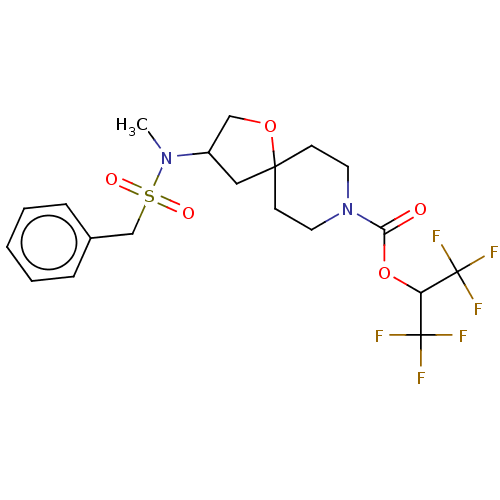
(US10858373, Example 42)Show SMILES CN(C1COC2(C1)CCN(CC2)C(=O)OC(C(F)(F)F)C(F)(F)F)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C20H24F6N2O5S/c1-27(34(30,31)13-14-5-3-2-4-6-14)15-11-18(32-12-15)7-9-28(10-8-18)17(29)33-16(19(21,22)23)20(24,25)26/h2-6,15-16H,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.587 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
T = 30 minutes. |
US Patent US10858373 (2020)
BindingDB Entry DOI: 10.7270/Q2B27ZCK |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395911
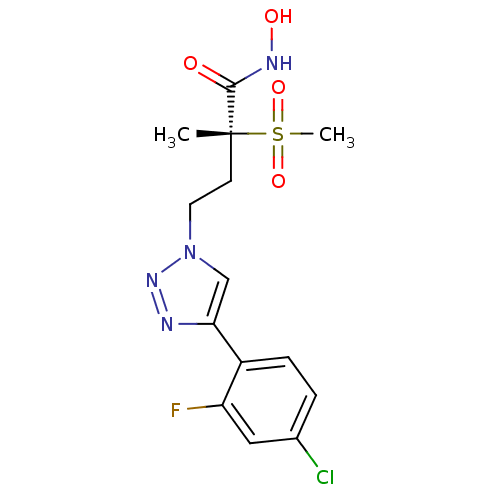
(CHEMBL2164521)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(Cl)cc1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16ClFN4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)10-4-3-9(15)7-11(10)16/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.657 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM474454

(US10858373, Example 52)Show SMILES CN(C1COC2(C1)CCN(CC2)C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)C(C)(C)C Show InChI InChI=1S/C18H26F6N2O4/c1-15(2,3)13(27)25(4)11-9-16(29-10-11)5-7-26(8-6-16)14(28)30-12(17(19,20)21)18(22,23)24/h11-12H,5-10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.666 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
T = 30 minutes. |
US Patent US10858373 (2020)
BindingDB Entry DOI: 10.7270/Q2B27ZCK |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50250859

(CHEMBL4097203)Show SMILES Fc1ccc(cc1F)-n1ccc(n1)C1CN(C1)C(=O)OC(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C16H11F8N3O2/c17-10-2-1-9(5-11(10)18)27-4-3-12(25-27)8-6-26(7-8)14(28)29-13(15(19,20)21)16(22,23)24/h1-5,8,13H,6-7H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM474429

(US10858373, Example 27)Show SMILES CC[C@H]1C[C@H]1S(=O)(=O)N1CCOC2(CCN(CC2)C(=O)OC(C(F)(F)F)C(F)(F)F)C1 |r| Show InChI InChI=1S/C17H24F6N2O5S/c1-2-11-9-12(11)31(27,28)25-7-8-29-15(10-25)3-5-24(6-4-15)14(26)30-13(16(18,19)20)17(21,22)23/h11-13H,2-10H2,1H3/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.679 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
T = 30 minutes. |
US Patent US10858373 (2020)
BindingDB Entry DOI: 10.7270/Q2B27ZCK |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250854
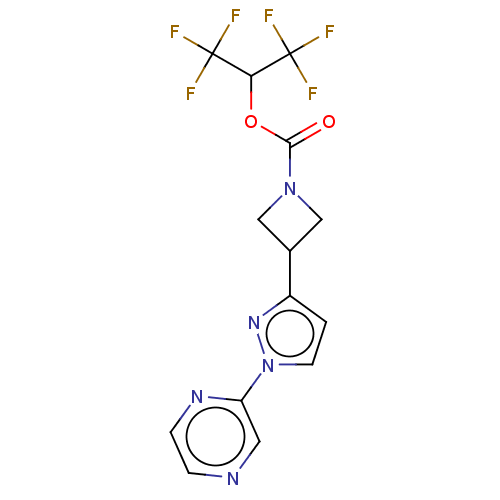
(CHEMBL4079190)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1ccn(n1)-c1cnccn1)C(F)(F)F Show InChI InChI=1S/C14H11F6N5O2/c15-13(16,17)11(14(18,19)20)27-12(26)24-6-8(7-24)9-1-4-25(23-9)10-5-21-2-3-22-10/h1-5,8,11H,6-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395920
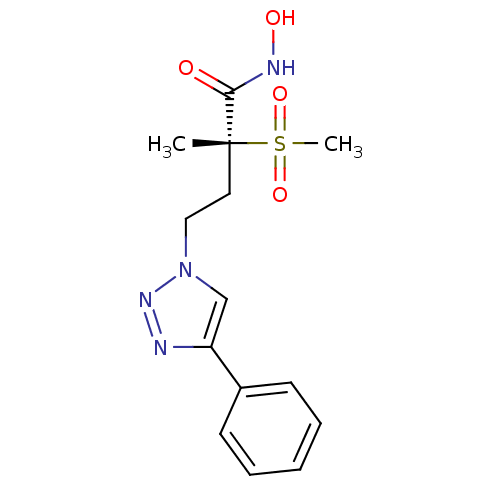
(CHEMBL2164512)Show SMILES C[C@@](CCn1cc(nn1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H18N4O4S/c1-14(13(19)16-20,23(2,21)22)8-9-18-10-12(15-17-18)11-6-4-3-5-7-11/h3-7,10,20H,8-9H2,1-2H3,(H,16,19)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395910
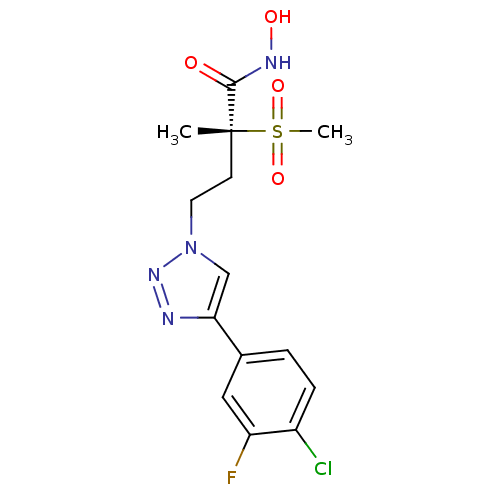
(CHEMBL2164522)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(Cl)c(F)c1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16ClFN4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)9-3-4-10(15)11(16)7-9/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM474416
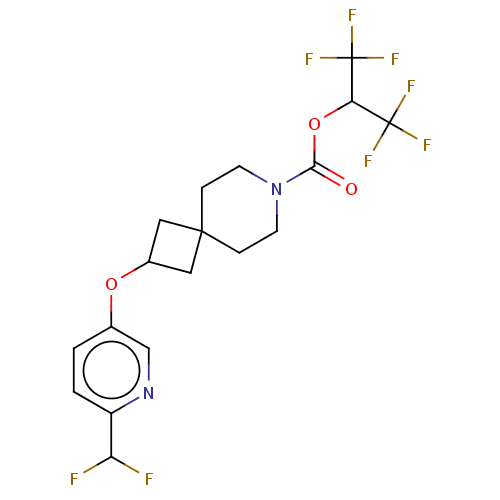
(1,1,1,3,3,3-Hexafluoropropan-2-yl 2-{[6-(difluorom...)Show SMILES FC(F)c1ccc(OC2CC3(C2)CCN(CC3)C(=O)OC(C(F)(F)F)C(F)(F)F)cn1 Show InChI InChI=1S/C18H18F8N2O3/c19-13(20)12-2-1-10(9-27-12)30-11-7-16(8-11)3-5-28(6-4-16)15(29)31-14(17(21,22)23)18(24,25)26/h1-2,9,11,13-14H,3-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.942 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
T = 30 minutes. |
US Patent US10858373 (2020)
BindingDB Entry DOI: 10.7270/Q2B27ZCK |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485056
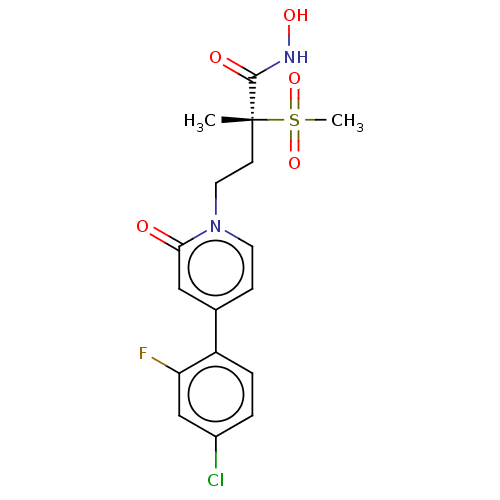
(CHEMBL2023515)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(Cl)cc1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C17H18ClFN2O5S/c1-17(16(23)20-24,27(2,25)26)6-8-21-7-5-11(9-15(21)22)13-4-3-12(18)10-14(13)19/h3-5,7,9-10,24H,6,8H2,1-2H3,(H,20,23)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM474404
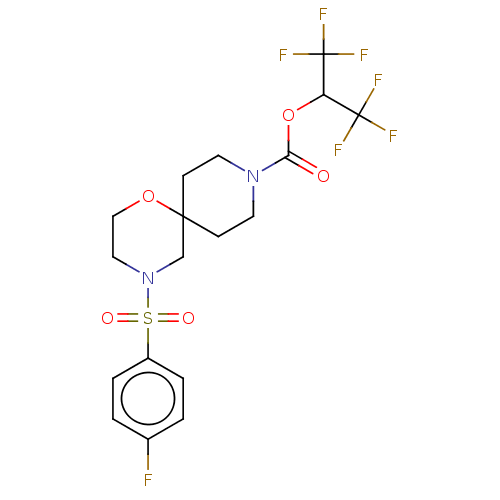
(1,1,1,3,3,3-Hexafluoropropan-2-yl 4-[(4-fluorophen...)Show SMILES Fc1ccc(cc1)S(=O)(=O)N1CCOC2(CCN(CC2)C(=O)OC(C(F)(F)F)C(F)(F)F)C1 Show InChI InChI=1S/C18H19F7N2O5S/c19-12-1-3-13(4-2-12)33(29,30)27-9-10-31-16(11-27)5-7-26(8-6-16)15(28)32-14(17(20,21)22)18(23,24)25/h1-4,14H,5-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
T = 30 minutes. |
US Patent US10858373 (2020)
BindingDB Entry DOI: 10.7270/Q2B27ZCK |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50255776
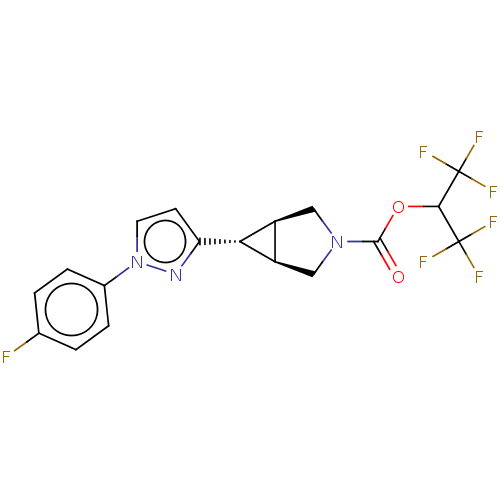
(CHEMBL4075310)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1ccn(n1)-c1ccc(F)cc1)C(=O)OC(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C18H14F7N3O2/c19-9-1-3-10(4-2-9)28-6-5-13(26-28)14-11-7-27(8-12(11)14)16(29)30-15(17(20,21)22)18(23,24)25/h1-6,11-12,14-15H,7-8H2/t11-,12+,14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... |
J Med Chem 61: 3008-3026 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00070
BindingDB Entry DOI: 10.7270/Q2S46VD4 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM474434
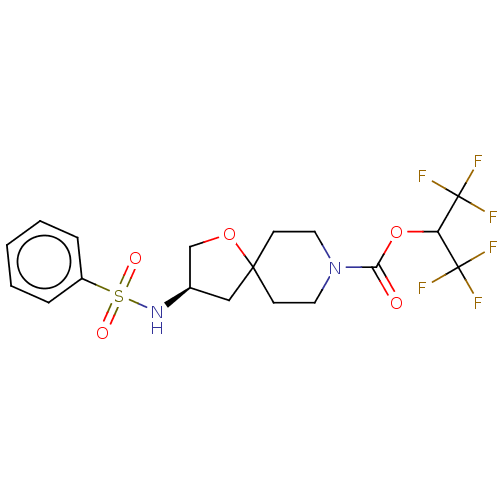
(US10858373, Example 32)Show SMILES FC(F)(F)C(OC(=O)N1CCC2(C[C@H](CO2)NS(=O)(=O)c2ccccc2)CC1)C(F)(F)F |r| Show InChI InChI=1S/C18H20F6N2O5S/c19-17(20,21)14(18(22,23)24)31-15(27)26-8-6-16(7-9-26)10-12(11-30-16)25-32(28,29)13-4-2-1-3-5-13/h1-5,12,14,25H,6-11H2/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
T = 30 minutes. |
US Patent US10858373 (2020)
BindingDB Entry DOI: 10.7270/Q2B27ZCK |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485060
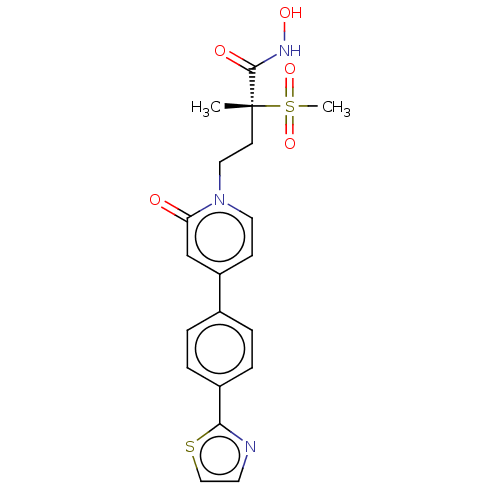
(CHEMBL2023522)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-c1nccs1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C20H21N3O5S2/c1-20(19(25)22-26,30(2,27)28)8-11-23-10-7-16(13-17(23)24)14-3-5-15(6-4-14)18-21-9-12-29-18/h3-7,9-10,12-13,26H,8,11H2,1-2H3,(H,22,25)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485076
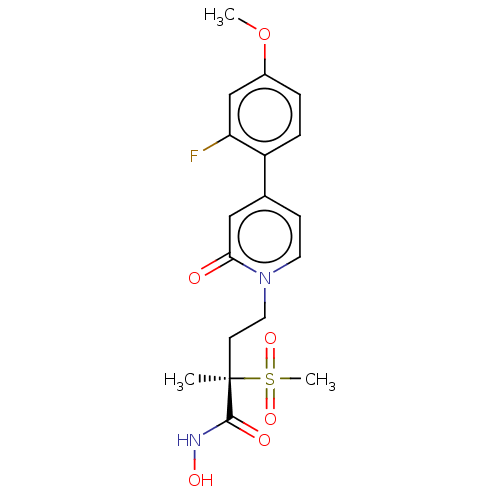
(CHEMBL2023402)Show SMILES COc1ccc(c(F)c1)-c1ccn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)c(=O)c1 |r| Show InChI InChI=1S/C18H21FN2O6S/c1-18(17(23)20-24,28(3,25)26)7-9-21-8-6-12(10-16(21)22)14-5-4-13(27-2)11-15(14)19/h4-6,8,10-11,24H,7,9H2,1-3H3,(H,20,23)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50250854

(CHEMBL4079190)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1ccn(n1)-c1cnccn1)C(F)(F)F Show InChI InChI=1S/C14H11F6N5O2/c15-13(16,17)11(14(18,19)20)27-12(26)24-6-8(7-24)9-1-4-25(23-9)10-5-21-2-3-22-10/h1-5,8,11H,6-7H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485083
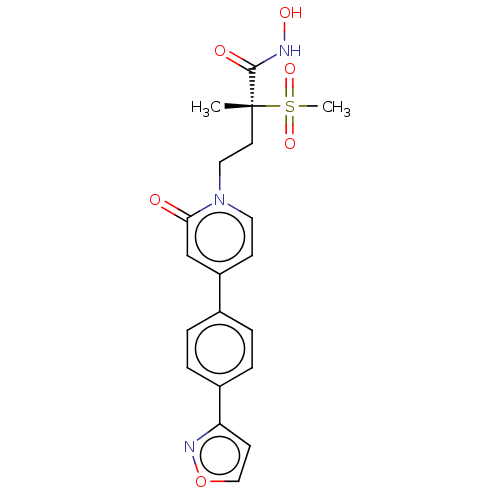
(CHEMBL2023523)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-c1ccon1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C20H21N3O6S/c1-20(19(25)21-26,30(2,27)28)9-11-23-10-7-16(13-18(23)24)14-3-5-15(6-4-14)17-8-12-29-22-17/h3-8,10,12-13,26H,9,11H2,1-2H3,(H,21,25)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485078
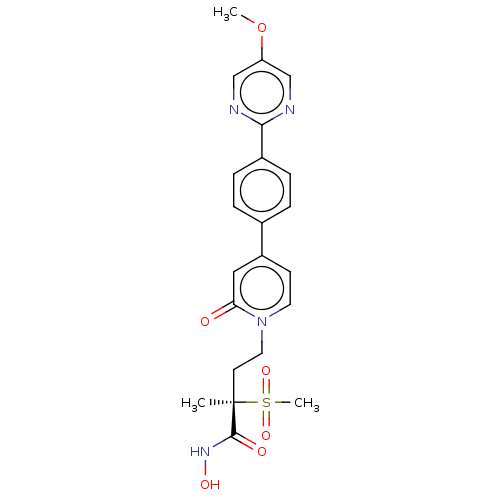
(CHEMBL2023521)Show SMILES COc1cnc(nc1)-c1ccc(cc1)-c1ccn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)c(=O)c1 |r| Show InChI InChI=1S/C22H24N4O6S/c1-22(21(28)25-29,33(3,30)31)9-11-26-10-8-17(12-19(26)27)15-4-6-16(7-5-15)20-23-13-18(32-2)14-24-20/h4-8,10,12-14,29H,9,11H2,1-3H3,(H,25,28)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485059
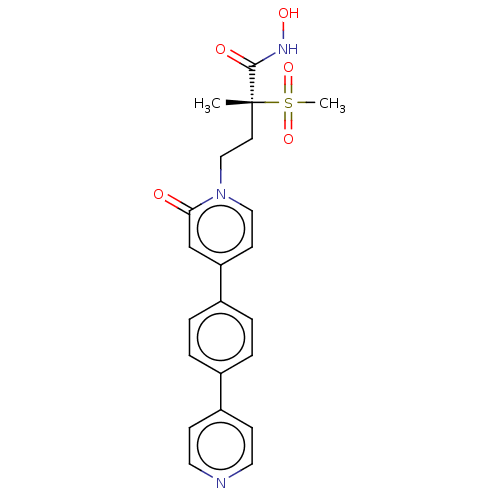
(CHEMBL2023520)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-c1ccncc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C22H23N3O5S/c1-22(21(27)24-28,31(2,29)30)10-14-25-13-9-19(15-20(25)26)17-5-3-16(4-6-17)18-7-11-23-12-8-18/h3-9,11-13,15,28H,10,14H2,1-2H3,(H,24,27)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485084
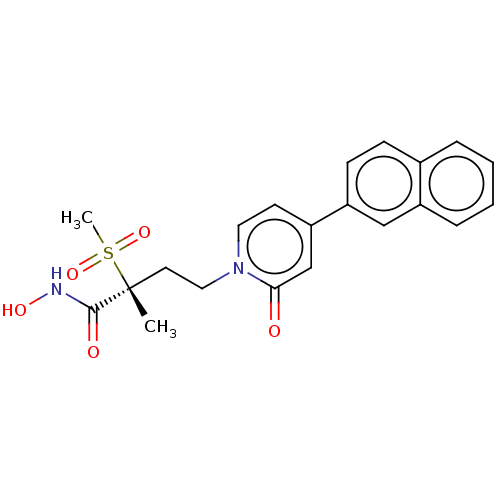
(CHEMBL2023518)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc2ccccc2c1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C21H22N2O5S/c1-21(20(25)22-26,29(2,27)28)10-12-23-11-9-18(14-19(23)24)17-8-7-15-5-3-4-6-16(15)13-17/h3-9,11,13-14,26H,10,12H2,1-2H3,(H,22,25)/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395909

(CHEMBL2164523)Show SMILES COc1ccc(-c2cn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)nn2)c(F)c1 |r| Show InChI InChI=1S/C15H19FN4O5S/c1-15(14(21)18-22,26(3,23)24)6-7-20-9-13(17-19-20)11-5-4-10(25-2)8-12(11)16/h4-5,8-9,22H,6-7H2,1-3H3,(H,18,21)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM474435

(US10858373, Example 33)Show SMILES FC(F)(F)C(OC(=O)N1CCC2(C[C@@H](CO2)NS(=O)(=O)c2ccccc2)CC1)C(F)(F)F |r| Show InChI InChI=1S/C18H20F6N2O5S/c19-17(20,21)14(18(22,23)24)31-15(27)26-8-6-16(7-9-26)10-12(11-30-16)25-32(28,29)13-4-2-1-3-5-13/h1-5,12,14,25H,6-11H2/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
T = 30 minutes. |
US Patent US10858373 (2020)
BindingDB Entry DOI: 10.7270/Q2B27ZCK |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM474443
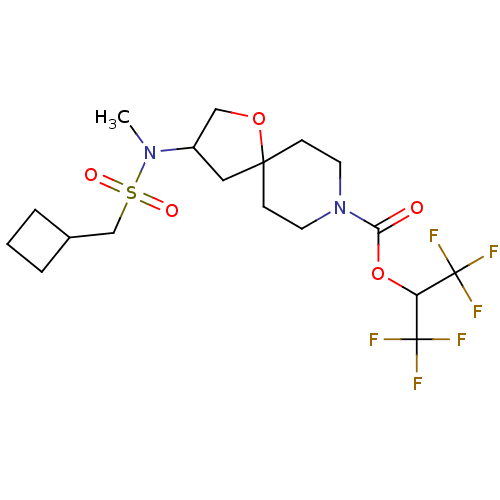
(US10858373, Example 41)Show SMILES CN(C1COC2(C1)CCN(CC2)C(=O)OC(C(F)(F)F)C(F)(F)F)S(=O)(=O)CC1CCC1 Show InChI InChI=1S/C18H26F6N2O5S/c1-25(32(28,29)11-12-3-2-4-12)13-9-16(30-10-13)5-7-26(8-6-16)15(27)31-14(17(19,20)21)18(22,23)24/h12-14H,2-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
T = 30 minutes. |
US Patent US10858373 (2020)
BindingDB Entry DOI: 10.7270/Q2B27ZCK |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485058
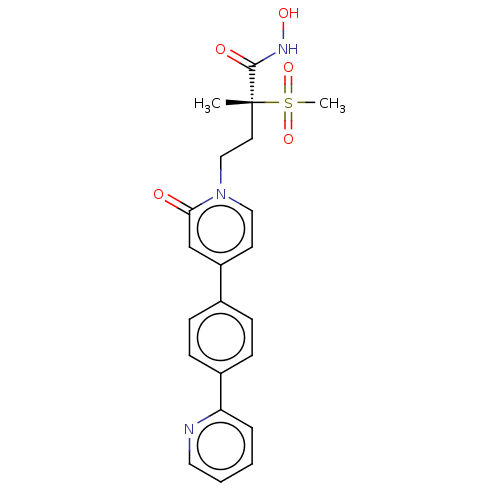
(CHEMBL2023519)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-c1ccccn1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C22H23N3O5S/c1-22(21(27)24-28,31(2,29)30)11-14-25-13-10-18(15-20(25)26)16-6-8-17(9-7-16)19-5-3-4-12-23-19/h3-10,12-13,15,28H,11,14H2,1-2H3,(H,24,27)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395908
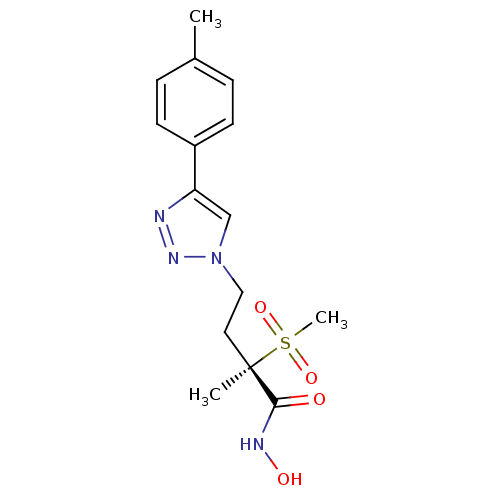
(CHEMBL2164524)Show SMILES Cc1ccc(cc1)-c1cn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)nn1 |r| Show InChI InChI=1S/C15H20N4O4S/c1-11-4-6-12(7-5-11)13-10-19(18-16-13)9-8-15(2,14(20)17-21)24(3,22)23/h4-7,10,21H,8-9H2,1-3H3,(H,17,20)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data