

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stimulator of interferon genes protein (Human) | BDBM490791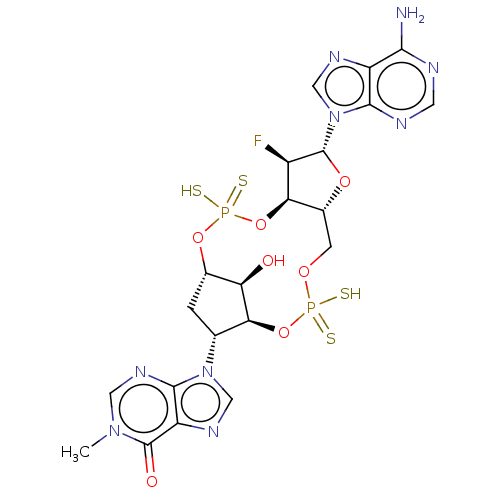 (9-((4S,6R,7S,11aR,13R,14R,14aR,15R)-13-(6-amino-9H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490773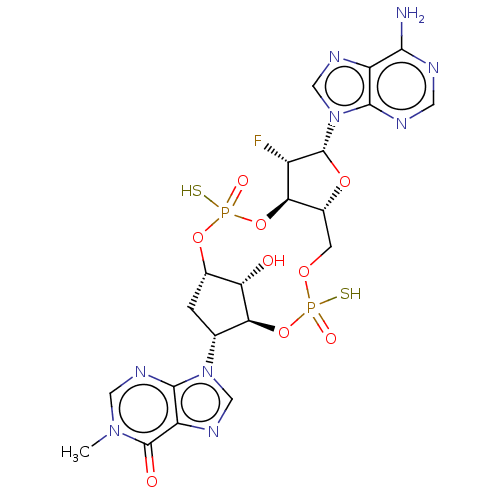 (US10968242, Example 2 Peak 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490777 (US10968242, Example 3 Peak 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490790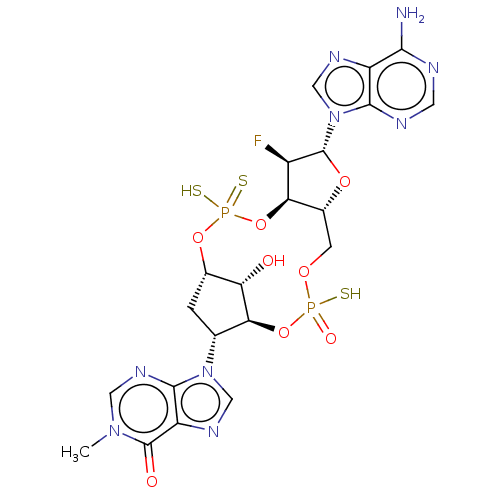 (US10968242, Example 17 Peak 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490786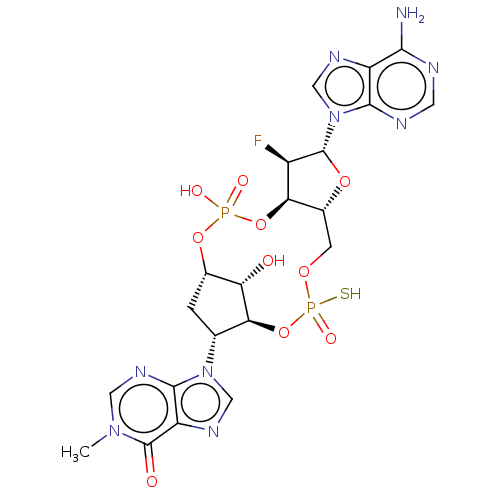 (US10968242, Example 15 Peak 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490787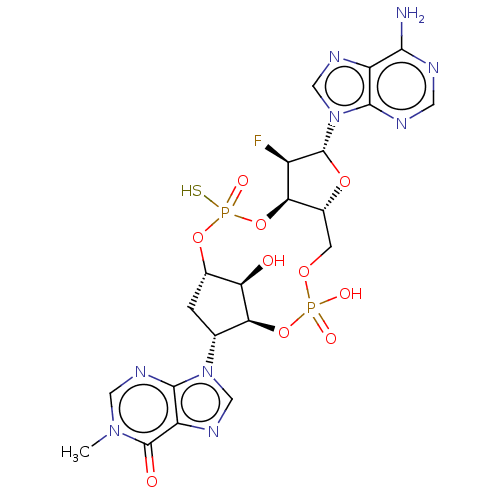 (US10968242, Example 16 Peak 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490776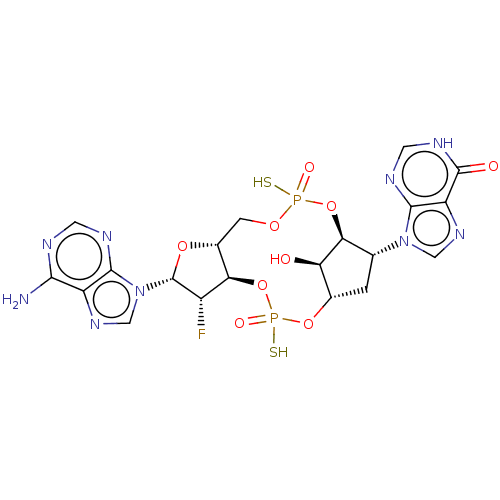 (US10968242, Example 3 Peak 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490794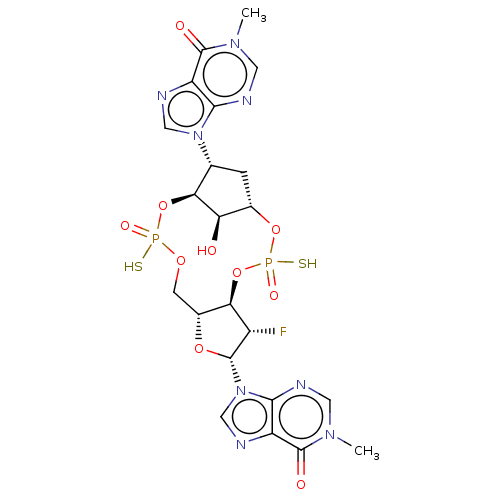 (US10968242, Example 19 Peak 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490772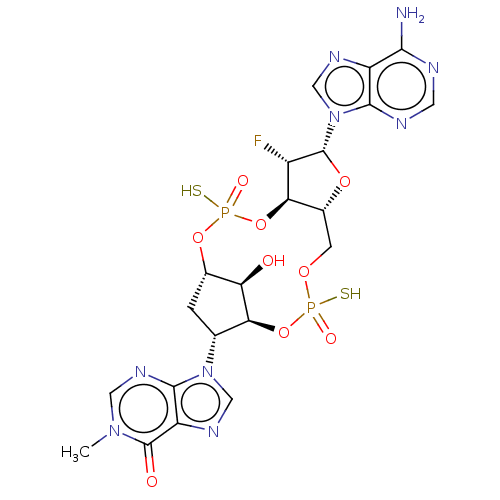 (US10968242, Example 2 Peak 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490789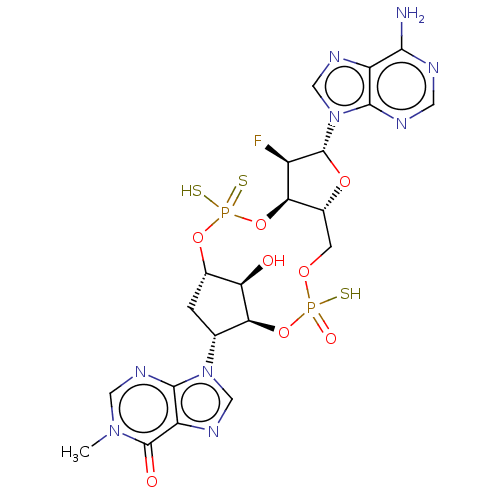 (US10968242, Example 17 Peak 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490781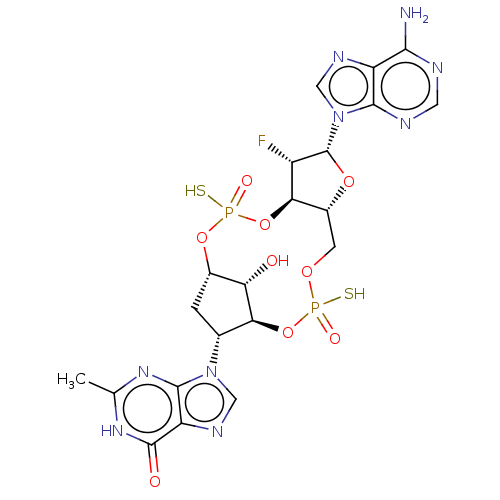 (US10968242, Example 7 Peak 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490774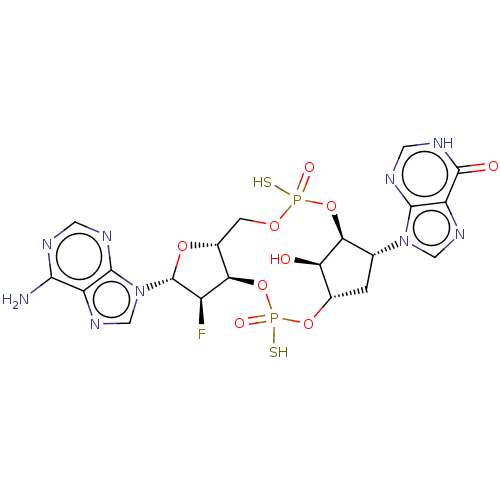 (US10968242, Example 3 Peak 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490770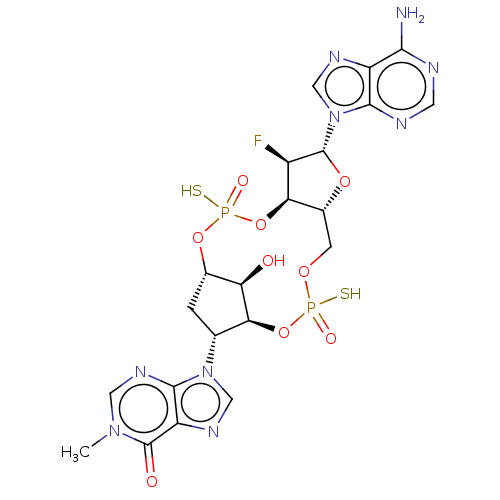 (US10968242, Example 2 Peak 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490784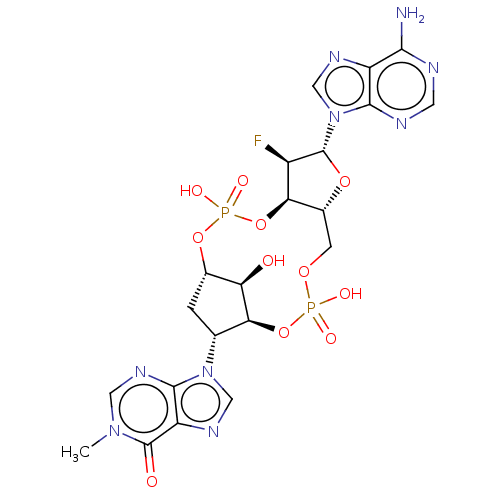 (9-[(4S,6R,7S,11aR,13R,14R,14aR,15R)-13-(6-amino-9H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490799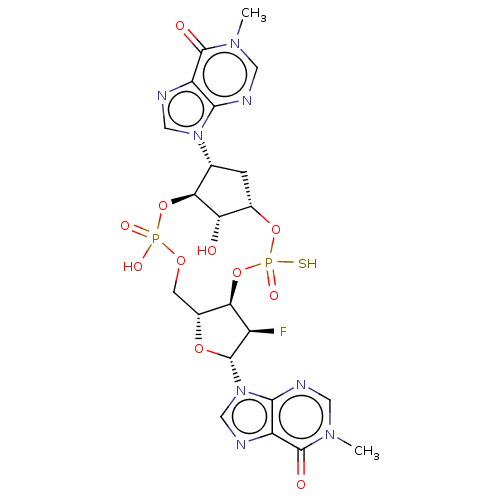 (US10968242, Example 21 Peak 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490788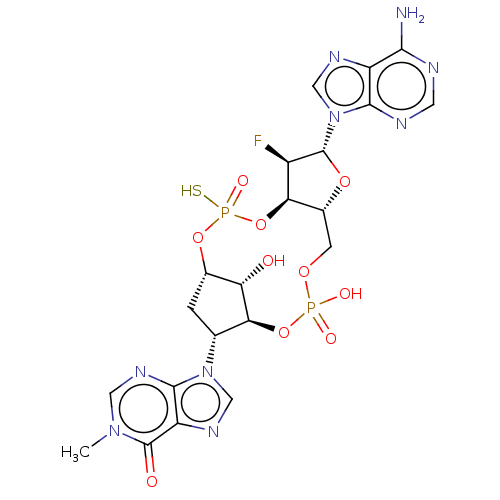 (US10968242, Example 16 Peak 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490780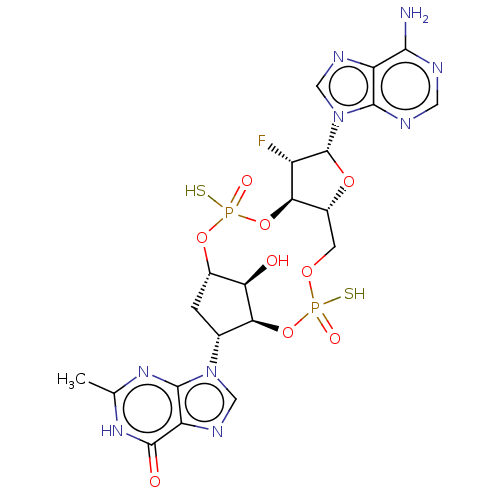 (US10968242, Example 7 Peak 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490775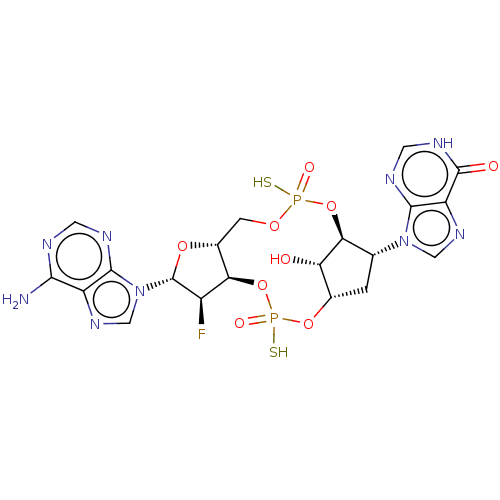 (US10968242, Example 3 Peak 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490792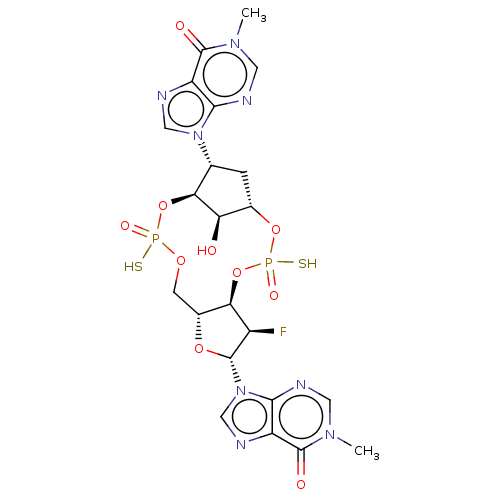 (US10968242, Example 19 Peak 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490785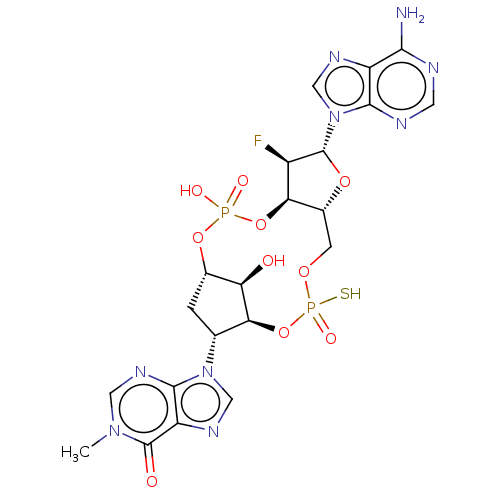 (US10968242, Example 15 Peak 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490797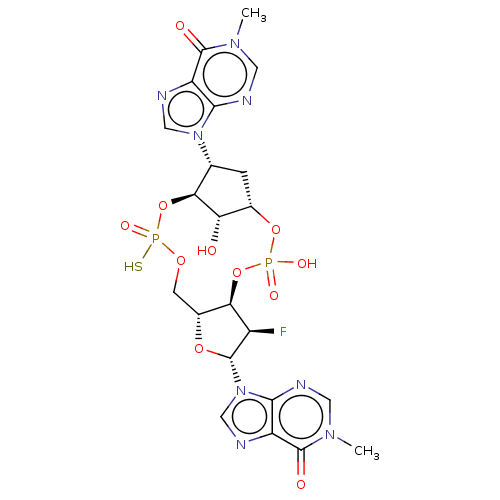 (US10968242, Example 20 Peak 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490769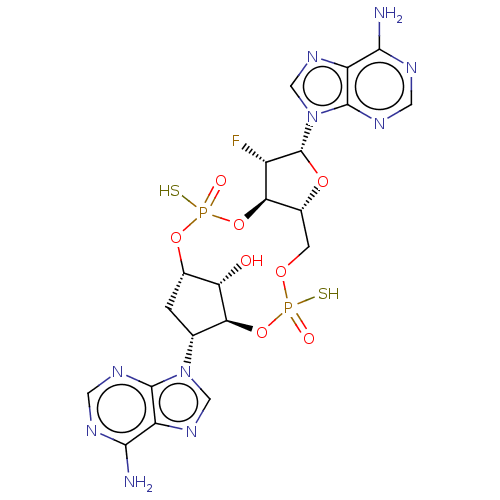 (US10968242, Example 1 Peak 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490778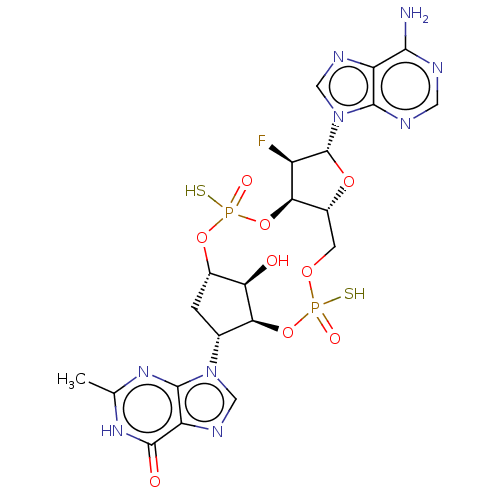 (US10968242, Example 7 Peak 1 | US10968242, Example...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490795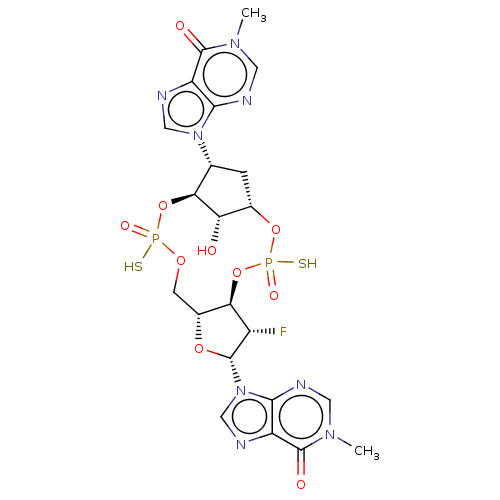 (US10968242, Example 19 Peak 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490771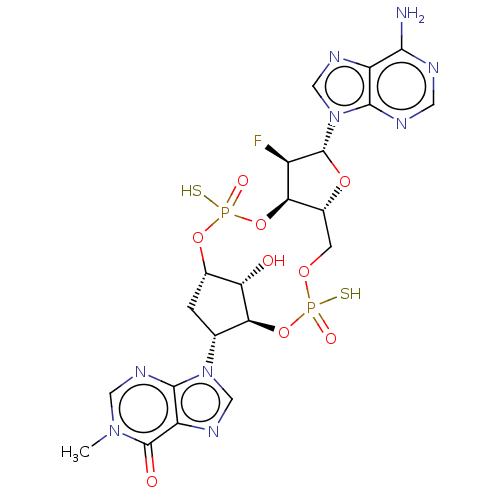 (US10968242, Example 2 Peak 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490767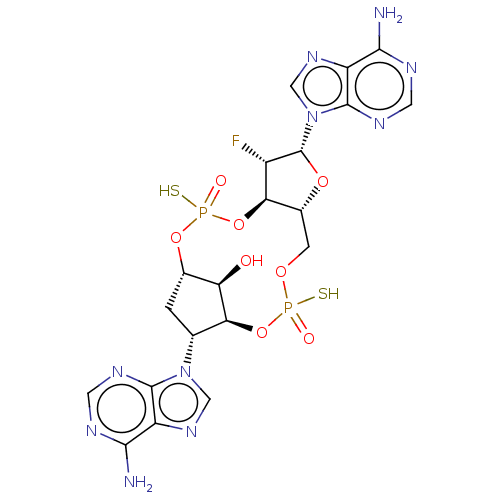 (US10968242, Example 1 Peak 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490778 (US10968242, Example 7 Peak 1 | US10968242, Example...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490798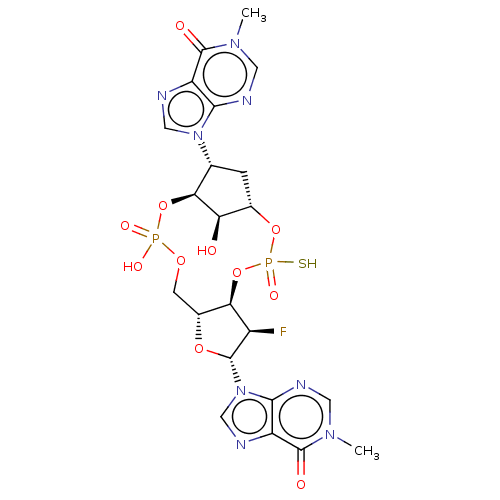 (US10968242, Example 21 Peak 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490800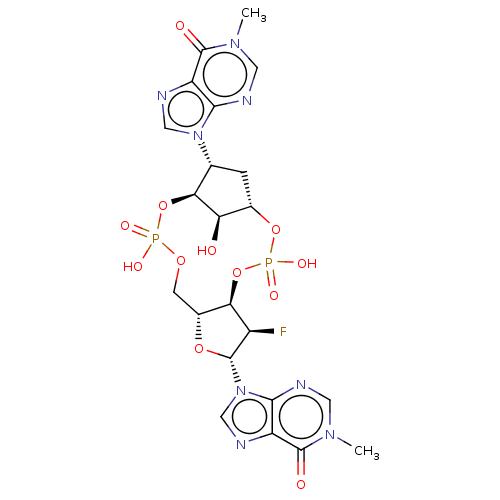 (9,9′-((4S,6R,7S,11aR,13R,14R,14aR,15R)-14-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490796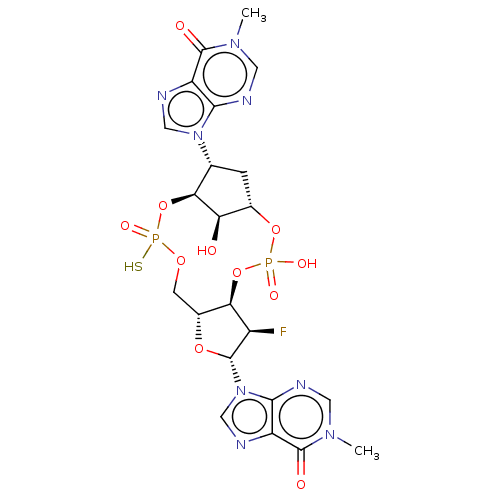 (US10968242, Example 20 Peak 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 294 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490783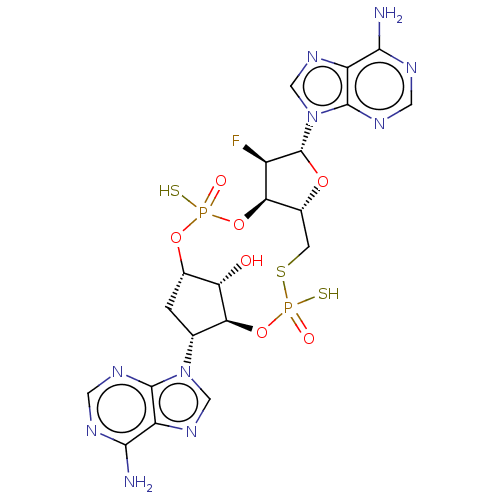 (US10968242, Example 13 Peak 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490793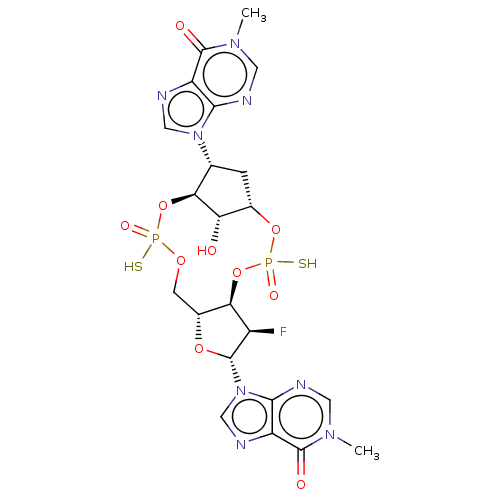 (US10968242, Example 19 Peak 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490782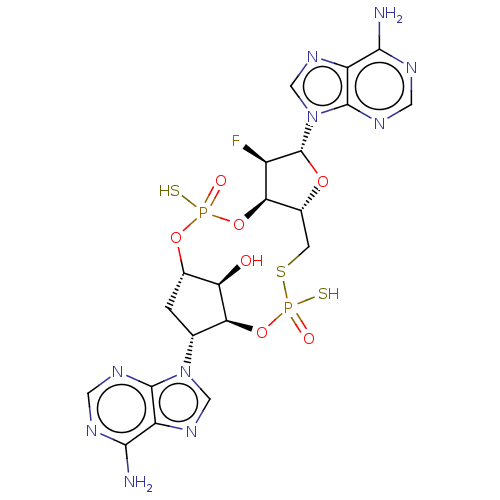 (US10968242, Example 13 Peak 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stimulator of interferon genes protein (Human) | BDBM490801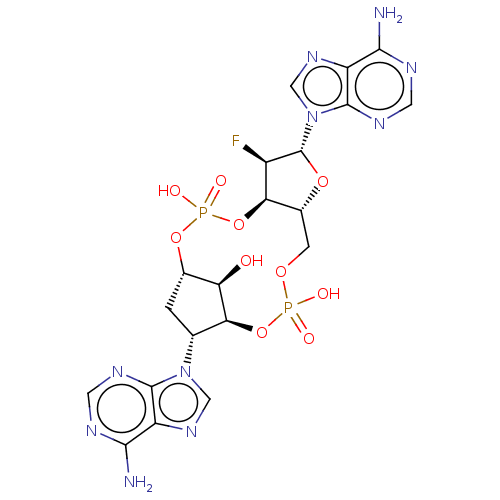 ((4S,6R,7S,11aR,13R,14R,14aR,15R)-6,13-bis(6-amino-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A radioligand binding assay was developed to determine compound interactions were competitive with a tritium-labeled version of the native STING liga... | US Patent US10968242 (2021) BindingDB Entry DOI: 10.7270/Q27W6GB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM451353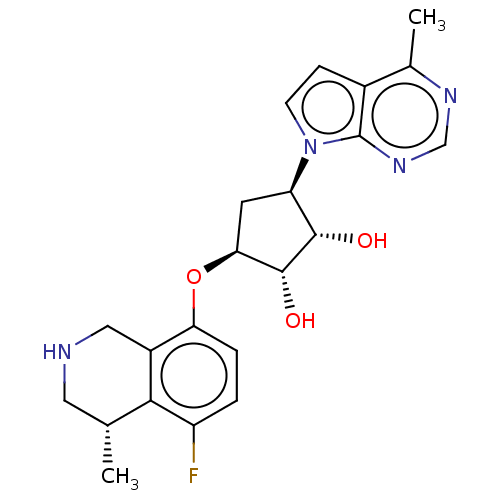 (US10709709, Example 183) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM451354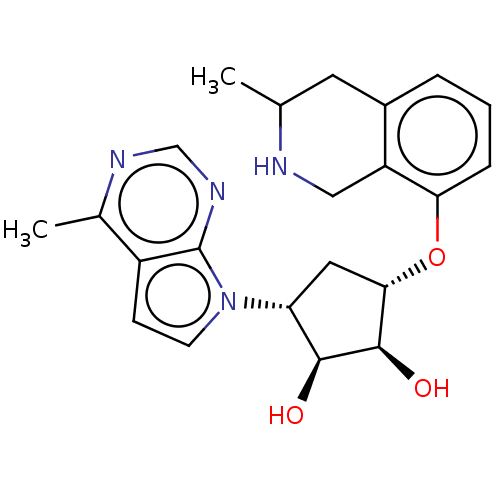 (US10709709, Example 184 | US10709709, Example 185) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM451360 (US10709709, Example 190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM451361 (US10709709, Example 191) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM451364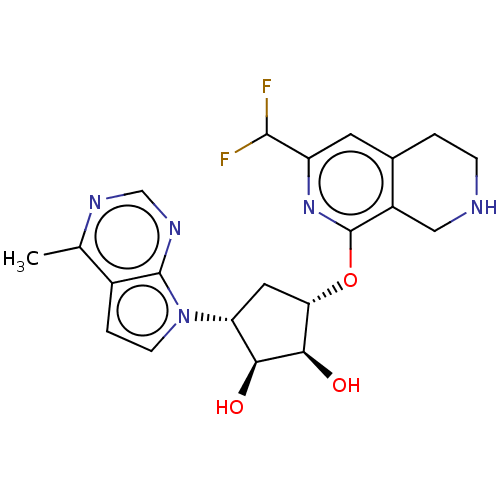 (US10709709, Example 194) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM451365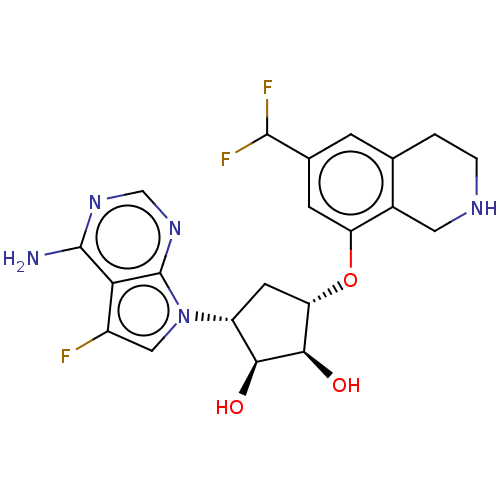 (US10709709, Example 195) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM451366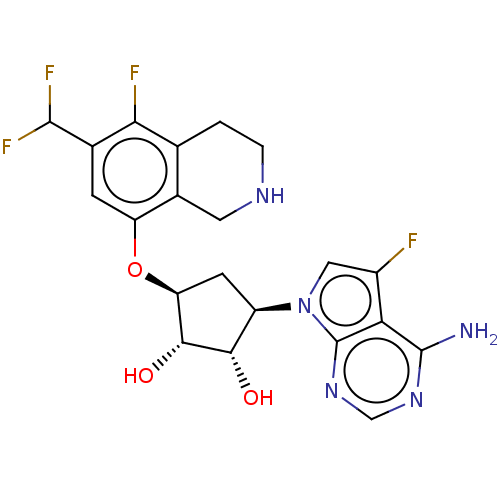 (US10709709, Example 196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM451367 (US10709709, Example 197) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50523649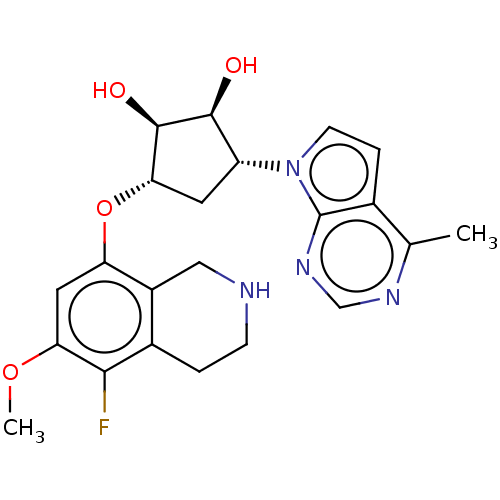 (CHEMBL4448266 | US10709709, Example 198) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50523638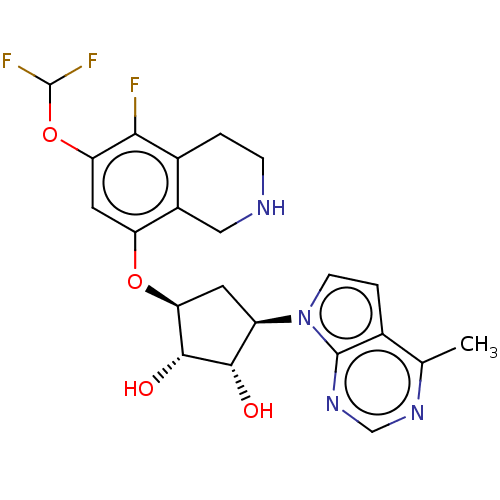 (CHEMBL4470815 | US10709709, Example 200) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM451372 (US10709709, Example 202) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM451373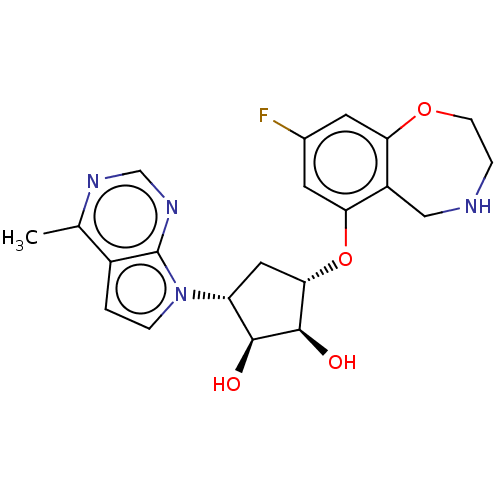 (US10709709, Example 203) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM451375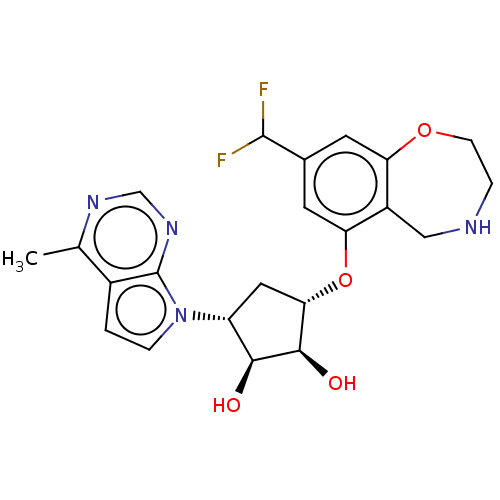 (US10709709, Example 205) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM451380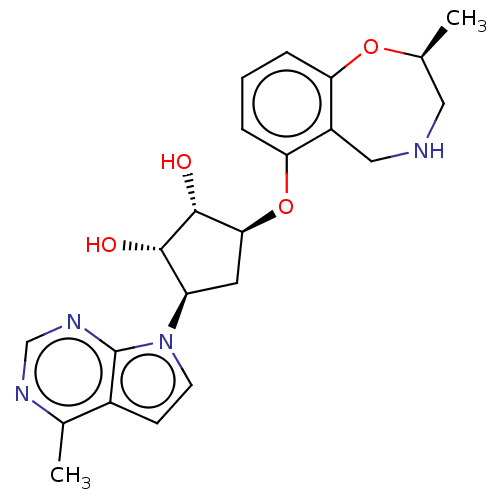 (US10709709, Example 210) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50523654 (CHEMBL4455651 | US10709709, Example 212) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50523654 (CHEMBL4455651 | US10709709, Example 212) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were solubilized in DMSO and serially diluted, using 3-fold dilutions, into 100% DMSO at a concentration 50-fold greater than the desired a... | US Patent US10709709 (2020) BindingDB Entry DOI: 10.7270/Q26113CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1274 total ) | Next | Last >> |