

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50140341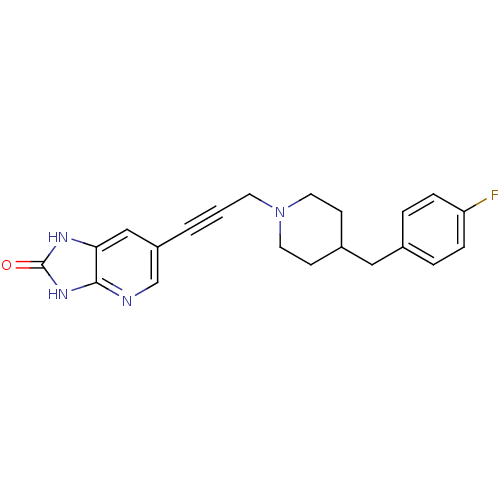 (6-(3-(4-(4-fluorobenzyl)piperidin-1-yl)prop-1-ynyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50091636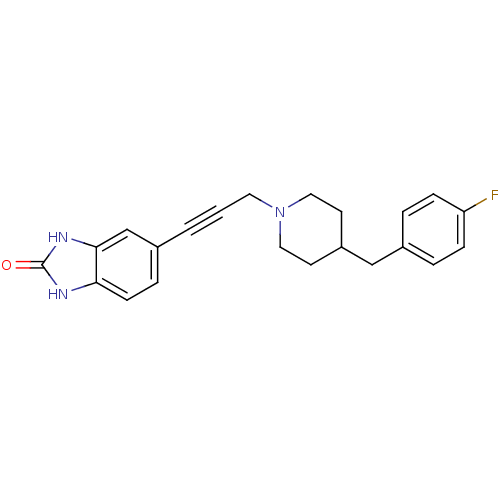 (5-{3-[4-(4-Fluoro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052343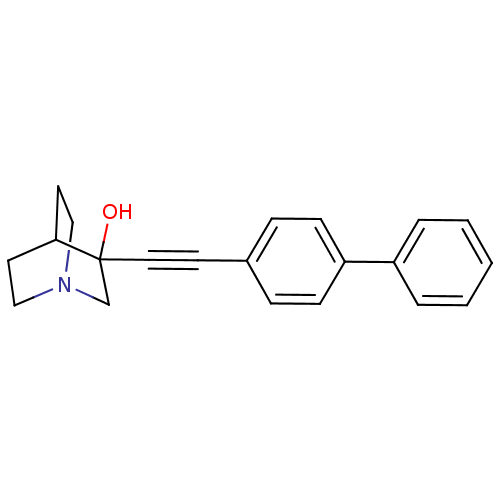 (3-Biphenyl-4-ylethynyl-1-aza-bicyclo[2.2.2]octan-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052350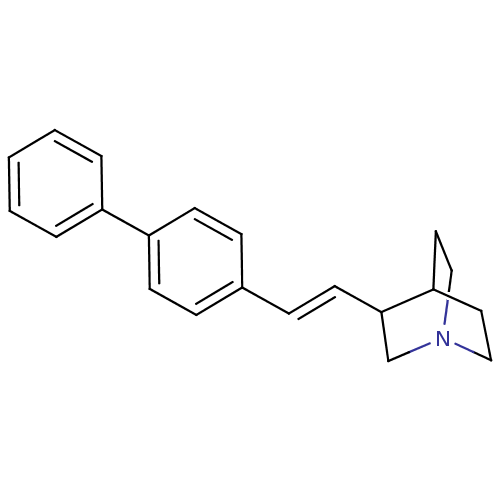 (3-((E)-2-Biphenyl-4-yl-vinyl)-1-aza-bicyclo[2.2.2]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50140339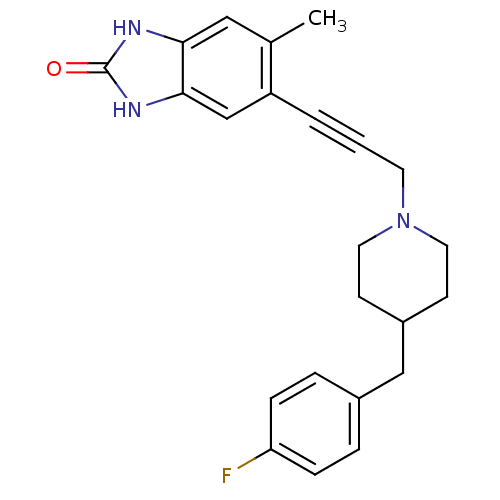 (5-{3-[4-(4-Fluoro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM50068794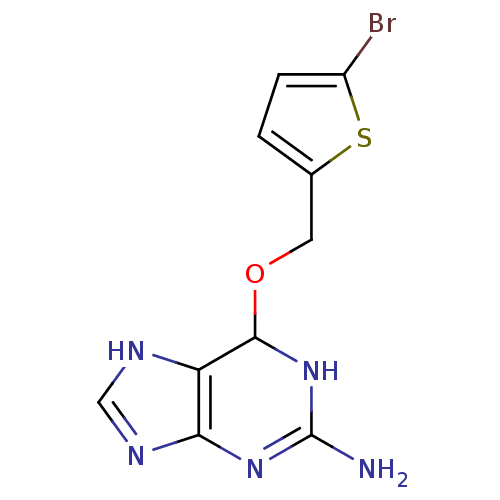 (6-(5-Bromo-thiophen-2-ylmethoxy)-6,9-dihydro-1H-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052342 (3-(2-Biphenyl-4-yl-ethyl)-1-aza-bicyclo[2.2.2]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052353 (3-(4-Benzothiazol-2-yl-phenyl)-1-aza-bicyclo[2.2.2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052355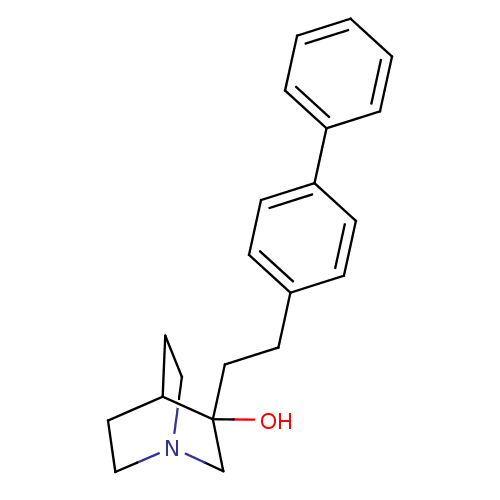 (3-(2-Biphenyl-4-yl-ethyl)-1-aza-bicyclo[2.2.2]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052377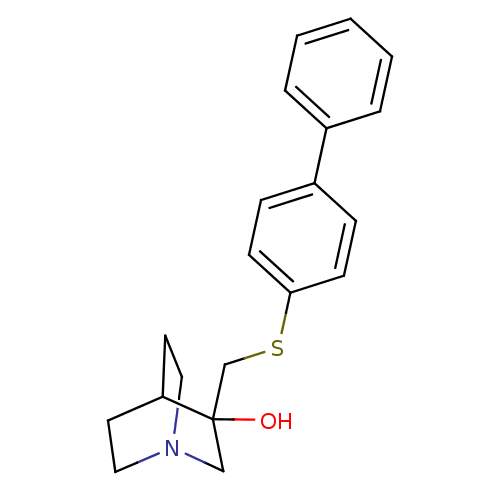 (3-(Biphenyl-4-ylsulfanylmethyl)-1-aza-bicyclo[2.2....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50140333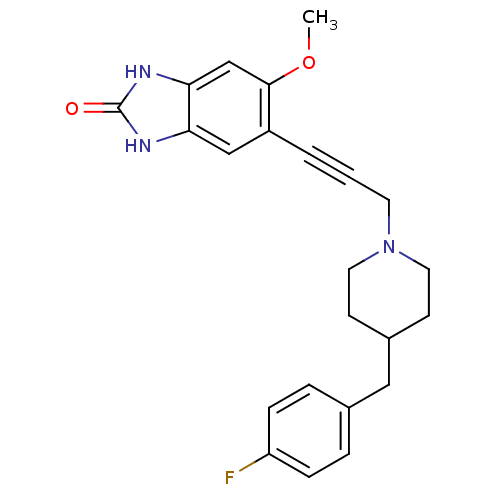 (5-{3-[4-(4-Fluoro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM50068794 (6-(5-Bromo-thiophen-2-ylmethoxy)-6,9-dihydro-1H-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac... | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052376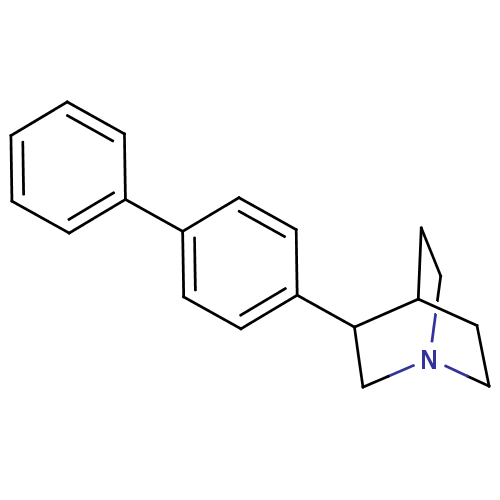 (3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]octane | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052358 (3-(Biphenyl-4-yloxymethyl)-1-aza-bicyclo[2.2.2]oct...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50091635 (5-{3-[4-(3-Fluoro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50140331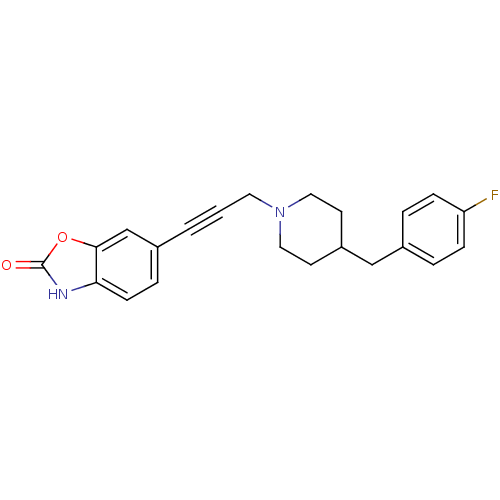 (6-{3-[4-(4-Fluoro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052351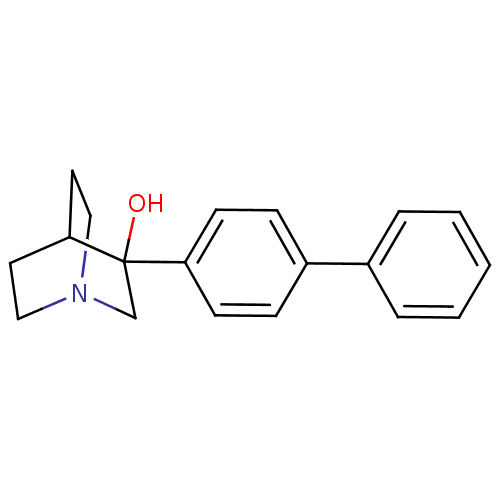 (3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]octan-3-ol | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM50068792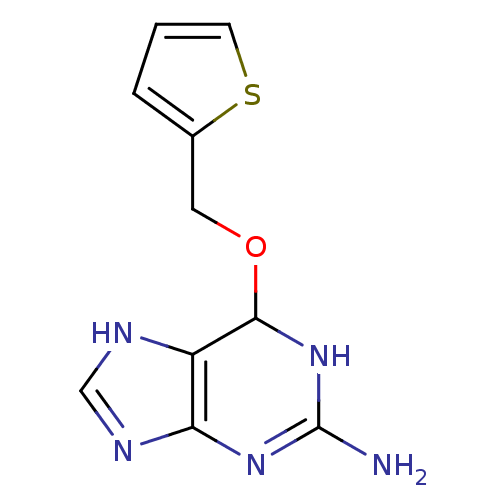 (6-(Thiophen-2-ylmethoxy)-6,9-dihydro-1H-purin-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052366 (3-(4'-Fluoro-biphenyl-4-yl)-1-aza-bicyclo[2.2.2]oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052352 (3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]oct-2-ene | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50140334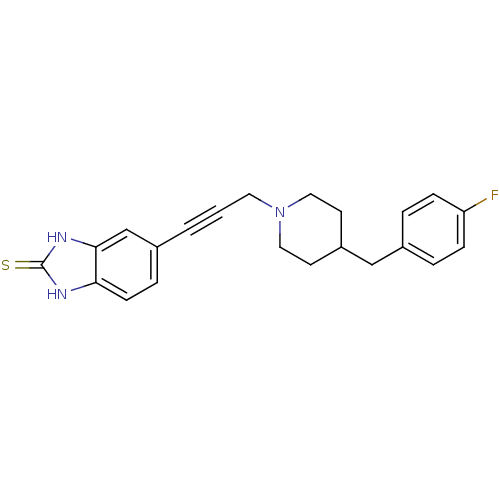 (5-{3-[4-(4-Fluoro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50140327 (6-{3-[4-(4-Fluoro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50091629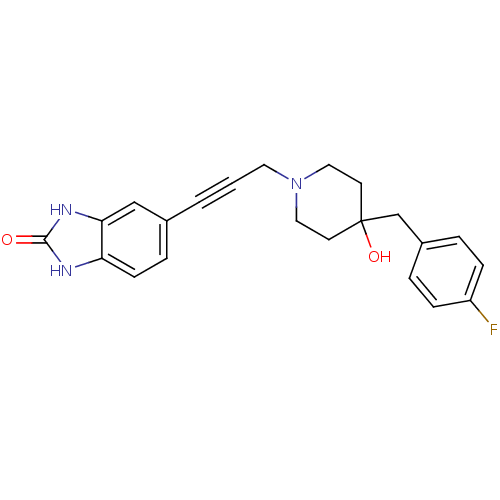 (5-{3-[4-(4-Fluoro-benzyl)-4-hydroxy-piperidin-1-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052367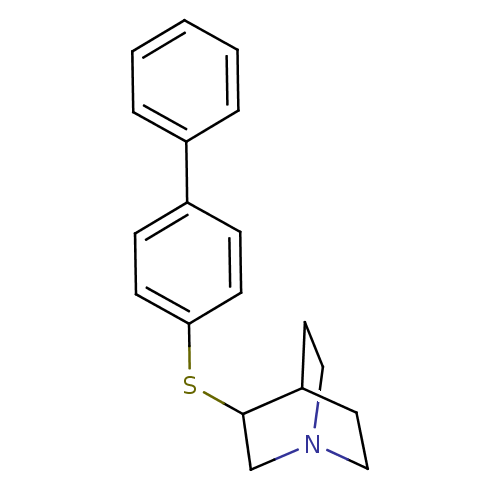 (3-(Biphenyl-4-ylsulfanyl)-1-aza-bicyclo[2.2.2]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50140335 (6-{3-[4-(4-Fluoro-benzyl)-4-hydroxy-piperidin-1-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052357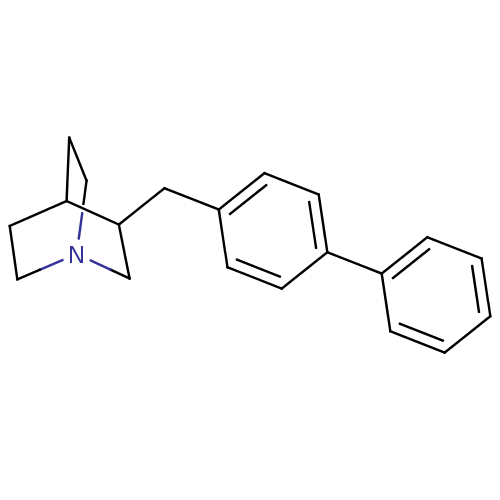 (3-Biphenyl-4-ylmethyl-1-aza-bicyclo[2.2.2]octane |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052372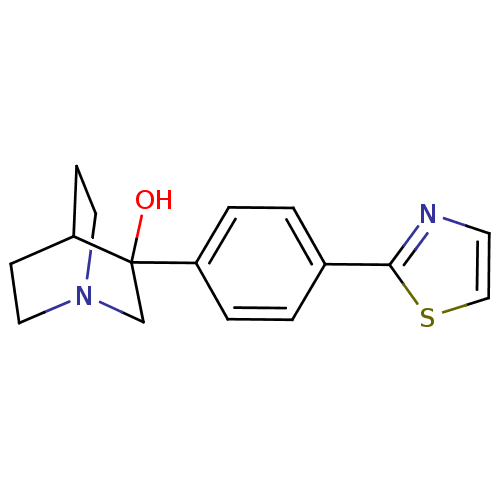 (3-(4-Thiazol-2-yl-phenyl)-1-aza-bicyclo[2.2.2]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052341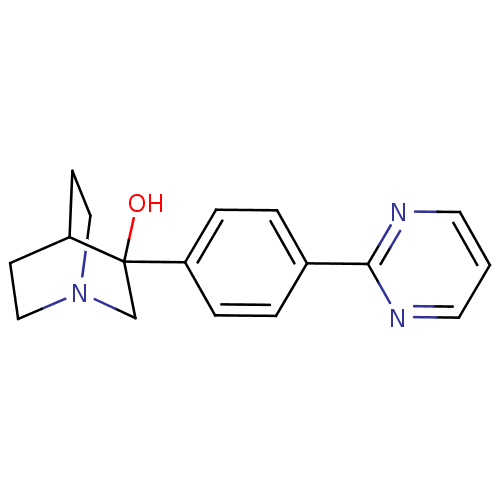 (3-(4-Pyrimidin-2-yl-phenyl)-1-aza-bicyclo[2.2.2]oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM50068792 (6-(Thiophen-2-ylmethoxy)-6,9-dihydro-1H-purin-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac... | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50140325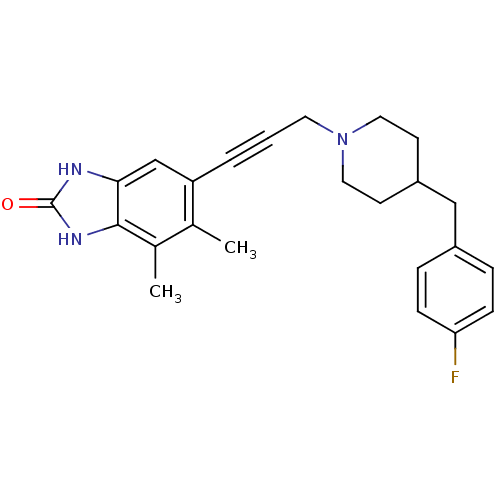 (6-{3-[4-(4-Fluoro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM50068785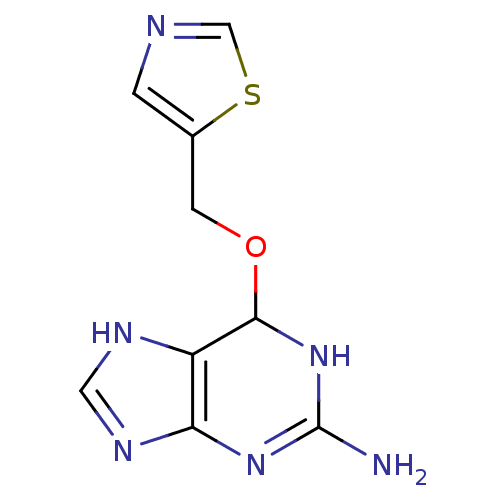 (6-(Thiazol-5-ylmethoxy)-6,9-dihydro-1H-purin-2-yla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052364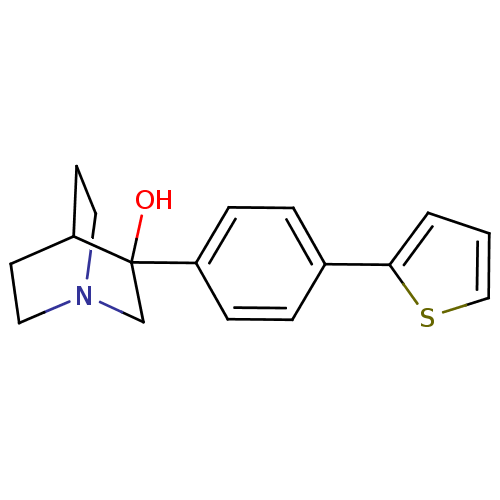 (3-(4-Thiophen-2-yl-phenyl)-1-aza-bicyclo[2.2.2]oct...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM50068786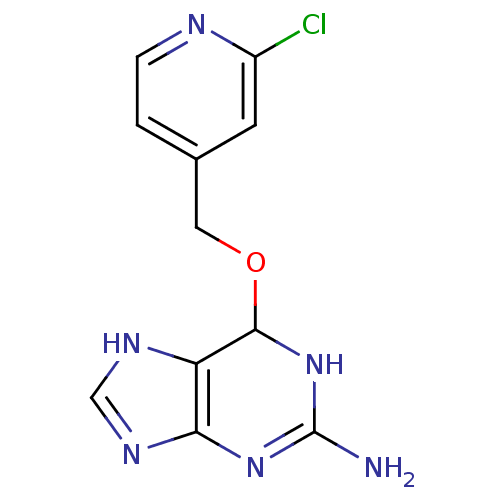 (6-(2-Chloro-pyridin-4-ylmethoxy)-6,9-dihydro-1H-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM5491 (6-(benzyloxy)-9H-purin-2-amine | CHEMBL407874 | O6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM50068786 (6-(2-Chloro-pyridin-4-ylmethoxy)-6,9-dihydro-1H-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac... | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50140336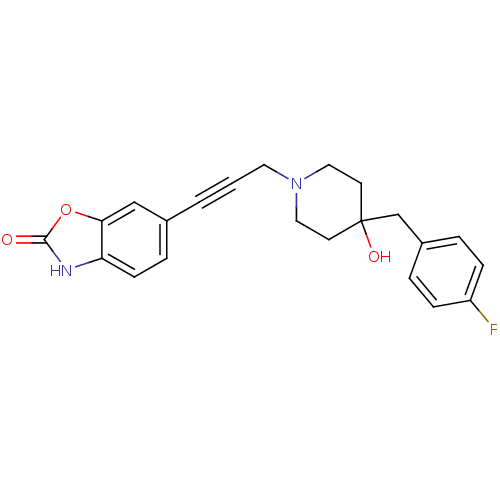 (6-{3-[4-(4-Fluoro-benzyl)-4-hydroxy-piperidin-1-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50140340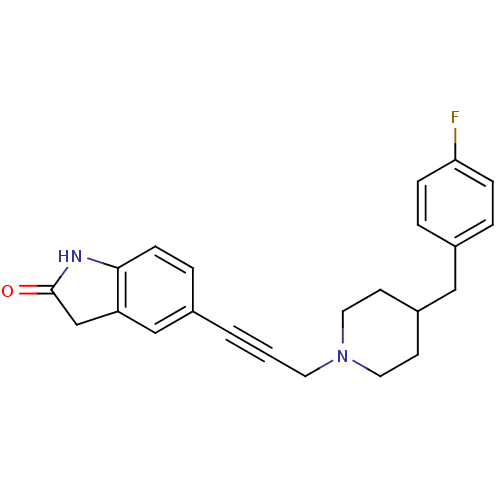 (5-{3-[4-(4-Fluoro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052344 (3-Biphenyl-3-yl-1-aza-bicyclo[2.2.2]octan-3-ol | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM50068785 (6-(Thiazol-5-ylmethoxy)-6,9-dihydro-1H-purin-2-yla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac... | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052373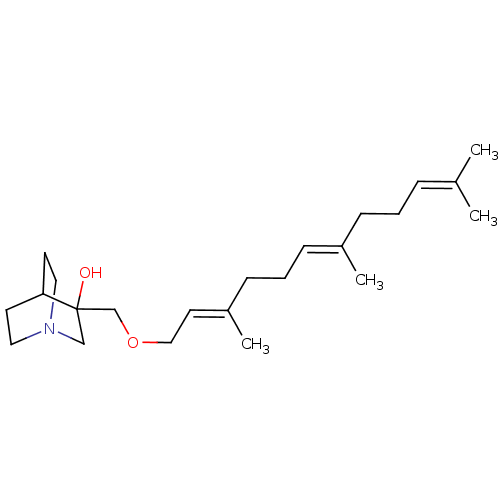 (3-((2E,6E)-3,7,11-Trimethyl-dodeca-2,6,10-trienylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052362 (3-(2-Phenyl-thiazol-5-yl)-1-aza-bicyclo[2.2.2]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM50068784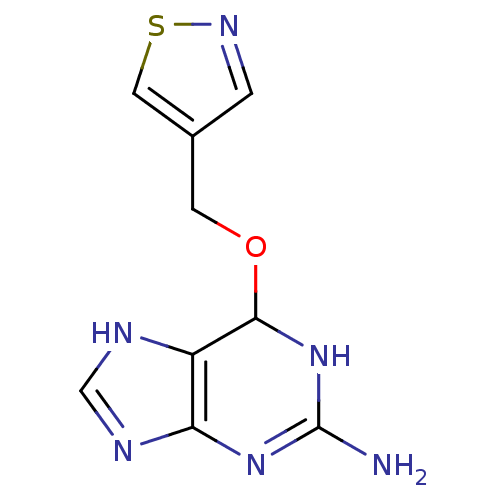 (6-(Isothiazol-4-ylmethoxy)-6,9-dihydro-1H-purin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052361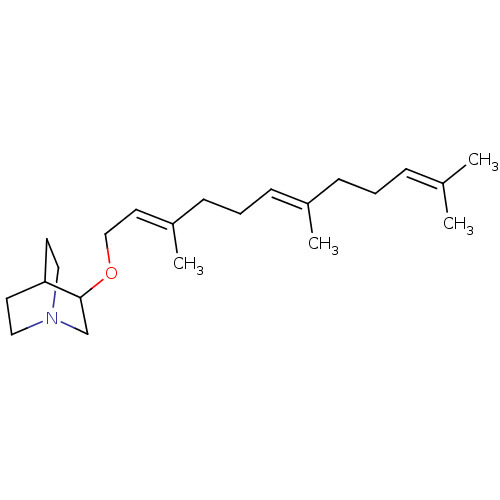 (3-((2E,6E)-3,7,11-Trimethyl-dodeca-2,6,10-trienylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM50068784 (6-(Isothiazol-4-ylmethoxy)-6,9-dihydro-1H-purin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac... | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM50068783 (6-(Furan-2-ylmethoxy)-6,9-dihydro-1H-purin-2-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM50068789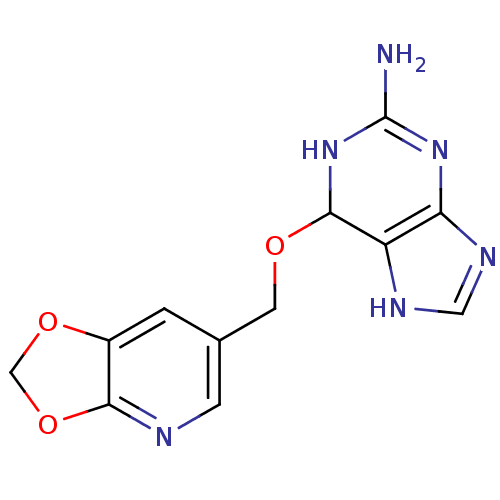 (6-([1,3]Dioxolo[4,5-b]pyridin-6-ylmethoxy)-6,9-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac... | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50140328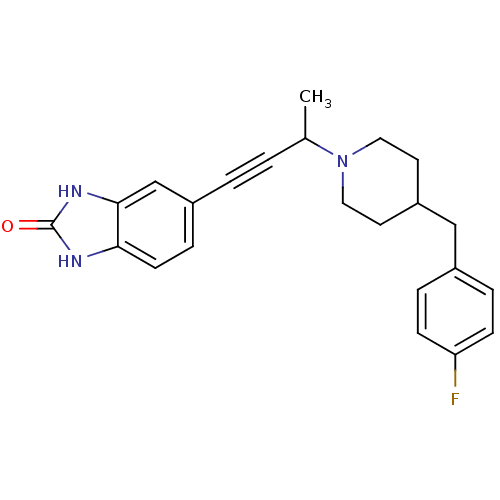 (5-{3-[4-(4-Fluoro-benzyl)-piperidin-1-yl]-but-1-yn...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM5491 (6-(benzyloxy)-9H-purin-2-amine | CHEMBL407874 | O6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac... | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Rattus norvegicus (Rat)-RAT) | BDBM50140332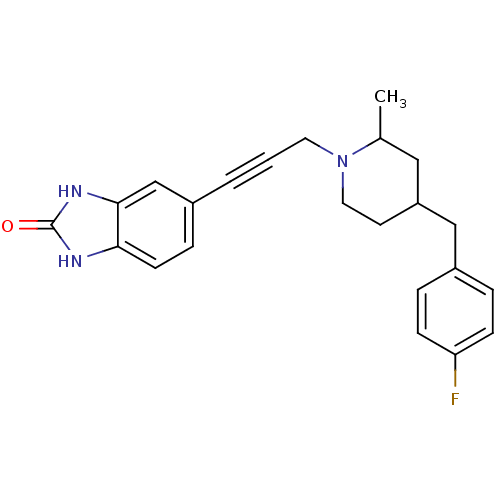 (5-{3-[4-(4-Fluoro-benzyl)-2-methyl-piperidin-1-yl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity against NR1A/2B receptor in rat brains by [3H]-ifenprodil displacement. | Bioorg Med Chem Lett 14: 1213-6 (2004) Article DOI: 10.1016/j.bmcl.2003.12.049 BindingDB Entry DOI: 10.7270/Q2057FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylated-DNA--protein-cysteine methyltransferase (Homo sapiens (Human)) | BDBM50068783 (6-(Furan-2-ylmethoxy)-6,9-dihydro-1H-purin-2-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac... | J Med Chem 41: 5265-71 (1999) Article DOI: 10.1021/jm9708644 BindingDB Entry DOI: 10.7270/Q2DJ5G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 165 total ) | Next | Last >> |